Abstract
Objectives
Compare serum biomarker levels between children with mild traumatic brain injury (mTBI) and orthopaedic injury (OI), following injury. Secondarily, to explore the association between biomarker levels and symptom burden over one month post injury.
Methods
Prospective cohort study of children ages 11-16 years who presented to the emergency department within six hours of sustaining mTBI or isolated extremity OI. Serum was drawn at time of study enrollment, and levels of ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1) and glial fibrillary acid protein (GFAP) were analysed. Symptom burden was assessed by the Post-Concussion Symptom Scale (PCSS) acutely following injury and at three subsequent time points over one month.
Results
Twenty-five children with mTBI and 20 children with OI were enrolled. The average age for the overall cohort was 13 (±1.6) years, and the majority was male and injured playing sports. GFAP and PCSS scores were significantly higher acutely following mTBI versus OI (p<0.01). There was no significant group difference in UCH-L1. Neither GFAP nor UCH-L1 was predictive of PCSS over the one month post-injury.
Conclusions
GFAP may be a promising diagnostic tool for children with mTBI. Additional approaches are needed to predict symptom severity and persistence.
Keywords: pediatric, concussion, biomarkers, diagnosis
INTRODUCTION
Over half a million children and adolescents present to emergency departments (EDs) in the USA each year for evaluation of mild traumatic brain injury (mTBI).1 The diagnosis of mTBI is challenging, particularly for children, because self-reported symptoms can be subjective and difficult to articulate. It is important to accurately diagnose mTBI acutely following injury given that up to 30% of children with mTBI experience somatic, behavioural, and cognitive symptoms for weeks to months following injury.2 Early and accurate diagnosis can augment the recovery process by leading to improved counseling on the expected recovery trajectory and specific activity restrictions.3,4 Computed tomography (CT) is commonly used to diagnose intracranial injuries such as hemorrhages and contusions; however, it is normal in the vast majority of children with mTBI.5 The poor correspondence between symptoms and CT findings suggests that many children with mTBI have underlying brain injury that is undetectable by CT. Although sensitive clinical decision rules for identifying children at very low risk for a clinically important TBI (e.g. needing neurosurgical intervention), have been published,5 we continue to lack a clinical test or decision rule which discerns children with mTBI, as well as identifies which children are at risk for persistent symptomatology. Alternative methodologies, such as serum biomarkers or advance magnetic resonance imaging (MRI) techniques, may be more sensitive to identifying signs of brain injury following mTBI. An objective biomarker or combination of clinical variables and biomarkers of brain injury would potentially transform our approach to diagnosis, stratification, and prognosis for children with mTBI.
Previous adult studies have demonstrated that the neuronal biomarker, ubiquitin carboxyl-terminal hydrolase L1 (UCH-L1), and the glial biomarker, glial fibrillary acid protein (GFAP), have diagnostic utility by distinguishing patients with mild to moderate traumatic brain injury (TBI) from both healthy and orthopaedic injured control patients, as well as detecting intracranial injuries on cranial CT, with GFAP displaying greater sensitivity and specificity.6-8 Both markers have also demonstrated the potential to differentiate patients with the mildest of head injuries, (i.e. TBI with a Glasgow Coma Scale (GCS) score of 15) from uninjured control patients.7-9 Finally, these two markers were significantly associated with head injury severity and predictive of six-month mortality following severe TBI.10
There is very limited research investigating the diagnostic use of these biomarkers in children with TBI.11 In one study, Berger et al found that UCH-L1 was significantly elevated in children acutely following moderate to severe head injury, relative to healthy, non-injured control patients, and that UCH-L1 levels significantly correlated with six-month outcomes.12 Similarly, levels of GFAP acutely following severe pediatric TBI were significantly correlated with injury severity and poor clinical outcomes at six months post injury. 13,14 Evidence examining the utility of these biomarkers specifically following mTBI in children is even more limited. In a recent preliminary study of children with mTBI, acutely elevated GFAP levels were associated with initial and follow-up symptom burden up to one month post injury.15 In the aforementioned study by Berger et al, which evaluated acute levels of UCH-L1 following TBI, there was not a significant difference between children with mTBI and control patients.
This investigation sought to explore the association of acute elevations of serum biomarkers, UCH-L1 and GFAP with injury type (i.e. mTBI or orthopaedic injury) and reported symptom burden across the initial month post injury. This study is unique because it investigates the diagnostic and prognostic utility of these biomarkers in children with the mildest of brain injuries within hours of sustaining injury.
