Abstract
The photosynthetic carbon reduction cycle, or Calvin–Benson–Bassham (CBB) cycle, is now contained in every standard biochemistry textbook. Although the cycle was already proposed in 1954, it is still the subject of intense research, and even the structure of the cycle, i.e. the exact series of reactions, is still under debate. The controversy about the cycle's structure was fuelled by the findings of Gibbs and Kandler in 1956 and 1957, when they observed that radioactive 14CO2 was dynamically incorporated in hexoses in a very atypical and asymmetrical way, a phenomenon later termed the ‘photosynthetic Gibbs effect’. Now, it is widely accepted that the photosynthetic Gibbs effect is not in contradiction to the reaction scheme proposed by CBB, but the arguments given have been largely qualitative and hand-waving. To fully appreciate the controversy and to understand the difficulties in interpreting the Gibbs effect, it is illustrative to illuminate the history of the discovery of the CBB cycle. We here give an account of central scientific advances and discoveries, which were essential prerequisites for the elucidation of the cycle. Placing the historic discoveries in the context of the modern textbook pathway scheme illustrates the complexity of the cycle and demonstrates why especially dynamic labelling experiments are far from easy to interpret. We conclude by arguing that it requires sound theoretical approaches to resolve conflicting interpretations and to provide consistent quantitative explanations.
Keywords: Calvin cycle, carbon fixation, Gibbs effect, mathematical model
Introduction
The Calvin–Benson–Bassham (CBB) cycle [1,2] — often simply termed ‘Calvin cycle’ or ‘Calvin–Benson cycle’ [3] or, as preferred by James Alan Bassham, the ‘photosynthetic carbon reduction (PCR) cycle’ [4] — is undoubtedly one of the most important biochemical pathways on earth. Plants and many other photosynthetic organisms employ it to fix carbon dioxide and reduce it to sugars. The key enzyme RuBisCO, which catalyses the carboxylation reaction, is probably the most abundant enzyme on earth [5,6], responsible for over 99% of global carbon dioxide fixation [7]. Not surprisingly, the biochemical mechanisms of inorganic carbon fixation have been the subject of intense scientific investigations since the early days of metabolic research.
Since first suggested by the German chemist Adolf Baeyer in 1870 [8], it was widely assumed that the primary product of carbon fixation should be formaldehyde, formed by photo-excited chlorophylls, and that formaldehyde molecules undergo subsequent polymerisation to sugars [9]. This hypothesis even received experimental support by Klein and Werner in 1926 [10] using the chemical dimedone (dimethylhydroresorcinol), which allows detecting even small quantities of formaldehyde. However, when repeating these experiments to address a few unsolved questions, such as the observation that formaldehyde was mainly found in the extracellular medium, Barton-Wright and Pratt could show, in 1930 [11], that formaldehyde production was an experimental artefact and ruled out that it is connected to photosynthesis. Lacking a plausible alternative theory, the formaldehyde hypothesis was nevertheless still widely accepted.
The interdisciplinary breakthrough: radioactive isotopes
It took the intuition of interdisciplinary scientists to apply techniques from nuclear physics to photosynthesis research to falsify the formaldehyde hypothesis. With the invention and actual construction of the first cyclotron [12] at the University of California, Berkeley, in 1932, it became possible to generate high-energy particles and use them to produce radionuclides. Applying this new technology to produce the radioactive isotope 11C (a positron emitter with a half-life of 20 min) opened dramatically new perspectives, because it allowed marking single carbon atoms to follow their fate, regardless of in which molecules these atoms were bound and which chemical or biochemical reactions they underwent. The scientists had developed ‘an eye, which could look into the plant cells’ [13]. Using 11C, Ruben and Kamen showed that the primary product of carboxylation must contain a carboxyl group [14]. These experiments not only started the long and tedious elucidation of the complete CBB cycle but also represent the birth of using radiolabelling techniques in biological research. Owing to the short half-life of 11C, which limited the possible experiment time to just a few hours, it was not suited to reveal more details about the series of reactions now known as the CBB cycle. Experimenting with different targets bombarded with high-energy particles in the cyclotron, Ruben and Kamen discovered different ways to produce 14C as early as 1941 [15]. Partly due to the low beta emission and low specific activity, but probably mainly due to the obvious hindrances to carry out research during World War II, they never used it in photosynthetic experiments [16]. However, with the discovery of the long-lived isotope 14C (half-life of ∼5700 years), they set the foundation for the major breakthrough of Melvin Calvin and his team after the end of the war.
Melvin Calvin's quest for the photosynthetic carbon fixation pathway
Only after World War II, starting in 1945, photosynthesis experiments with 14C were systematically carried out by Melvin Calvin and Andrew A. Benson, who operated their photosynthesis research laboratory adjacent to the cyclotron, in which 14C could be produced [16,17]. Benson first confirmed with 14C what Ruben had conjectured already with 11C that the primary product of carboxylation contains a carboxyl group. Experiments with preilluminated Chlorella and Scenedesmus algae provided evidence that the first product of carbon fixation is phosphoglyceric acid (PGA) [13,18]. Elucidating the path of photosynthesis was also strongly facilitated by the clever experimental set-up devised by Andrew Bassham, which was often dubbed ‘lollipop’ due to its appearance. In an upright flat glass disc, algae were illuminated from both sides. Through a stopper at the top, radioactive carbon could be applied and the suspension bubbled with gas, while a stopper at the bottom allowed harvesting directly into boiling alcohol, immediately killing the algae and stopping the reaction [19]. Refining the technique of two-dimensional paper chromatography [13], and varying the illumination time, during which Chlorella and Scenedesmus were fed with radio-labelled carbon dioxide, it became possible to identify the organic compounds containing the radioactive carbon atoms.
