Summary
Objective
Identifying early predictors of weight loss is key for developing personalized treatment. However, few individual factors have been identified that predict weight loss during intervention, other than early weight loss itself.
Methods
Women with overweight or obesity (n = 186, mean ± SD age 50.0 ± 10.6 years, body mass index 34.0 ± 4.2 kg m−2) participated in the Portion‐Control Strategies Trial, a 1‐year randomized controlled weight‐loss trial with three intervention groups. Early changes in eating behaviours and psychological factors were evaluated by questionnaires at baseline and Month 1. The influence of these early changes on the trajectory of weight loss from baseline to Months 3 and 12 was assessed by random coefficients models.
Results
Although there were no differences in weight loss between intervention groups at the end of the trial, certain individual factors were shown to predict both early weight loss at Month 3 and longer‐term weight loss at Month 12. Across all participants, increases in dietary restraint and healthy lifestyle ratings in the first month predicted more rapid weight loss from baseline to Month 3 (P < 0.05) and also predicted more rapid weight loss and slower regain from baseline to Month 12 (both P < 0.01). Early attendance and changes in disinhibition were not associated with subsequent weight loss.
Conclusions
Changes in psychological and behavioural measures, such as restraint, in the first month of weight loss intervention predicted longer‐term weight loss in women. Early additional support or tailored treatment could promote long‐term success by reinforcing these behaviours.
Keywords: Adults, eating behaviours, obesity treatment, weight loss
Introduction
Extensive research has focused on identifying effective weight loss treatments to benefit adults with excess weight or obesity 1, 2. Despite this work, long‐term outcomes vary substantially across individuals 2, 3. No single intervention works well for everyone, so identifying changes in individual factors, such as eating behaviours, that predict success is key for developing effective personalized interventions. Assessment of these changes in the initial months of intervention would allow treatment tailoring through provision of additional support for individuals who are less responsive and reinforcement for those who respond well. However, interventions are seldom designed to evaluate such early changes, and many factors are assessed only at baseline or baseline and post‐intervention 4. This study repeatedly assessed individual factors during a year‐long weight‐loss intervention to determine whether changes early in treatment predicted subsequent weight loss across individuals.
The evidence identifying early predictors of subsequent weight outcomes is limited; however, early weight loss itself has consistently been shown to predict later weight loss. One trial found that weight loss after 2 months of behavioural intervention was correlated with weight loss 8 years later and that individuals who did not achieve a given weight loss in this initial period failed to respond to treatment long‐term 5. Multiple shorter‐term weight‐loss trials have also found that greater initial weight loss predicts greater long‐term weight loss 6, 7, 8, 9.
In addition to weight loss itself, certain psychological and behavioural factors have been associated with weight loss during intervention, such as programme attendance 10, 11, self‐efficacy 12, 13, dietary restraint 14, and disinhibition 15. However, assessment of changes early in treatment is rare. Thus, in a secondary analysis, early measures of behaviour change were tested to determine whether they predicted weight loss in a year‐long trial of different dietary strategies 16. Specifically, analyses focused on changes in ratings of eating behaviours and psychological factors from baseline to Month 1 and their ability to predict either early weight loss (baseline to Month 3) or longer‐term weight loss (baseline to Month 12). It was hypothesized that early, beneficial change in eating behaviours such as disinhibition and restraint would predict success in short‐term and longer‐term weight loss.
Methods
Study design
The Portion‐Control Strategies Trial was a 1‐year randomized controlled trial in women with overweight and obesity comparing two portion‐control strategies to standard dietary advice for weight loss. The trial design and main outcomes have been previously reported 16.