MATERIALS AND METHODS
This study was an ancillary study of a prospective cohort study comparing children, ages 11-16 years, with either an isolated first-time mTBI or an isolated single extremity orthopaedic injury (OI) who presented within six hours of injury to the ED of a large tertiary care children’s hospital.16 Enrollment occurred between December 2010 and August 2012. Children with head injuries were eligible if they had sustained either a direct blow to the head or an acceleration/deceleration movement of the head and had a GCS score of either 14 or 15 on presentation to the ED, as well as any one of the following: i. loss of consciousness <30 minutes, ii. amnesia, or iii. any alteration in mental state at the time of the injury (which included agitation, irritability, sleepiness, lethargy, slow to respond, dazed, or asking repetitive questions).17 Children with mTBIs were excluded if they had at least two or more extracranial injuries, which were defined as an injury with an Abbreviated Injury Scale (AIS) score ≥1 to that region.18 Children were eligible for the control group of the study if they presented with an isolated extremity trauma requiring radiography and an AIS of ≤3. Individuals with OI were excluded if they had an abnormal neurologic examination, symptoms of concussion, or required immediate surgical care. Children were excluded from either group for the following reasons: inability to understand English, prior history of concussion, any condition that precluded undergoing MRI (e.g. orthodontic braces), prescription of a drug that impaired cognition (e.g. narcotics) that could not be skipped four hours prior to follow-up visits, any neurologic, psychiatric, or cognitive conditions (including attention-deficit disorder or developmental delay), or receipt of a T-score of greater than 65 on the Child Behaviour Checklist (CBCL)19 completed in the ED, due to probable pre-existing neurocognitive or behavioural impairment.
Eligible children/guardians were recruited and assented/consented by trained research assistants in the ED during the hours of 8am to midnight, seven days a week. Patient and injury characteristics and a venous blood sample were collected in ED. Treating clinicians assigned an AIS score for each body region for all patients and completed a standardised form for patients with mTBI regarding the nature and characteristics of the mTBI (e.g. mechanism, signs, and symptoms). Patients underwent routine medical care. Cranial CTs for patients with mTBI and radiographs for patients with OI were ordered at the discretion of the treating physicians. As part of the study protocol to explore novel indices of neuronal injury, all patients underwent cranial MRI one to four days after injury on a Philips Achieva 3T scanner (Philips Medical Systems, Best, The Netherland). 3D T1-weighted anatomical images were acquired using a MPRAGE sequence with the following specifications: TR/TE=8.1/3.7 msec; FOV = 256x256; acquisition matrix = 256x256; slice thickness = 1mm; number of slices = 180. The T2-weighted images were acquired with a TSE: TR/TE=3000/100 msec; FOV=240x240mm; acquisition matrix = 240x238; slice thickness =3mm; number of slices = 23; 2 averages. 3D T2-FLAIR sequences were obtained with the following parameters: TR/TE=8.1/3.7 msec; FOV = 256x256; acquisition matrix = 232 x 230; slice thickness = 1mm; number of slices = 180. Susceptibility-weighted images (3D Venous BOLD) were obtained with TR/TE= 8,000 / 365 msec; FOV = 220 x 220; acquisition matrix = 220 x 220; slice thickness = 2 mm; number of slices = 63; 1 average. Anatomical T1and T2, T2-FLAIR, and susceptibility weighted images were independently reviewed for structural abnormalities by two board-certified paediatric neuroradiologists who were blinded to study group membership and clinical presentation. The MRI results are included here to characterise the mTBI and OI cohorts with respect to neuroimaging findings.
The CBCL u is a 120-item parent-report questionnaire assessing both internalising and externalising behavioural symptoms, and as noted above: based on normative data collected on healthy young adults, a T-score of greater than 65 is thought to reflect premorbid emotional or behavioural issues.19 To evaluate symptom burden, all patients completed a self-reported symptom survey, the Post-Concussion Symptom Scale (PCSS), in the ED and then again at the in-person clinic follow-ups at an average of three days, one week, and one month post-injury. The PCSS is a validated 22-item inventory of symptoms associated with mTBI graded on 7-point scale (0 none to 6 severe). It provides a total score (0-132) as well as four symptom cluster subscores based on physical, cognitive, behavioural, and sleep disturbance symptoms. Based on normative data collected on healthy young adults, a score of ≤5 is normal for males, and a score of ≤9 is normal for females.20
In the ED, each patient had approximately 10mL of blood drawn via venipuncture, and within 30-60 minutes after collection, the samples were centrifuged for 20 minutes at 1100-1300 g at room temperature; serum was then pipetted into cryovials, and samples were frozen at -80°C. All samples were processed in accordance to the Serum Standard Operating Procedures published by Early Detection Research Network of the National Cancer Institute, a method that has shown optimal reproducibility and stability of serum samples for biomarker profiling studies.21 Frozen samples were shipped to Banyan Biomarkers, Inc. where levels of UCH-L1 and GFAP were measured using enzyme immunoassays (ELISA) as per their protocols in their Good Laboratory Practice certified laboratory. These are two-site, sandwich type immunoassays using monoclonal antibodies with standardised reference ranges. All concentrations in this study are reported in nanogram/milliliter (ng/mL). The upper limits of quantification for the GFAP and UCH-L1 assays are 50 ng/ml and 9ng/ml, respectively. The lower limits of detection and quantification for the GFAP assay are 0.008 ng/mL and 0.03 ng/mL, respectively. The lower limits of detection and quantification for the UCH-L1 assay are 0.045 ng/mL and 0.1 ng/mL, respectively. The investigators at Banyan were blinded to the clinical characteristics of the patients and their injury type.