Nevertheless, the long-standing question of the nature of the acceptor of the carbon atom from carbon dioxide was still unsolved. Observations by Calvin that the pool sizes of ribulose 1,5-bisphosphate (RuBP) and PGA changed in a reciprocal fashion when algae were subjected to changes in light intensity [20] gave strong hints that RuBP was indeed the substrate providing the 2-carbon backbone to which the carbon atom from carbon dioxide was fixed. In their seminal paper from 1954, Melvin Calvin and his co-workers proposed their famous scheme, which is now known as the CBB cycle. There they confirmed the reciprocal behaviour of RuBP and PGA also under changes in carbon dioxide concentration. Apparently, they had no doubt regarding the nature of the carbon acceptor and it is therefore even more remarkable that the enzyme catalysing the formation of PGA from RuBP and carbon dioxide was isolated and characterised [21] only 2 years later by Weissbach et al. Interestingly, it took another year until it was realised by Dorner et al. [22] that this enzyme, termed ‘carboxylation enzyme’ by Horecker and ‘carboxydismutase’ by Calvin, was actually the same as the ‘Fraction 1 protein’, already isolated in 1947 at CalTech (Pasadena) by Wildman and Bonner by ammonium sulphate fractionation [23]. The now commonly used name RuBisCO was only coined more than 20 years later by David Eisenberg in a talk at a symposium honouring Wildman [24].
However, even the strong indication Calvin and co-workers had regarding the nature of the carbon dioxide acceptor molecule, the problem was still open by which series of reactions this substrate could be recycled. Here, the pioneering biochemical research by Racker and Horecker set the foundation to derive a plausible reaction scheme. Both researchers investigated the series of reactions, now known as the pentose phosphate pathway (PPP), by which pentose phosphates are converted into hexose phosphates. In the early 1950s, both groups independently published the action of the enzyme transketolase (a name coined by Racker), isolated from rat liver [25], yeast [26] and spinach [27], demonstrating that it catalyses the transfer of two carbons from a keto-sugar to an aldo-sugar. Simultaneously, Horecker isolated and characterised the enzyme aldolase from rat liver [28], which together with his isolation of transaldolase from rat liver and yeast [29] formed the core of the PPP. Knowing these results, and excluding many chemically feasible alternatives by a series of careful experiments with short pulses of radioactive carbon dioxide, Calvin and co-workers finally proposed their famous reaction scheme in 1954 [2]. However, in contrast with the carboxylation, the authors were apparently uncertain regarding the existence and activity of transketolase in chloroplasts, as is nicely illustrated by the question marks at the respective locations in the proposed scheme in Figure 7 of the 1954 publication.
Challenging Calvin's scheme: the photosynthetic Gibbs effect
The 1954 paper by Melvin Calvin and his collaborators is an illustrative example of rigorous scientific work. Experimental evidence is provided in various forms, foremost in short-pulse 14CO2 labelling experiments to identify in which sugars the radioactive label is found, and — by chemical degradation — also the position of the labels in pentoses and heptoses was determined. By logical reasoning, various potential pathways were excluded, so that the paper concludes with the scheme of the CBB cycle as a logically derived, plausible hypothesis. However, the authors are also critical of their own results and suggest further experiments to test how the reaction scheme could be verified. Here is where Martin Gibbs enters the scene. Appointed in 1947 as a junior scientist in the Brookhaven National Laboratory, his task was to synthesise radiocarbon-labelled sugars and to provide them to other researchers. Because the only available compound with radioactive carbon at that time was barium carbonate, an obvious solution for Martin Gibbs (chemist by training, PhD in botany) was to produce these sugars from radioactive carbon dioxide using photosynthesis. One of the early problems he was facing was to localise the radioactive carbon in the produced sugars. Together with Irwin C. Gunsalus, this problem was solved by exploiting the unusual fermentation route of Leuconostoc mesenteroides, which degrades glucose into carbon dioxide (from carbon 1), ethanol (carbons 2 and 3) and lactic acid (carbons 4, 5 and 6) [30]. This fermentation pathway allowed a unique determination of the positions of the radioactive carbon atoms, a decisive advantage over the degradation by Lactobacillus casei, introduced by Wood et al. in 1945 [31], which only allowed a separation into 2-carbon groups (3–4, 2–5, 1–6). The Leuconostoc method soon became a standard for many laboratories interested in carbon metabolism. This method was regularly employed before the modern powerful 13C NMR spectroscopy technique was successfully applied to microbial metabolism in 1976 [32]. With 13C NMR spectroscopy, individual isotopomers can be identified and quantified in a complex mixture, based on changes in the spectrum depending on the positioning of the 13C-labelled atoms. This ability to give a detailed time-resolved description of metabolic events makes 13C NMR spectroscopy superior to all previously applied tracer techniques [33].
Bernard Horecker initially used the Leuconostoc method to investigate the conversion of pentose phosphates into hexose phosphates in rat liver [34] by applying pentose phosphate that was labelled either in position 1 or in positions 2 and 3. The results gave rise to suggesting a series of reactions, now known as the PPP, in which reactions catalysed by transketolase, transaldolase and aldolase inter-convert six pentoses in five hexoses. In the same year, Gibbs and Horecker [35] studied the same pathway in extract from pea roots and leaves. While they found that the root extract showed essentially the same isotope label pattern as rat liver extract, the experiments with leaf extract were significantly different. Aware of the ongoing studies by Calvin to unravel the path of carbon in photosynthesis, who have demonstrated that ribulose, sedoheptulose, fructose and glucose phosphates are rapidly labelled during photosynthesis [36], he concluded that the path to recycle pentoses into hexoses is of significant importance in photosynthesis.