Participants
Eligible women were aged 20–65 years with a body mass index (BMI) of 28–45 kg m−2. They were recruited through local advertisements and websites in State College, PA and surrounding areas. Exclusion criteria included blood pressure > 160/100 mm Hg; following a special diet or weight‐loss programme; weight change >4.5 kg in the past 3 months; a medical condition that prevented participation; pregnancy or lactation; or scoring >19 on the Eating Attitudes Test 17 or >25 on the Beck Depression Inventory 18. Inclusion required completion of three daily food and activity diaries and a 2‐week run‐in period. Participants provided signed informed consent and were financially compensated for their time. The trial protocol was approved by the Office for Research Protections at The Pennsylvania State University.
Interventions
Participants were randomly assigned to one of three parallel intervention groups 16. The Standard Advice group was instructed to follow dietary recommendations that focused on eating less and selecting healthy options from different food groups. The Portion Selection group was taught to choose food portions based on energy density and was given portion‐control tools such as food scales. The Pre‐Portioned Foods group was taught to use pre‐portioned foods to structure meals; they were also given vouchers for single‐serving main dishes. The instructional sessions focused on applying the principles of the assigned programme when selecting the types and amounts of food at meals and snacks. The principles were reinforced in individual lessons on specific food groups, meal planning, and eating away from home. Participants in all groups received similar instruction on increasing physical activity, keeping records for self‐monitoring, and managing behaviour change.
All participants met individually with trained interventionists weekly during Month 1, biweekly during Months 2–6, and monthly during Months 7–12. In addition to 19 instructional sessions, there were assessment sessions at baseline and Months 1, 3, 6, and 12 that included computer‐administered questionnaires. Body weight was measured at baseline and all 23 sessions.
Measures
This study examined four questionnaires, which assess eating behaviours and psychological factors that have been suggested in the literature to be related to weight loss. The questionnaires are summarized in Table 1 and described further below.
Table 1.
Questionnaires, scales, score ranges, and time points administered
| Questionnaire (total number of items) | Scale | Scale number of items | Score range | Time points administered |
|---|---|---|---|---|
| Three‐Factor Eating Questionnaire 19 (51 items) | Dietary restraint | 21 | 0–21 | Baseline and Months 1, 3, 6, & 12 |
| Disinhibition | 16 | 0–16 | ||
| Susceptibility to hunger | 14 | 0–14 | ||
| Diet‐Satisfaction Questionnaire 22 (45 items) | Healthy lifestyle | 8 | 1–5 | Baseline and Months 1, 3, 6, & 12 |
| Convenience | 9 | 1–5 | ||
| Cost | 5 | 1–5 | ||
| Family dynamics | 6 | 1–5 | ||
| Preoccupation with food | 6 | 1–5 | ||
| Negative aspects | 6 | 1–5 | ||
| Meal planning and preparation | 5 | 1–5 | ||
| Dieting Beliefs Scale 23 (16 items) | Internal locus of control | 6 | 6–36 | Baseline and Months 6 & 12 |
| External (individual) locus of control | 5 | 5–30 | ||
| External (environmental) locus of control | 4 | 4–24 | ||
| Weight Efficacy Lifestyle Questionnaire 24 (20 items) | Negative emotions | 4 | 0–36 | Baseline and Months 6 & 12 |
| Food availability | 4 | 0–36 | ||
| Social pressure | 4 | 0–36 | ||
| Physical discomfort | 4 | 0–36 | ||
| Positive activities | 4 | 0–36 |
The Three‐Factor Eating Questionnaire (TFEQ) 19 assesses three cognitive and behavioural aspects of eating behaviour: dietary restraint, disinhibition, and susceptibility to hunger. Dietary restraint measures the tendency to restrict food intake as a means of weight management, disinhibition evaluates overeating in response to palatable foods or negative emotions, and hunger assesses susceptibility to feelings of hunger. Subsequent to the development of the TFEQ, other researchers have proposed subscales of the main scales, such as flexible and rigid restraint 20 and internal and external disinhibition 21, which were also assessed in this study.