Analysis
Descriptive analyses with means and proportions were used to describe patient demographic variables (e.g. race, age), injury-related variables (e.g. mechanism of injury), and outcome variables (e.g. biomarker levels and PCSS scores). All data were assessed for normal distribution, and the level of statistical significance was set at a p-value of 0.05. T-tests or chi-square tests were used to compare patients in the mTBI and OI groups on demographic characteristics and medical outcomes: the two serum biomarker levels and the PCSS at each visit. To account for the unreliable measurements due to the limit of quantification (LLOQ), we performed sensitivity analyses by substituting the values below LLOQ by 0, LLOQ/2, LLOQ/√2, respectively,22 in addition to primary analyses using the raw measurements. When the assumptions for T-tests or chi-square tests were violated, Wilcoxon two-sample tests were used to compare continuous variables and Fisher’s exact tests were used to compare discrete variables. Mixed modeling was used to evaluate the relationship between each serum biomarker level with the total PCSS score in the ED and at the three follow-ups that occurred approximately three days, one week, and one month post injury. Initially, covariates of interest including race, age, gender, household income were included in the models, with only significant covariates retained in the final models. Additionally, Spearman’s correlation analyses were performed to assess for relationships between GFAP and UCH-L1 levels and the total PCSS scores at each of the four visits. Correlational analyses for the entire cohort were also conducted for the four cluster subscores of the PCSS: Physical, Cognitive, Behavioural, and Sleep. As a secondary analyses, we also examined the between-group difference in race for the two serum biomarkers, as well as the effect of hemolysis on biomarker levels, using Friedman’s nonparametric two-way analysis of variance (ANOVA).23 Least-square means from traditional ANOVA were used to simplify interpretation of the comparisons of group mean serum biomarkers. All analyses were conducted by using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Twenty-five children with mTBI and 20 children with OI were included in this study. One child with mTBI and one with OI were lost to follow up after the initial ED enrollment, and by one month, four additional children with mTBI and one with OI were lost to follow up. We screened 91 children with mTBI: 65 declined to participate and of the 26 that consented, one was subsequently excluded by the primary investigator due to failure to meet the study’s definition of mTBI. Similarly, we screened 149 children with OI: 127 declined to participate and of the 22 that consented, two were excluded from this analysis due to insufficient blood samples. For mTBI cases, there were no significant differences between those children who consented and those who declined with regards to gender or race, however, those who consented were more likely to have a household income over $50,000 and have been injured playing sports (both p<0.01). For OI controls, there was no significant difference between those children who consented and those who declined with regards to gender; however, those who consented were significantly more likely to be a race other than white, have a household income over $50,000, and have been injured playing sports (all p<0.01). Table 1 compares demographics of the enrolled children, denoting significant differences between the mTBI and OI groups.
Table 1.
Characteristics of Patients by Group
| Characteristics | mTBI n=25 | OI n=20 | p-value |
|---|---|---|---|
| Age in years (mean +/-sd) | 13.6 +/- 1.6 | 13.2 +/- 1.6 | 0.42 |
| Sex (% male) | 92 | 75 | 0.12 |
| Race (% white) | 72 | 30 | <.01* |
| Ethnicity (% non-Hispanic) | 96 | 100.0 | 0.43 |
| Household median income (% > $50, 000) | 96 | 65 | <.01* |
| Mechanism of injury (% sports) | 72 | 85 | 0.29 |
| Time from injury to ED presentation in hours (mean+/- sd) | 4.3 +/- 1.1 | 4 +/- 1 | 0.44 |
| Baseline total T-score CBCL (mean +/- sd) | 44.7 +/-10.9 | 43.3 +/-12.5 | 0.69 |
Legend: CBCL: child behavior checklist, ED: emergency department, mTBI: mild traumatic brain injury, OI: orthopedic injury, sd: standard deviation
Significantly different based on T-test or Chi-square test (p<0.05)
The majority of children with mTBI had a GCS of 15 (92%), although almost half (44%) were described as having signs of altered mental status. The most commonly endorsed symptom in the ED was headache (80%) and almost one-third had vomiting. Eight (32%) children had a loss of consciousness and 13 (52%) endorsed some sort of amnesia surrounding the injury. Children in the OI group had a mean AIS score of 2.1 and 74% had fractures on radiography, with forearm fractures being the most common injury pattern. Five patients with mTBI had cranial CTs performed and all were normal. Two children (one child with mTBI and one with OI) were lost to follow up prior to obtaining anatomical T1 and T2 MRI neuroimaging. Forty-three subjects had structural MRI (23 mTBI, 20 OI). Four children with mTBI and four children with OI were noted to have small non-specific foci of white matter signal without associated signal changes on susceptibility weighted sequences or associated cortical signal abnormalities, and their MRI reads and biomarker levels are listed in Table 2. These foci were similar in morphology, distribution, and prevalence to incidental white matter signal changes that have been previously reported in neurologically normal children,24 and these findings were not felt to suggest a traumatic etiology by the study staff. There was similar prevalence of these white matter findings among the groups (17% mTBI, 20% OI). Three children had incidental findings: two with a low cerebellar tonsillar position (one mTBI, one OI) compatible with a Chiari I anomaly, and one child from the OI group with a left middle cranial fossa arachnoid cyst. No evidence of parenchymal, intraventricular, or subarachnoid hemorrhage was noted in any subjects on the susceptibility weighted sequences.
Table 2.