Otto Kandler, a German botanist from Munich, was sceptical about the carbon fixation scheme proposed by Calvin and visited Martin Gibbs to test the proposed pathway with the newly developed Leuconostoc method. Before the establishment of this new method, various researchers [37–39] reported that photosynthetically derived hexoses are rapidly labelled in the 3,4 positions, while the label appeared later in the 2,5 and 1,6 positions, where sometimes an equal and sometimes an unequal label intensity were observed [40]. Comparing these results with experiments of carbon fixation in the dark [40] or non-photosynthetic tissues like rat liver [31], in which the label was almost exclusively found in the 3,4 positions, led to the assumption that the initial steps of carbon fixation are identical in the light and dark, and that the presence of light was responsible only for the further distribution of the labels; a hypothesis that was rapidly refuted after the initial experiments of Kandler and Gibbs.
Applying the new Leuconostoc method, Kandler and Gibbs observed, to their great surprise, that the distribution of labels in glucose phosphate [41] and starch-derived glucose [42] formed during photosynthesis was, in fact, very asymmetric. Regardless of the precise experimental conditions and the botanical origin of the hexoses or hexose phosphates investigated, they found a persistent asymmetry in that the 4-carbon was always labelled before the 3-carbon, while the 1-carbon was labelled stronger than the 6-carbon and the 2-carbon stronger than the 5-carbon. This surprising finding of asymmetric label incorporation during photosynthetic carbon fixation is now often referred to as the ‘Gibbs effect’, which, as stressed by Chrispeels [43] in his biographical memoirs of Martin Gibbs, ‘is yet to be explained’.
Kandler and Gibbs observed these asymmetries in various algae and plant species, but the exact order, in which carbons 1, 2, 5 and 6 are labelled, differed. So how can the common features, such as the 4/3, 1/6 and 2/5 asymmetries, be interpreted and how can it be understood, which processes determine the exact labelling dynamics?
Some aspects can indeed be simply explained. Bassham noted already in 1964 [44] that the asymmetry in the 3 and 4 positions can be explained by different pool sizes of the triose phosphates. Radioactive carbon from carbon dioxide is incorporated into glyceraldehyde phosphate (GAP) in the carboxyl carbon (carbon 1). GAP is isomerised to dehydroxy acetone phosphate (DHAP) in a reversible reaction, which is close to equilibrium [45]. However, the DHAP pool is ∼20 times larger than the GAP pool. Therefore, the fraction of radioactive carbon atoms in position 3 of DHAP (which results from the 1-carbon of GAP after isomerisation) increases more slowly than the label in GAP. During condensation of GAP and DHAP by aldolase, the 1-carbon of GAP becomes the 4-carbon of fructose 1,6-bisphosphate (FBP), while the 3-carbon of DHAP enters position 3. Since the further conversion to hexose phosphates and hexoses stored in starch, the position of carbon atoms is unchanged, it is obvious that Gibbs and Kandler always observed a higher radioactivity in position 4 compared with position 3.
In contrast, explaining the 1/6 and 2/5 asymmetries is far more challenging. Although Bassham [44] explained the observed pattern by the ‘reversibility of transketolase’, it remains difficult to find verbal explanations for the exact appearance of labels in the various intermediates of the CBB cycle. Indeed, Trebst and Fiedler [46] argued in 1962 that the observations by Kandler and Gibbs ‘are in full agreement’ with the proposed reaction scheme, whereas Stiller concludes in the same year [47] that the ‘entire pathway from triose phosphate to pentose phosphate … is inadequate in a wide selection of organisms’.
The rediscovery of quantitative biology as an interdisciplinary science
Traditionally, biological researchers have often sought theoretical explanations of their observations, and mathematical formulae have been commonly found in many publications to support interpretations. Also, Bassham employed quantitative arguments in his 1964 publication [44] in an attempt to explain the asymmetric label distribution observed by Gibbs and Kandler [41,42]. His mathematical considerations are, in fact, simplifying mathematical models, which attempt to explain observations based on theoretical considerations. Quantitative predictions can best be generated by mathematical models, which are based on a sound theory.
Indeed, the validation of pathway schemes with detailed mathematical models and the explanation of seemingly contradictory experimental findings have a long history. For example, the observation that ornithine added to liver slices [48] did not increase urea production was used as an argument against the structure of the urea cycle, as proposed originally by Krebs and Henseleit [49,50]. However, with computer models, Kuchel et al. [51] have shown in 1977 that these observations are actually in agreement with the proposed pathway scheme, explaining the observation in the context of metabolic control analysis [52–54] with an extremely low control coefficient.
The non-oxidative PPP is closely related to the CBB cycle, with many enzymes shared between the pathways. Also, the structure of the PPP was heavily debated. In 1978, Williams proposed an alternative reaction scheme for the non-oxidative PPP in rat liver, the so-called F-type pathway, involving five additional intermediates (arabinose 5-phosphate, d-glycero-d-ido-octulose1,8-bisphosphate, d-glycero-d-altro-octulose1,8-bisphosphate, altro-heptulose1,7-bisphosphate and mannoheptulose 7-phosphate) [55]. However, with a dynamic model including many different isotopomers, built to investigate the ‘label scrambling’, in particular by the transketolase and transaldolase reactions [56], it was shown that the observed data are well in agreement with the classical (L-type) pathway (if one considers the reversibility of the transketolase, transaldolase and aldolase reactions) and that the F-type pathway, while biochemically possible, carries a negligible flux.