The Diet Satisfaction Questionnaire (D‐Sat) 22 evaluates satisfaction with the current diet and identifies potential barriers to change by assessing seven aspects: healthy lifestyle, convenience, cost, family dynamics, preoccupation with food, negative aspects, and meal planning and preparation. For example, the healthy lifestyle scale assesses the degree to which the current diet supports a healthy lifestyle and promotes positive feelings about life, using agreement with statements such as ‘I am satisfied with my diet’ and ‘I believe that I am reducing my risk for disease by the way that I eat’.
The Dieting Beliefs Scale 23 measures three types of beliefs about weight‐related locus of control: internal locus, which is controlled by internal factors (e.g. willpower), external locus, which is controlled by individual characteristics outside that individual's influence (e.g. genetics), and external locus, which is controlled by factors outside the individual (e.g. environment).
The Weight Efficacy Lifestyle Questionnaire 24 assesses the ability to resist eating in response to certain environmental situations or emotional states. It evaluates self‐efficacy in five contexts: negative emotions, food availability, social pressure, physical discomfort, and positive activities.
Attendance was determined by summing the total number of instructional and assessment visits attended by each participant during the first month of treatment (a maximum of four).
Statistical analysis
Weight loss from baseline was modelled as a polynomial curve incorporating multiple measurements across time using a random coefficients model. The linear coefficient of the trajectory characterized the initial rate of weight loss, and the quadratic coefficient characterized the deceleration of weight loss and the beginning of weight regain 16. Questionnaire completion rates were 100% at baseline and Month 1, 94% at Month 3, 83% at Month 6, and 76% at Month 12. In an intention‐to‐treat analysis, the model included all available data for randomized participants and used maximum likelihood methods to handle missing data.
Changes in individual factors from baseline to Month 1 were tested for influence on the weight‐loss trajectories from baseline to Month 3 (10 measurements) and from baseline to Month 12 (23 measurements). Baseline levels and initial change in each of the questionnaire scales, initial participant attendance, and initial weight loss were tested in individual, univariate models. The predictors found to significantly associate (P < 0.05) with weight loss were used to build the subsequent, hierarchical models. Those variables that no longer remained significant or marginally significant in the multivariate model were removed in a stepwise fashion before building the subsequent model. For Month 3 weight loss, three models were built: the first paralleling the model used in the main trial paper 16 and used to establish a reference for testing additional predictors, the second adding the main effects of the covariates of interest and their interactions with linear rate of weight loss, and the third that added Month 1 weight loss as a fixed effect. For Month 12 weight loss, four models were built: the first paralleling the model used in the main trial paper, the second adding the main effects of the covariates of interest and their interactions with linear rate of weight loss, the third adding the interactions between these covariates and quadratic change (deceleration) in weight loss, and the fourth adding Month 1 weight loss as a fixed effect. The TFEQ subscales were evaluated in the same set of multivariate, hierarchical models, but in order to streamline the models and enable comparison across a larger literature base, the overall restraint and disinhibition scales were retained in the final models and the tables shown here. Results for the subscales are reported in the text.
The reference model for both time points included the fixed effects of intervention group, baseline BMI, age, and the linear and quadratic effects of time (trial week). The effects for intercept and linear coefficient were included as random effects in all models to account for within‐subject correlation across assessments. The data were analyzed using SAS software (version 9.4, 2013, SAS Institute Inc., Cary, NC). Results are reported as mean ± SD for demographic data and mean ± SEM for modelled data.
Results
Participant characteristics and overall weight loss
There were 186 women with overweight and obesity enrolled in the trial (age 50.0 ± 10.6 years). The majority of participants had obesity (BMI 34.0 ± 4.2 kg m−2) were Caucasian (98%) and had at least some college education (88%). As reported previously 16, there were differences in weight‐loss trajectories across intervention groups. The Pre‐Portioned Foods group lost weight at a faster rate than the other groups during the initial months and then regained at a faster rate than the other groups during later months. Consequently, no differences were found in weight loss between groups at Months 6 or 12. On average, participants had lost 5.2 ± 0.4 kg at Month 6 and 4.5 ± 0.5 kg at Month 12. None of the effects reported below differed significantly across groups nor were there significant effects of baseline age or BMI on the weight‐loss trajectory.