Abnormal MRI Dictations and Corresponding Biomarker Levels
| Study ID Number | Radiology Dictation | UCH-L1 Level (ng/mL) | GFAP Level (ng/mL) |
|---|---|---|---|
| mTBI | |||
| 1 | There are small foci of increased signal intensity identified within the anterior temporal lobes bilaterally. There is no evidence of hemorrhage. They are non-specific. Although potentially related to trauma, the distribution is unusual for this etiology and there is no associated hemorrhage. No other abnormalities identified. | 0.89036 | 0.02126 |
| 2 | There are scattered areas of increased FLAIR signal in the bilateral centrum semiovale regions. The DTI and SWI are normal. Etiology is indeterminate, although could be traumatic, demyelinating, or ischemic. No evidence of hemorrhage. | 0.26738 | 0.08194 |
| 8 | Small focus of increased signal in the subcortical WM of the left inferior occipital lobe. Significance uncertain, unlikely traumatic. Clinical significance is likely minimal. No mass effect. | 0.02885* | 0.03978 |
| 44 | Nonspecific punctate bright foci base of R frontal lobe on Flair | 0.00000* | 0.04607 |
| OI | |||
| 5 | No clinically significant findings requiring further evaluation. Several small foci of increased signal in the right > left parietal periventricular WM. Non-specific. | 0.20914 | 0.00165* |
| 13 | Single non-specific region of increased FLAIR signal in right frontal WM | 0.23921 | 0.00737* |
| 14 | 1.2 × 1.1 cm. simple pineal cyst. Likely incidental. Multiple small foci of increased FLAIR signal in the right frontal subcortical WM. No SWI abnormality. No DTI abnormality. Questionable etiology. Could be traumatic. | 0.00000* | 0.01042 |
| 16 | No clinically significant findings requiring further evaluation. Several small foci of increased signal in the right > left parietal periventricular WM. Non-specific. No follow-up needed unless related clinical symptoms. | 0.19228 | 0.00591* |
Legend:GFAP: glial fibrillary acid protein, ID: identification; OI: orthopedic injury, MRI: magnetic resonance imaging, mTBI: mild traumatic brain injury, UCH-L1: ubiquitin carboxyl-terminal hydrolase L1
The biomarker concentration in this sample is below the Limit of Detection of this assay (0.008 ng/ml for GFAP and 0.045 ng/ml for UCH-L1).
None of the blood processing time measures (in hours) were significantly different between the mTBI and OI groups, including time between injury and blood draw (4.3±1.1 versus 4.0±1.0), time from blood draw to centrifuge (1.49±2.42 versus 0.82±0.15); time in centrifuge (0.36±0.05 versus 0.38±0.07), and time from centrifuge to freezer (0.12±0.06 versus 0.09±0.05) Although Banyan Biomarkers has not detected any statistically significant effects of hemolysis on their human UCH-L1 and GFAP assays, it was identified that one sample from the mTBI group and five samples from the OI group had some degree of serum sample hemolysis. No children within our cohort were above the upper limit of quantification. For UCH-L1, eight cases and two controls were below our designated lower limit of detection. For GFAP, three cases and ten controls were below our designated lower limit of detection.
Distributions of both biomarkers were skewed to the right and failed the Shapiro-Wilk test for normality. Wilcoxon two-sample test indicated that children with mTBI had significantly higher mean GFAP levels than children with OI (0.09±0.10 versus 0.03±0.03, p<0.01), however, mean UCH-L1 levels were not statistically different between the mTBI and OI groups (0.19±0.2 versus 0.26±0.03, p=0.29). Figures 1 and 2 illustrate the comparison of the biomarker levels between the two groups. Sensitivity analyses substituting the raw values below the LLOQ by 0, LLOQ/2, and LLOQ/√2 yielded consistent results with those from the raw values. The effect of injury type on the group differences for both biomarkers did not change after adjusting for hemolysis (p=0.12).
Figure 1. Group Comparisons of GFAP Levels Acutely Following Injury.
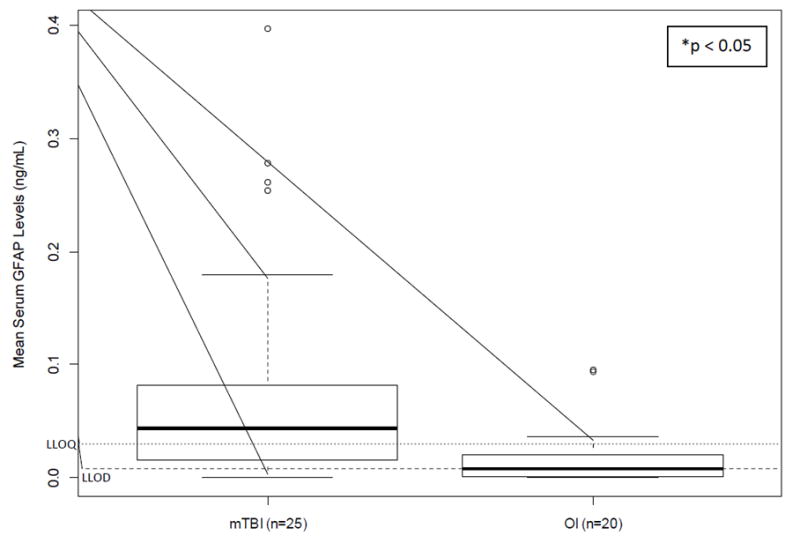
LLOD: lower limit of detection (LLOD); LLOQ: lower limit of quantification; mTBI: mild traumatic brain injury, OI: orthopedic injury
*Significant group difference based on t-test
Figure 2. Group Comparisons of UCH-L1 Levels Acutely Following Injury.