More conceptionally, Kuchel and Philp [57] argued that a model does not necessarily need to include all isotopomers, but that a lot can be learned about the underlying pathway scheme by calculating the label patterns that may be generated at all by a certain series of reactions. For this, they presented a formal algebraic description of isotopomer-exchange reactions, which allows determination of the subspace of all isotopomers, which can, in principle, be produced by a given pathway scheme. By predicting the limitations of the isotopomer space, this method provides a powerful generic tool to distinguish between different proposed pathway schemes and comparing the predictions to experimentally determined isotopomers, when substrates with different label patterns are supplied. For the Gibbs effect, however, this elegant approach is not easily applicable, because it turns out that providing the label through CO2, containing only a single carbon atom, in principle all isotopomers can be produced. Consequently, to explain the Gibbs effect, a dynamic model including all possible isotopomers is needed.
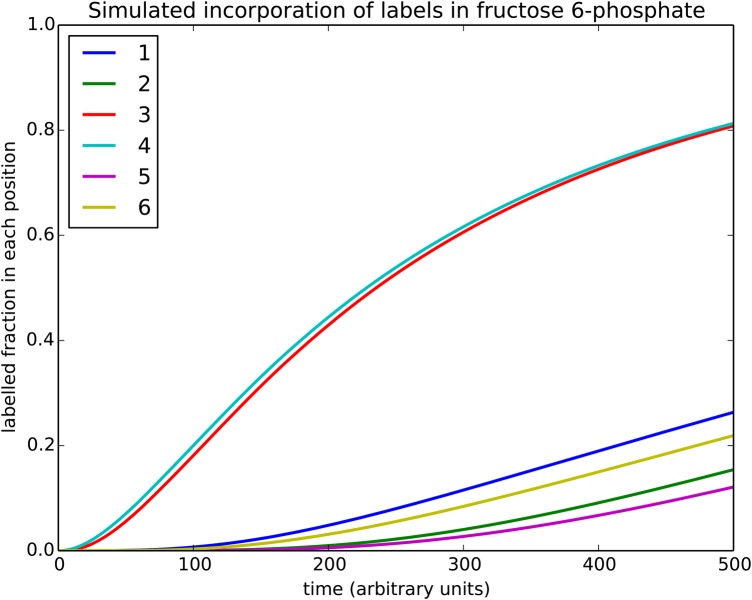
At the Metabolic Pathway Analysis conference in Bozeman, MT in 2017, we presented preliminary results of a dynamic model that simulates the label incorporation dynamics of the CBB cycle. The model incorporates all reactions originally proposed by Calvin and co-workers [2], and the parameters were chosen, such that the model exhibits experimentally determined steady-state pool sizes [45]. Together with the thermodynamic properties of the reactions, the pool sizes determine the degree of reversibility, in particular for those reactions catalysed by transketolase, aldolase, isomerases and epimerases. Simulating the label distribution involved definition of dynamic variables representing all possible labelling patterns for all intermediates. For example, 23 = 8 variables describe the patterns of GAP, while 27 = 128 versions of sedoheptulose 1,7-bisphosphate (SBP) are included. Reactions were included reflecting the exact carbon transition maps for all transformations, resulting in 3904 isotope-specific rate equations. Under the chosen conditions, the model predicts the dynamic appearance of radioactive labels in hexose phosphate as shown in Figure 1. Here, the x-axis displays the time in arbitrary units. In this preliminary study, this was chosen because we are interested in generic properties of the network. Moreover, the experimentally observed labelling dynamics differ quite substantially between different species [42,58]. The robustly observed orders of label appearance (4 before 3, 1 before 6 and 2 before 5) are clearly reproduced. While it should be stressed that these results are only reflecting one particular experimental condition, and generality still remains to be demonstrated, the theoretical results indicate that the consistently observed asymmetries are a direct consequence of the structure and the thermodynamics of the CBB cycle.
Figure 1. Simulated dynamic incorporation of radioactive carbon during photosynthesis.
Displayed is a simulated time course of the incorporation of radioactive carbon dioxide into the CBB cycle intermediate fructose 6-phosphate. The six lines indicate the percentage of carbons labelled in each position with colours indicated in the legend. As observed in the Gibbs effect, labels are introduced in position 4 before position 3 and appear earlier in position 1 (2) than in 6 (5). For the simulation, the standard reaction scheme of the CBB cycle is assumed, where the transketolase, transaldolase and aldolase are considered to be reversible. Time on the x-axis is in arbitrary units.
So, is the scheme of Calvin finally proved? Not by a long shot!