Early predictors of weight loss
After a month of treatment, initial changes in several individual factors were found to predict the trajectory of weight loss at both Months 3 and 12 (Tables 2 and 3). As described below, changes in TFEQ and D‐Sat Questionnaire scales (Figure 1) were significantly associated with the rate of weight loss, as was initial weight loss. No significant relationships to weight loss were found for the Dieting Beliefs Scale or the Weight Efficacy Lifestyle Questionnaire.
Table 2.
Hierarchical random coefficients models of the influence of individual factors* of 186 women on the trajectory of weight loss across the first 3 months of a 1‐year trial
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Coefficient ± SEM | P value | Coefficient ± SEM | P value | Coefficient ± SEM | P value | |
| Age | 0.01 ± 0.01 | P = 0.01 | 0.01 ± 0.01 | P = 0.01 | −0.002 ± 0.003 | P = 0.55 |
| BMI | 0.04 ± 0.02 | P < 0.01 | 0.04 ± 0.02 | P < 0.01 | 0.006 ± 0.007 | P = 0.37 |
| Group assignment | −0.27 ± 0.15 | P = 0.08 | −0.27 ± 0.15 | P = 0.08 | −0.12 ± 0.07 | P = 0.07 |
| Week | 0.43 ± 0.03 | P < 0.001 | 0.24 ± 0.04 | P < 0.001 | 0.11 ± 0.03 | P < 0.01 |
| Week * week | −0.01 ± 0.002 | P < 0.001 | −0.01 ± 0.002 | P < 0.001 | −0.01 ± 0.002 | P < 0.001 |
| TFEQ restraint change in Month 1 | 0.02 ± 0.02 | P = 0.35 | −0.01 ± 0.02 | P = 0.46 | ||
| TFEQ restraint change * week | 0.02 ± 0.01 | P < 0.01 | 0.005 ± 0.004 | P = 0.22 | ||
| TFEQ disinhibition change in Month 1 | −0.01 ± 0.02 | P = 0.82 | 0.001 ± 0.02 | P = 0.60 | ||
| TFEQ disinhibition change * week | −0.003 ± 0.007 | P = 0.66 | −0.003 ± 0.005 | P = 0.43 | ||
| D‐Sat healthy lifestyle change in Month 1 | 0.16 ± 0.08 | P = 0.04 | −0.03 ± 0.07 | P = 0.69 | ||
| Healthy lifestyle change * week | 0.09 ± 0.02 | P < 0.001 | 0.02 ± 0.02 | P = 0.43 | ||
| Weight loss in Month 1 | 0.17 ± 0.02 | P < 0.001 | ||||
| Weight loss in Month 1 * week | 0.06 ± 0.005 | P < 0.001 | ||||
| Model fit indices | AIC: 3,983.4 | AIC: 3,951.5 | AIC: 3,558.7 | |||
| BIC: 4,028.5 | BIC: 4,016.0 | BIC: 3,629.6 | ||||
Asterisks indicate the influence of the factor on the linear coefficient of the weight loss trajectory (* week). None of the factors significantly influenced the quadratic coefficient of the weight loss curve at Month 3. Factor coefficients are not directly comparable due to differences in scoring ranges.
Note. BMI, body mass index; D‐Sat, Diet Satisfaction Questionnaire; SEM, standard error of the mean; TFEQ, Three‐Factor Eating Questionnaire.
Table 3.