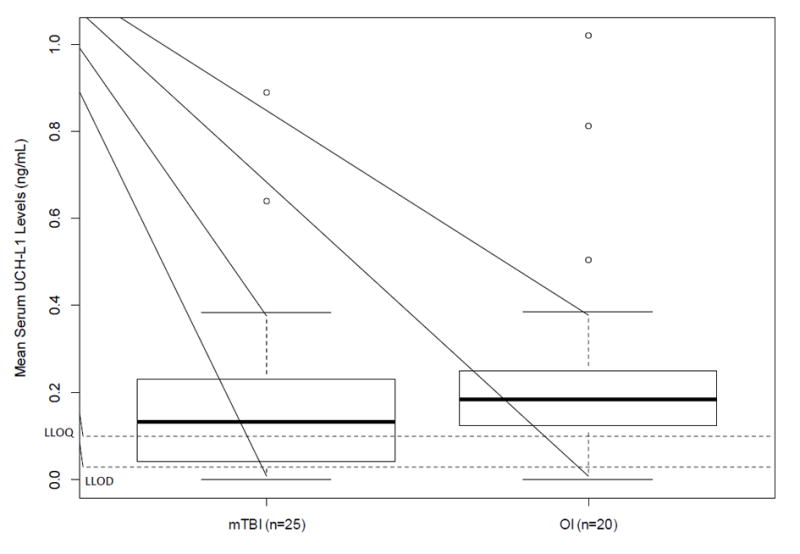
LLOD: lower limit of detection (LLOD); LLOQ: lower limit of quantification; mTBI: mild traumatic brain injury, OI: orthopedic injury
*No significant group difference based on t-test
Since there was a difference between enrolled children with mTBI and OI, the effect of race on biomarker levels was explored. There was a significant difference in biomarker levels after controlling for injury type, with non-white children relative to white children having higher serum levels of UCH-L1 (0.29±0.27 vs. 0.15±0.17, p-value = 0.01), and yet lower serum levels of GFAP (0.05±0.10 vs. 0.06±0.08, p-value <0.01). Nonetheless, after statistically controlling for race, there was still a significant difference in GFAP levels between the mTBI and OI groups (0.08 ± 0.11 vs. 0.02 ± 0.03, p<0.01), and the group difference for UCH-L1 remained nonsignificant (p-value = 0.1).
Almost all study participants completed the PCSS at each visit, although due to technical difficulties there was a missing PCSS score for one child with mTBI at the ED visit, and one child with OI developed a headache during testing and was unable to complete her PCSS at the one-month visit. Symptom burden, as defined by the total PCSS, was initially high and declined over the one month period following injury. Children with mTBI had statistically significant higher total PCSS scores than children with OI until the one month post-injury visit (Figure 3). Acutely following injury, among the four PCSS cluster scores, children with mTBI had significantly (p<0.01) higher Physical and Cognitive scores relative to children with OI (14±9 versus 4.2±7 and 15.7±8 versus 7±5, respectively). There were no significant differences between the two groups for the Sleep (1.9±3 versus 1.5±3) or Behavioural (4.1±4 versus 4.4±5) scores. Significant group differences resolved by one week and one month post injury for the Cognitive and Physical scores, respectively.
Figure 3. Group Comparisons of Total PCSS Scores Over 1 Month Post-Injury.
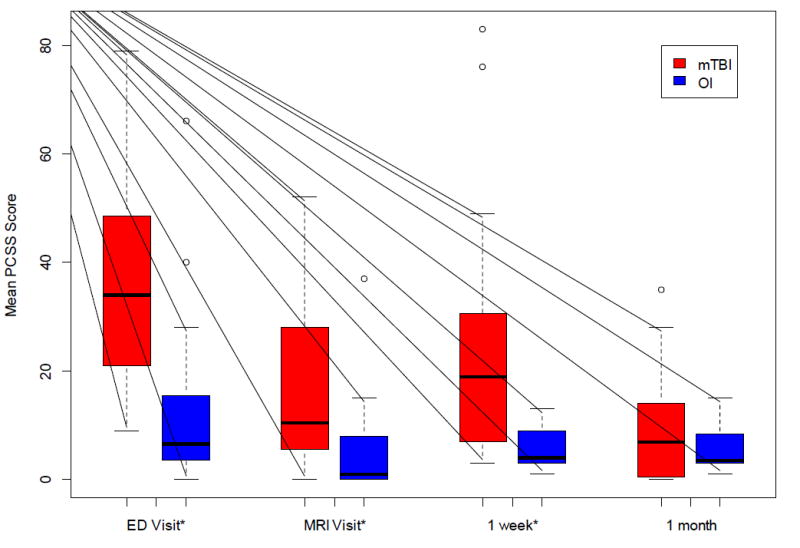
ED: Emergency Department; MRI: Magnetic Resonance Imaging; mTBI: mild traumatic brain injury, OI: orthopedic injury; PCSS: Post-Concussion Sumptom Scale
*Significant group difference based on t-test (p<0.05)
In mixed models analyses, neither biomarker was predictive of the total PCSS in the ED or over the one month post injury. Age, gender, race, and income were also not significant predictors of total PCSS score in these models.