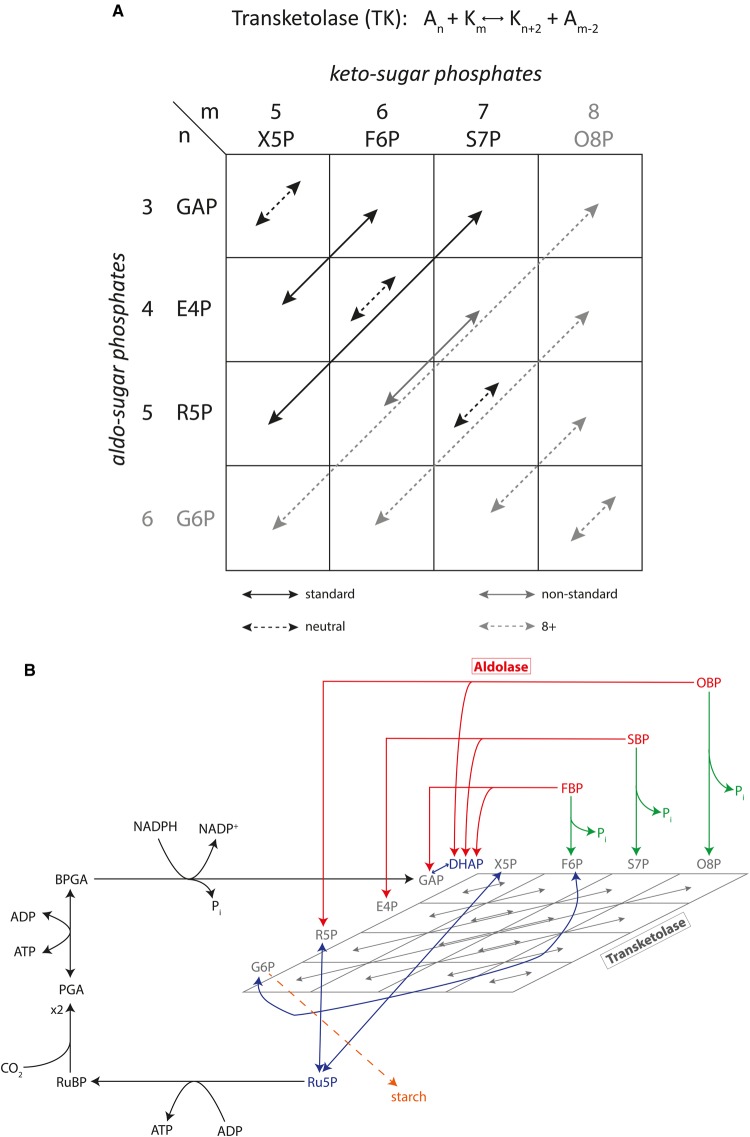
One of the key enzymes in the recycling process from triose phosphates to pentose phosphates is transketolase, which — as already subsumed by Calvin in 1954 — catalyses two key steps: the transformation of a triose phosphate and a hexose phosphate into a tetrose phosphate and a pentose phosphate, and of a triose phosphate and a heptose phosphate into two pentose phosphates. Both reactions occur according to the same scheme: carbons 1 and 2 from a ketose are transferred to an aldose. Considering that the catalysed reactions are readily reversible (see, e.g. [45]), the donor ketose is a pentose, hexose or heptose, while the acceptor aldose is a triose, tetrose or pentose. There is no convincing argument why the four (two reactions in two directions) transformations should be the only ones transketolase can catalyse. Why should not all nine possible combinations of ketose and aldose phosphates serve as substrates? In fact, using radiocarbon labelling, Clark et al. [59] showed in vitro that rat liver transketolase catalyses also the neutral exchange reaction erythrose 4-phosphate (E4P) + glucose 6-phosphate (G6P) <=> G6P + E4P, indicating clearly that the specificity of transketolase is not limited to the two reactions in the classical CBB cycle scheme. Moreover, it has long been debated whether longer sugars, such as eight carbon octoses, play a role in the recycling reactions. Although theoretical studies by Philip Kuchel and co-workers indicate that the F-type non-oxidative PPP carries only a negligible flux, in 2006, Williams and Flanigan demonstrated transketolase activity for C8 sugars and measured the distribution of labelled 13CO2 in these octoses using a new gas chromatography and electron ionisation mass spectrometry methodology combined with selected ion monitoring (GC/EIMS/SIM) [60,61]. This clearly indicates that transketolase presents a far more complex picture than originally assumed by Calvin. Seemingly complex, all possible activities by transketolase can easily be explained by the basic mechanism of transferring a 2-carbon group from a ketose to an aldose phosphate. All possible combinations can be compactly depicted by the scheme in Figure 2. Potentially, all 16 reactions will contribute to distributing the label around the different positions in the intermediate sugar phosphates.
Figure 2. Broad substrate specificity of transketolase.
(A) Schematic representation of all transketolase-catalysed reactions. Columns represent ketose-phosphates with m = 5.8 carbons, while rows represent aldo-phosphates with n = 3.6 carbons. Every possible reaction is indicated by an arrow, where the start and end squares stand for the substrates/products of the respective reaction. For example, the arrow leading from (n,m) = (4, 5) to (n,m) = (3, 6) represents the reaction E4P + X5P = GAP + F6P. The solid black arrows indicate the two standard reactions in the CBB cycle, the solid grey arrow a third reaction, which is also in agreement with the general transketolase reaction scheme, and is, in fact, a linear combination of the two solid black reactions. ‘Neutral reactions’ are neutral in the sense that substrates and products are the same chemical compounds (e.g. E4P + F6P = F6P + E4P), but isotopes are exchanged. Dashed grey arrows indicate reactions that are chemically feasible if 8-carbon sugars are included. (B) Alternative representation of the CBB cycle. This representation includes the full complexity introduced by the broad substrate specificity of the transketolase and aldolase enzymes. The rectangular scheme from A is ‘tilted’, thus appearing as a lozenge, to indicate a layer, in which isotope scrambling occurs. The black reactions include carbon fixation and reduction and are driven by external energy input. Green reactions represent phosphatases, which also display a high-energy gradient and are practically irreversible under physiological conditions. Red arrows represent the reversible aldolase reactions, and blue arrows represent isomerases and epimerases. In this representation, the separation of the cycle into energy-driven (black and green) and entropy-driven (grey, red and blue) reactions becomes visible.