In bivariate analyses, there were no significant correlations between initial levels of GFAP and the total or cluster PCSS scores over one month post injury. UCH-L1 was significantly inversely correlated (r= -0.31, p=0.04) with the total PCSS score in the ED, but not at subsequent follow ups. UCH-L1 was also significantly inversely correlated with PCSS Physical cluster score in the ED (r= -0.33, p=0.03), but no correlation was found at subsequent follow ups.
DISCUSSION
In children ages 11-16 years, we found that levels of the serum biomarker GFAP, drawn within six hours after injury, discriminated children with mTBI from those with OI, yet UCH-L1 levels did not. Neither biomarker was associated with acute symptom burden following injury, nor was either biomarker predictive in identifying children at risk for persistent symptoms following mTBI, although definitive conclusions on their prognostic ability are limited given that only three children with mTBI were still symptomatic by one month post injury. These findings support the need for further investigation to determine the diagnostic utility of GFAP in the acute assessment of a larger heterogeneous cohort of children with mTBI.
Current literature examining the diagnostic and prognostic utility of these biomarkers in children with mTBI is limited.11,25 Our study addresses a gap in the literature by assessing levels of these biomarkers within hours of sustaining injury while focusing on children with the mildest of brain injuries. Similar to adult literature evaluating mild to moderate TBI, our study found that GFAP was able to distinguish between patients with OI and those with mTBI.6,7 Recently GFAP has been shown to outperform the serum biomarker S100B in identifying intracranial lesions in children with mild to moderate TBI,26,27 although none of the children in our study had abnormal CT neuroimaging. Non-specific white matter signal foci were identified by MRI in 16.3% of all subjects, with no difference in prevalence between injury types. Our findings were similar in prevalence, distribution, and morphology to foci seen in children with no neurologic disease.24 Although etiology and clinical significance are uncertain, the distribution and morphology were not thought to be typical for a traumatic etiology, but rather representative of small areas of gliosis from prior ischemia, inflammation, or demyelination. No intracranial hemorrhage was identified in any case, despite performance of high resolution susceptibility weighted sequences. Additionally, incidental, non-traumatic findings (two Chiari I anomalies and one arachnoid cyst) were identified, which are not uncommon in paediatric neuroimaging research, and it unlikely that any of our cohort’s MRI findings would result in alteration of the serum biomarkers assessed in this study.24
Similar to Berger et al., who evaluated 11 children within 24 hours of being admitted to the hospital for mTBI, we found no significant difference in levels of UCH-L1 between children with mTBI and control patients,12,27 with our OI group actually having slightly, but not significantly, higher levels of UCH-L1 than the mTBI group. Notably, our control group levels were substantially higher than those reported by Papa et al. in adults (mean 0.08±0.01, range 0.02-0.49), although only 12% of their control group had sustained trauma, with the remainder being healthy volunteers.8 The failure of UCH-L1 to discriminate children with mTBI and OI may be attributable to the timing of blood sampling, mechanism and type of injury, or the degree of injury. Based on our calculations, we did not appreciate an effect from sample hemolysis. Given significantly different racial composition between the mTBI and OI groups, we examined differences in biomarker levels between racial groups and found significant group differences for both biomarkers between non-white and white children. However, after controlling for race, GFAP levels still differed significantly between the mTBI and OI groups, and UCH-L1 group differences remained insignificant. Although there is no literature to support racial differences among these biomarkers, this issue should be further examined with larger cohorts. Levels of GFAP were not correlated with PCSS scores in the ED or at subsequent follow-ups, nor were they correlated with any of the PCSS cluster scores. Contrary to our findings, a pilot study evaluating 13 children with mTBI reported that GFAP levels within 24 hours (mean 7.6 ±3.9 hours) of mTBI correlated with self-reported symptom burden both in the ED and at one month post injury.15 Key differences in the current study were a shorter time between injury and biomarker collection and an overall lower serum level of GFAP. However, both studies had relatively small sample sizes, and a larger prospective evaluation that would be sufficient for ROC analyses28 is still needed. While we did find a significant correlation between levels of UCH-L1 and the total and Physical cluster PCSS scores in the ED (p<0.01), surprisingly it was an inverse relationship. Previous studies examining UCH-L1 have shown significant direct correlations with 3-8 month outcomes in children with TBI, however, the majority of this cohort (72%) had moderate to severe brain injury.12 These contradictory findings highlight the need for further investigations examining levels of GFAP and UCH-L1 in children with various types and severities of brain injury assessed at multiple time points post injury in association with symptoms over time, as it is possible that persistent elevations in these biomarkers are a better predictor of post-concussive symptoms.
This study possesses a number of strengths including a relatively homogeneous sample, a short and narrow interval between injury and blood draw, as well as minimal variation in blood sample processing procedures. Limitations to our study include the small sample size, limiting our abilities to detect differences between the two groups, and the fact biomarker levels were only assessed at single time point given the time to peak for these markers is not definitively known. The low consent rate, primarily because of the complexity of study design, may reduce the generalizability of the results. Additionally, our cohort was a convenience sample from a single centre, and while patients with mTBI and OI were comparable on most characteristics, there were differences in race and income that may have confounded results. Finally, the lack of gold standard tests to accurately diagnose the disease and stratify injury severity is a limitation, although using a standardised clinical definition of mTBI was the best available method for diagnosis of mTBI.17
This study provides preliminary evidence that may support the use of GFAP as diagnostic tool to identify children who have sustained a head injury. This tool could be especially useful in the field of paediatrics when the injury is often unwitnessed in a pre-verbal child, when there is a question of non-accidental trauma, or in the setting of poly-trauma during which an altered mental status could be due to a myriad of injuries. While neither biomarker was a strong predictor of PCSS scores over our one month period, additional work using a larger sample size and baseline PCSS scores would greatly contribute to future work evaluating the prognostic capabilities of UCH-L1 and GFAP.
Acknowledgments
The authors would like to acknowledge the following people for their contributions: Jeff Bazarian, MD, MPH, (Scientific Advisor, Department of Emergency Medicine, University of Rochester Medical Center); Terri Byczkowski, PhD, MBA, (Scientific Advisor, Division of Pediatric Emergency Medicine, Cincinnati Children’s Hospital Medical Center); Richard Hornung, PhD, (Statistical Oversight, Division of Pediatric Emergency Medicine, Cincinnati Children’s Hospital Medical Center); Blaise Jones, MD, (Clinical Neuroradiologist, Division of Radiology, Cincinnati Children’s Hospital Medical Center); Lynn Mullins, BA, (Data Manager, Division of Pediatric Emergency Medicine, Cincinnati Children’s Hospital Medical Center); Brad Kurowski, MD, MS, (Patient Care, Division of Physical Medicine and Rehabilitation, Cincinnati Children’s Hospital Medical Center); Richard Ruddy, MD, (Division Chair, Scientific Advisor, Division of Pediatric Emergency Medicine, Cincinnati Children’s Hospital Medical Center); Imaging Research Coordinators at the Pediatric Neuroimaging Research Consortium at the Cincinnati Children’s Hospital Medical Center (Data Collection); and the Pediatric Emergency Medicine Physicians and Clinical Research Coordinators in the Division of Pediatric Emergency Medicine at Cincinnati Children’s Hospital Medical Center (Data Collection, Data Entry).
This study was funded in part by (1) National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR000078 (KL2 RR026315) (Babcock); and (2) Cincinnati Children’s Hospital Medical Center Division of Emergency Medicine.
Footnotes
DECLARATION OF INTEREST
The additional authors have no funding sources or conflicts to disclose for this work.
Contributor Information
Lynn Babcock, Division of Pediatric Emergency Medicine, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center.
Nanhua Zhang, Division of Biostatistics, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center.
James Leach, Division of Medical Imaging, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center.
Shari L. Wade, Division of Physical Medicine and Rehabilitation Department of Pediatrics, Cincinnati Children’s Hospital Medical Center.
References
- 1.Faul M, Xu L, Wald MW, Coronado VG. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths 2002-2006. GA: Centers for Disease Control and Prevetnion, National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 2.Babcock L, Byczkowski T, Wade SL, Ho M, Mookerjee S, Bazarian JJ. Predicting postconcussion syndrome after mild traumatic brain injury in children and adolescents who present to the emergency department. JAMA Pediatr. 2013;167(2):156–161. doi: 10.1001/jamapediatrics.2013.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponsford J, Willmott C, Rothwell A, Cameron P, Ayton G, Nelms R, Curran C, Ng K. Impact of early intervention on outcome after mild traumatic brain injury in children. Pediatrics. 2001;108(6):1297–303. doi: 10.1542/peds.108.6.1297. [DOI] [PubMed] [Google Scholar]