Conclusions
What do the experimental and theoretical results discussed here tell us about the true structure of the CBB cycle? The preliminary results from mathematical models indicate that assuming the CBB cycle in its strict form is in accordance with the experimentally observed asymmetries of labels initially reported by Kandler and Gibbs. However, the results certainly do not preclude the existence of additional reactions, which possibly only have a minor influence on label distributions. Experimental evidence and rational arguments allow us to re-interpret the activity of the key enzyme transketolase. Instead of viewing it as catalysing exactly two reactions, we need to understand it as an enzyme catalysing a highly specific transfer of two carbons from a ketose phosphate to an aldose phosphate, but for a broad range of substrates. Since the reactions are highly reversible, the enzyme constantly mixes sugar phosphates of different lengths, much alike the ‘entropic’ carbohydrate-active enzymes experimentally and theoretically described by Kartal et al. in 2011 [62]. The thermodynamic driving force is an increase in the mixing entropy of the substrate and product mixture. Because all of the 16 possible reactions are close to (or at) equilibrium, the individual forward and backward rates of the reversible processes must be considerably higher than the overall net conversion rate. Consequently, the scheme of the CBB cycle, as originally proposed, can still be considered as correct, understanding that it indicates the net conversion fluxes. However, it does ignore a large number of reversible transformations, which do not contribute to the net flux, but which certainly have some influence on the label incorporation dynamics. An attempt to depict the complexity caused by the reversible reactions with broad substrate specificity, catalysed particularly by transketolase (and to some degree by aldolase), is given in Figure 2B. Currently, there is no possibility to determine the rates of the individual reactions separately in vivo. Fortunately, mathematical models are ideally suited to test how all these ‘invisible’ reactions influence predicted labelling patterns. Like the discovery of radioactive carbon isotopes provided scientists a new ‘eye’ to look into processes inside leaves, so can mathematical models serve as yet another ‘eye’, by which internal details of metabolism can be made visible, that lie beyond our experimental observation capabilities.
It must be concluded, however, that despite the enormous power in theoretical approaches to discriminate between and derive novel hypotheses, only experiments can provide confidence that the theories realistically describe reality. Therefore, in addition to develop novel models and theories, also a constant improvement and further development of experimental techniques are required.
Funding
This work was financially supported by the Deutsche Forschungsgemeinschaft “Cluster of Excellence on Plant Sciences” CEPLAS (EXC 1028) to O.E. and by the European Union Marie Curie ITN AccliPhot (PITN-GA-2012-316427) to St.S.
Abbreviations
- CBB
Calvin–Benson–Bassham
- DHAP
dehydroxy acetone phosphate
- E4P
erythrose 4-phosphate
- G6P
glucose 6-phosphate
- GAP
glyceraldehyde phosphate
- PGA
phosphoglyceric acid
- PPP
Pentose Phosphate Pathway
- RuBP
ribulose 1,5-bisphosphate
Competing Interests
The Authors declare that there are no competing interests associated with the manuscript.
References
- 1.Bassham J.A., Benson A.A. and Calvin M. (1950) The path of carbon in photosynthesis. J. Biol. Chem. 185, 781–787 PMID: [PubMed] [Google Scholar]
- 2.Bassham J.A., Benson A.A., Kay L.D., Harris A.Z., Wilson A.T. and Calvin M. (1954) The path of carbon in photosynthesis. XXI. The cyclic regeneration of carbon dioxide acceptor. J. Am. Chem. Soc. 76, 1760–1770 10.1021/ja01636a012 [DOI] [Google Scholar]
- 3.Govindjee M. (2010) Celebrating Andrew Alm Benson's 93rd birthday. Photosynth. Res. 105, 201–208 10.1007/s11120-010-9591-3 [DOI] [PubMed] [Google Scholar]
- 4.Govindjee M., Bassham H. and Bassham S. (2016) Remembering James Alan Bassham (1922–2012). Photosynth. Res. 128, 3–13 10.1007/s11120-015-0201-2 [DOI] [PubMed] [Google Scholar]
- 5.Ellis R.J. (1979) The most abundant protein in the world. Trends Biochem. Sci. 4, 241–244 10.1016/0968-0004(79)90212-3 [DOI] [Google Scholar]
- 6.Raven J.A. (2013) Rubisco: still the most abundant protein of Earth? New Phytol. 198, 1–3 10.1111/nph.12197 [DOI] [PubMed] [Google Scholar]
- 7.Raven J.A. (2009) Contributions of anoxygenic and oxygenic phototrophy and chemolithotrophy to carbon and oxygen fluxes in aquatic environments. Aquat. Microb. Ecol. 56, 177–192 10.3354/ame01315 [DOI] [Google Scholar]
- 8.Baeyer A. (1870) Über die Wasserentziehung und ihre Bedeutung für das Pflanzenleben und die Gährung. Eur. J. Inorg. Chem. 3, 63–75 10.1002/cber.18700030123 [DOI] [Google Scholar]
- 9.Willstätter R. and Stoll A. (1918) Untersuchungen über die Assimilation der Kohlensäure. Springer, Berlin [Google Scholar]
- 10.Klein G. and Werner O. (1926) Formaldehyd als Zwischenprodukt bei der Kohlensäureassimilation. Biochem. Zeitschr. 168, 361–386 [Google Scholar]
- 11.Barton-Wright E.C. and Pratt M.C. (1930) Studies in photosynthesis: the formaldehyde hypothesis. Biochem. J. 24, 1210–1216 10.1042/bj0241210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrence E.O. and Livingston M.S. (1932) The production of high speed light ions without the use of high voltages. Phys. Rev. 40, 19–35 10.1103/PhysRev.40.19 [DOI] [Google Scholar]
- 13.Calvin M. (1949) The path of carbon in photosynthesis. J. Chem. Educ. 26, 639 10.1021/ed026p639 [DOI] [Google Scholar]
- 14.Ruben S., Kamen M.D., Hassid W.Z. and DeVault D.C. (1939) Photosynthesis with radio-carbon. Science 90, 570–571 10.1126/science.90.2346.570 [DOI] [PubMed] [Google Scholar]
- 15.Ruben S. and Kamen M.D. (1941) Long-lived radioactive carbon: C14. Phys. Rev. 59, 349–354 10.1103/PhysRev.59.349 [DOI] [Google Scholar]
- 16.Benson A.A. (2002) Following the path of carbon in photosynthesis: a personal story. Photosynth. Res. 73, 29–49 10.1023/A:1020427619771 [DOI] [PubMed] [Google Scholar]
- 17.Bassham J.A. (2003) Mapping the carbon reduction cycle: a personal retrospective. Photosynth. Res. 76, 35–52 10.1023/A:1024929725022 [DOI] [PubMed] [Google Scholar]
- 18.Benson A.A. and Calvin M. (1950) The path of carbon in photosynthesis: VII. Respiration and photosynthesis. J. Exp. Bot. 1, 63–68 10.1093/jxb/1.1.63 [DOI] [Google Scholar]
- 19.Buchanan B.B. and Wong J.H. (2013) A conversation with Andrew Benson: reflections on the discovery of the Calvin–Benson cycle. Photosynth. Res. 114, 207–214 10.1007/s11120-012-9790-1 [DOI] [PubMed] [Google Scholar]
- 20.Calvin M. and Massini P. (1952) The path of carbon in photosynthesis. Experientia 8, 445–457 10.1007/BF02139287 [DOI] [PubMed] [Google Scholar]
- 21.Weissbach A., Horecker B.L. and Hurwitz J. (1956) The enzymatic formation of phosphoglyceric acid from ribulose diphosphate and carbon dioxide. J. Biol. Chem. 218, 795–810 PMID: [PubMed] [Google Scholar]
- 22.Dorner R.W., Kahn A. and Wildman S.G. (1957) The proteins of green leaves. VII. Synthesis and decay of the cytoplasmic proteins during the life of the tobacco leaf. J. Biol. Chem. 229, 945–952 PMID: [PubMed] [Google Scholar]
- 23.Wildman S.G. and Bonner J. (1947) The proteins of green leaves. I. Isolation, enzymatic properties and auxin content of spinach cytoplasmic proteins. Arch. Biochem. 14, 381–413 PMID: [PubMed] [Google Scholar]
- 24.Wildman S.G. (2002) Along the trail from Fraction I protein to Rubisco (ribulose bisphosphate carboxylase-oxygenase). Photosynth. Res. 73, 243–250 10.1023/A:1020467601966 [DOI] [PubMed] [Google Scholar]
- 25.Horecker B.L. and Smyrniotis P.Z. (1952) The enzymatic formation of sedoheptulose phosphate from pentose phosphate. J. Am. Chem. Soc. 74, 2123–2123 10.1021/ja01128a524 [DOI] [Google Scholar]
- 26.Racker E., De La Haba G. and Leder I.G. (1953) Thiamine pyrophosphate, a coenzyme of transketolase. J. Am. Chem. Soc. 75, 1010–1011 10.1021/ja01100a526 [DOI] [Google Scholar]
- 27.Horecker B.L. and Smyrniotis P.Z. (1953) The coenzyme function of thiamine pyrophosphate in pentose phosphate metabolism. J. Am. Chem. Soc. 75, 1009–1010 10.1021/ja01100a525 [DOI] [Google Scholar]
- 28.Horecker B.L., Smyrniotis P.Z. and Klenow H. (1953) The formation of sedoheptulose phosphate from pentose phosphate. J. Biol. Chem. 205, 661–682 PMID: [PubMed] [Google Scholar]
- 29.Horecker B.L. and Smyrniotis P.Z. (1953) Transaldolase: the formation of fructose-6-phosphate from sedoheptulose-7-phosphate. J. Am. Chem. Soc. 75, 2021–2022 10.1021/ja01104a532 [DOI] [Google Scholar]
- 30.Gunsalus I.C. and Gibbs M. (1952) The heterolactic fermentation. II. Position of C14 in the products of glucose dissimilation by Leuconostoc mesenteroides. J. Biol. Chem. 194, 871–875 PMID: [PubMed] [Google Scholar]
- 31.Wood H.G., Lifson N. and Lorber V. (1945) The position of fixed carbon in glucose from rat liver glycogen. J. Biol. Chem. 159, 475. [PubMed] [Google Scholar]
- 32.Walker T.E., London R.E., Whaley T.W., Barker R. and Matwiyoff N.A. (1976) Carbon-13 nuclear magnetic resonance spectroscopy of [1-13C] enriched monosaccharides. Signal assignments and orientational dependence of geminal and vicinal carbon-carbon and carbon-hydrogen spin-spin coupling constants. J. Am. Chem. Soc. 98, 5807–5813 10.1021/ja00435a011 [DOI] [Google Scholar]
- 33.Jeffrey F.M.H., Rajagopal A., Malloy C.R. and Sherry A.D. (1991) C-NMR: a simple yet comprehensive method for analysis of intermediary metabolism. Trends Biochem. Sci. 16, 5–10 10.1016/0968-0004(91)90004-F [DOI] [PubMed] [Google Scholar]
- 34.Horecker B.L., Gibbs M., Klenow H. and Smyrniotis P.Z. (1954) The mechanism of pentose phosphate conversion to hexose monophosphate: I. With a liver enzyme preparation. J. Biol. Chem. 207, 393–404 PMID: [PubMed] [Google Scholar]
- 35.Gibbs M. and Horecker B.L. (1954) The mechanism of pentose phosphate conversion to hexose monophosphate: II. With pea leaf and pea root preparations. J. Biol. Chem. 208, 813–820 PMID: [PubMed] [Google Scholar]
- 36.Benson A.A., Bassham J.A., Calvin M., Hall A.G., Hirsch H., Kawaguchi S. et al. (1952) The path of carbon in photosynthesis, XV. Ribulose and sedoheptulose. J. Biol. Chem. 196, 703–716 PMID: [DOI] [PubMed] [Google Scholar]