- 4.Sady MD, Vaughan CG, Gioia GA. School and the concussed youth: recommendations for concussion education and management. Phys Med Rehabil Clin N Am. 2011;22(4):701–19. ix. doi: 10.1016/j.pmr.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuppermann N, Holmes JF, Dayan PS, Hoyle JD, Jr, Atabaki SM, Holubkov R, Nadel FM, Monroe D, Stanley RM, Borgialli DA, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374(9696):1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 6.Diaz-Arrastia R, Wang KK, Papa L, Sorani MD, Yue JK, Puccio AM, McMahon PJ, Inoue T, Yuh EL, Lingsma HF, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31(1):19–25. doi: 10.1089/neu.2013.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papa L, Lewis LM, Falk JL, Zhang Z, Silvestri S, Giordano P, Brophy GM, Demery JA, Dixit NK, Ferguson I, et al. Elevated Levels of Serum Glial Fibrillary Acidic Protein Breakdown Products in Mild and Moderate Traumatic Brain Injury Are Associated With Intracranial Lesions and Neurosurgical Intervention. Annals of Emergency Medicine. 2012;59(6):471–483. doi: 10.1016/j.annemergmed.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM, Demery JA, Liu MC, Mo J, Akinyi L, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg. 2012;72(5):1335–1344. doi: 10.1097/TA.0b013e3182491e3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMahon PJ, Panczykowski DM, Yue JK, Puccio AM, Inoue T, Sorani MD, Lingsma HF, Maas AI, Valadka AB, Yuh EL, et al. Measurement of the glial fibrillary acidic protein and its breakdown products GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging. J Neurotrauma. 2015;32(8):527–33. doi: 10.1089/neu.2014.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mondello S, Papa L, Buki A, Bullock MR, Czeiter E, Tortella F, Wang K, Hayes R. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care. 2011;15(3):R156. doi: 10.1186/cc10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa L, Ramia MM, Kelly JM, Burks SS, Pawlowicz A, Berger RP. Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J Neurotrauma. 2013;30(5):324–38. doi: 10.1089/neu.2012.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger RP, Hayes RL, Richichi R, Beers SR, Wang KK. Serum concentrations of ubiquitin C-terminal hydrolase-L1 and alphaII-spectrin breakdown product 145 kDa correlate with outcome after pediatric TBI. J Neurotrauma. 2012;29(1):162–7. doi: 10.1089/neu.2011.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser DD, Close TE, Rose KL, Ward R, Mehl M, Farrell C, Lacroix J, Creery D, Kesselman M, Stanimirovic D, et al. Severe traumatic brain injury in children elevates glial fibrillary acidic protein in cerebrospinal fluid and serum. Pediatr Crit Care Med. 2011;12(3):319–324. doi: 10.1097/PCC.0b013e3181e8b32d. [DOI] [PubMed] [Google Scholar]
- 14.Žurek J, Fedora M. Dynamics of Glial Fibrillary Acidic Protein During Traumatic Brain Injury in Children. J Trauma. 2011;71(4):854–859. doi: 10.1097/TA.0b013e3182140c8c. [DOI] [PubMed] [Google Scholar]
- 15.Mannix R, Eisenberg M, Berry M, Meehan WP, 3rd, Hayes RL. Serum biomarkers predict acute symptom burden in children after concussion: a preliminary study. J Neurotrauma. 2014;31(11):1072–5. doi: 10.1089/neu.2013.3265. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Wade SL, Babcock L. Structural connectivity abnormality in children with acute mild traumatic brain injury using graph theoretical analysis. Hum Brain Mapp. 2015;36(2):779–92. doi: 10.1002/hbm.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay T, Harrington DE, Adams R, Anderson T, Berrol S, Cicerone K, Dahlberg C, Gerber D, Goka R, Harley P, et al. Report of the Mild Traumatic Brain Injury. Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–87. [Google Scholar]
- 18.Civil ID, Schwab CW. The Abbreviated Injury Scale, 1985 revision: a condensed chart for clinical use. J Trauma. 1988;28(1):87–90. doi: 10.1097/00005373-198801000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Achenbach TM, Edelbrock CS. Manual for The Child Behavior Checklist and Revised Child Behavior Profile. Burlington: Department of Psychiatry, University of Vermont; 1983. [Google Scholar]
- 20.Lovell MR, Iverson GL, Collins MW, Podell K, Johnston KM, Pardini D, Pardini J, Norwig J, Maroon JC. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–74. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 21.Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2008;8(1):113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. The AAPS journal. 2009;11(2):371–380. doi: 10.1208/s12248-009-9112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. Journal of the American Statistical Association. 1937;32(200):675–701. [Google Scholar]
- 24.Kaiser D, Leach J, Vannest J, Schapiro M, Holland S. Unanticipated findings in pediatric neuroimaging research: prevalence of abnormalities and process for reporting and clinical follow-up. Brain Imaging Behav. 2015;9(1):32–42. doi: 10.1007/s11682-014-9327-7. [DOI] [PubMed] [Google Scholar]
- 25.Daoud H, Alharfi I, Alhelali I, Charyk Stewart T, Qasem H, Fraser D. Brain Injury Biomarkers as Outcome Predictors in Pediatric Severe Traumatic Brain Injury. Neurocrit Care. 2014;20(3):427–435. doi: 10.1007/s12028-013-9879-1. [DOI] [PubMed] [Google Scholar]
- 26.Papa L, Mittal MK, Ramirez J, Ramia M, Kirby S, Silvestri S, Giordano P, Weber K, Braga CF, Tan CNS, et al. In Children and Youth With Mild and Moderate Traumatic Brain Injury GFAP Out-performs S100B In Detecting Traumatic Intracranial Lesions On CT. J Neurotrauma. 2015 Mar 9; doi: 10.1089/neu.2015.3869. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papa L, Silvestri S, Brophy GM, Giordano P, Falk JL, Braga CF, Tan CN, Ameli NJ, Demery JA, Dixit NK, et al. GFAP Out-Performs S100B in Detecting Traumatic Intracranial Lesions on Computed Tomography in Trauma Patients with Mild Traumatic Brain Injury and Those with Extracranial Lesions. J Neurotrauma. 2014;31(22):1815–1822. doi: 10.1089/neu.2013.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obuchowski NA. Sample size tables for receiver operating characteristic studies. AJR Am J Roentgenol. 2000;175(3):603–8. doi: 10.2214/ajr.175.3.1750603. [DOI] [PubMed] [Google Scholar]