- 37.Benson A.A. and Calvin M. (1950) Carbon dioxide fixation by green plants. Annu. Rev. Plant Physiol. 1, 25–42 10.1146/annurev.pp.01.060150.000325 [DOI] [Google Scholar]
- 38.Varner J.E. and Burrell R.C. (1950) Use of C14 in the study of the acid metabolism of Bryophyllum calycinum. Arch. Biochem. 25, 280–287 PMID: [PubMed] [Google Scholar]
- 39.Vittorio P., Krotkov G. and Reed G.B. (1950) Labelling in the glucose deposited as starch during photosynthesis. Exp. Biol. Med. 74, 775–776 10.3181/00379727-74-18044 [DOI] [PubMed] [Google Scholar]
- 40.Gibbs M. (1951) The position of C14 in sunflower leaf metabolites after exposure of leaves to short period photosynthesis and darkness in an atmosphere of C14O2. Plant Physiol. 26, 549–556 10.1104/pp.26.3.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandler O. and Gibbs M. (1956) Asymmetric distribution of C14 in the glucose phosphates formed during photosynthesis. Plant Physiol. 31, 411–412 10.1104/pp.31.5.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibbs M. and Kandler O. (1957) Asymmetric distribution of C14 in sugars formed during photosynthesis. Proc. Natl Acad. Sci. U.S.A. 43, 446–451 10.1073/pnas.43.6.446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chrispeels M.J. (2016) Martin Gibbs 1922-2006. Bibliographical Memoirs. National Academy of Sciences. [Google Scholar]
- 44.Bassham J.A. (1964) Kinetic studies of the photosynthetic carbon reduction cycle. Annu. Rev. Plant Physiol. 15, 101–120 10.1146/annurev.pp.15.060164.000533 [DOI] [Google Scholar]
- 45.Bassham J.A. and Krause G.H. (1969) Free energy changes and metabolic regulation in steady-state photosynthetic carbon reduction. Biochim. Biophys. Acta, Bioener. 189, 207–221 10.1016/0005-2728(69)90048-6 [DOI] [PubMed] [Google Scholar]
- 46.Trebst A. and Fiedler F. (1962) On the cause of asymmetrical C-14 distribution in hexose during photosynthesis with chloroplasts. Z. Naturforsch. B. 17B, 553–558 PMID: [PubMed] [Google Scholar]
- 47.Stiller M. (1962) The path of carbon in photosynthesis. Annu. Rev. Plant Physiol. 13, 151–170 10.1146/annurev.pp.13.060162.001055 [DOI] [Google Scholar]
- 48.Bach S.J., Crook E.M. and Williamson S. (1944) On arginase and its participation in urea synthesis in the liver. Biochem. J. 38, 325–332 10.1042/bj0380325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krebs H.A. and Henseleit K. (1932) Untersuchungen über die Harnstoffbildung im Tierkörper. J. Mol. Med. 11, 757–759 10.1007/BF01757657 [DOI] [Google Scholar]
- 50.Krebs H.A. and Henseleit K. (1932) Untersuchungen über die Harnstoffbildung im Tierkörper. II. J. Mol. Med. 11, 1137–1139 10.1007/BF01758220 [DOI] [Google Scholar]
- 51.Kuchel P.W., Roberts D.V. and Nichol L.W. (1977) The simulation of the urea cycle: correlation of effects due to inborn errors in the catalytic properties of the enzymes with clinical-biochemical observations. Aust. J. Exp. Biol. Med. Sci. 55, 309–326 PMID: [DOI] [PubMed] [Google Scholar]
- 52.Kacser H. and Burns J.A. (1973) The control of flux. Symp. Soc. Exp. Biol. 27, 65–104 PMID: [PubMed] [Google Scholar]
- 53.Heinrich R. and Rapoport T.A. (1974) A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur. J. Biochem. 42, 89–95 10.1111/j.1432-1033.1974.tb03318.x [DOI] [PubMed] [Google Scholar]
- 54.Heinrich R. and Schuster S. (1996) The regulation of cellular systems, Chapman & Hall, London [Google Scholar]
- 55.Williams J.F., Blackmore P.F. and Clark M.G. (1978) New reaction sequences for the non-oxidative pentose phosphate pathway. Biochem. J. 176, 257–282 10.1042/bj1760257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berthon H.A., Bubb W.A. and Kuchel P.W. (1993) 13C NMR isotopomer and computer-simulation studies of the non-oxidative pentose phosphate pathway of human erythrocytes. Biochem. J. 296, 379–387 10.1042/bj2960379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuchel P.W. and Philp D.J. (2008) Isotopomer subspaces as indicators of metabolic-pathway structure. J. Theor. Biol. 252, 391–401 10.1016/j.jtbi.2007.05.039 [DOI] [PubMed] [Google Scholar]
- 58.Gibbs M. and Cynkin M.A. (1958) Conversion of carbon-14 dioxide to starch glucose during photosynthesis by spinach chloroplasts. Nature 182, 1241–1242 10.1038/1821241b0 [DOI] [PubMed] [Google Scholar]
- 59.Clark M.G., Williams J.F. and Blackmore P.F. (1971) The transketolase exchange reaction in vitro. Biochem. J. 125, 381–384 10.1042/bj1250381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams J.F. and MacLeod J.K. (2006) The metabolic significance of octulose phosphates in the photosynthetic carbon reduction cycle in spinach. Photosynth. Res. 90, 125–148 10.1007/s11120-006-9113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flanigan I.L., MacLeod J.K. and Williams J.F. (2006) A re-investigation of the path of carbon in photosynthesis utilizing GC/MS methodology. Unequivocal verification of the participation of octulose phosphates in the pathway. Photosynth. Res. 90, 149–159 10.1007/s11120-006-9114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kartal O., Mahlow S., Skupin A. and Ebenhöh O. (2011) Carbohydrate-active enzymes exemplify entropic principles in metabolism. Mol. Syst. Biol. 7, 542 10.1038/msb.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]