Hierarchical random coefficients models of the influence of individual factors* of 186 women on the trajectory of weight loss across the full year of a 1‐year trial
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient ± SEM | P value | Coefficient ± SEM | P value | Coefficient ± SEM | P value | Coefficient ± SEM | P value | |
| Age | 0.01 ± 0.01 | P = 0.24 | 0.01 ± 0.01 | P = 0.20 | 0.01 ± 0.008 | P = 0.19 | −0.003 ± 0.003 | P = 0.27 |
| BMI | 0.07 ± 0.02 | P < 0.01 | 0.05 ± 0.02 | P = 0.01 | 0.05 ± 0.02 | P = 0.01 | 0.003 ± 0.008 | P = 0.67 |
| Group assignment | −0.30 ± 0.21 | P = 0.17 | −0.28 ± 0.20 | P = 0.16 | −0.27 ± 0.20 | P = 0.17 | −0.13 ± 0.79 | P = 0.11 |
| Week | 0.27 ± 0.02 | P < 0.001 | 0.23 ± 0.03 | P < 0.001 | 0.06 ± 0.04 | P = 0.14 | −0.03 ± 0.04 | P = 0.50 |
| Week * week | −0.004 ± 0.00 | P < 0.001 | −0.004 ± 0.00 | P < 0.001 | −0.001 ± 0.00 | P = 0.06 | 0.0001 ± 0.0006 | P = 0.82 |
| TFEQ restraint change in Month 1 | 0.02 ± 0.02 | P = 0.38 | 0.01 ± 0.02 | P = 0.57 | −0.04 ± 0.02 | P < 0.05 | ||
| TFEQ restraint change * week | 0.007 ± 0.003 | P < 0.01 | 0.02 ± 0.006 | P < 0.001 | 0.01 ± 0.005 | P = 0.06 | ||
| TFEQ disinhibition change in Month 1 | −0.06 ± 0.03 | P = 0.07 | −0.06 ± 0.03 | P = 0.06 | −0.04 ± 0.02 | P = 0.08 | ||
| TFEQ disinhibition change * week | 0.0001 ± 0.003 | P = 0.98 | 0.006 ± 0.007 | P = 0.46 | 0.008 ± 0.007 | P = 0.23 | ||
| D‐Sat healthy lifestyle change in Month 1 | 0.36 ± 0.11 | P < 0.01 | 0.31 ± 0.11 | P < 0.01 | −0.03 ± 0.09 | P = 0.76 | ||
| D‐Sat healthy lifestyle change * week | 0.004 ± 0.01 | P = 0.75 | 0.09 ± 0.03 | P < 0.001 | 0.02 ± 0.02 | P = 0.41 | ||
| TFEQ restraint change * week * week | −0.0002 ± 0.00 | P = 0.01 | −0.00 ± 0.00 | P = 0.40 | ||||
| TFEQ disinhibition change * week * week | −0.00 ± 0.0001 | P = 0.42 | −0.0001 ± 0.0001 | P = 0.20 | ||||
| D‐Sat healthy lifestyle change * week * week | −0.002 ± 0.0004 | P < 0.001 | −0.0004 ± 0.0004 | P = 0.25 | ||||
| Weight loss in Month 1 | −0.24 ± 0.02 | P < 0.001 | ||||||
| Weight loss in Month 1 * week | 0.05 ± 0.006 | P < 0.001 | ||||||
| Weight loss in Month 1 * week * week | −0.0007 ± 0.00 | P < 0.001 | ||||||
| Model fit indices | AIC: 10731.9 | AIC: 10706.3 | AIC: 10686.5 | AIC: 10291.5 | ||||
| BIC: 10777.1 | BIC: 10770.8 | BIC: 10760.7 | BIC: 10375.4 | |||||
Asterisks indicate the influence of the factor on the linear (* week) and quadratic (* week * week) coefficients of the weight loss trajectory. Factor coefficients are not directly comparable due to differences in scoring ranges.
Note. BMI, body mass index; D‐Sat, Diet Satisfaction Questionnaire; SEM, standard error of the mean; TFEQ, Three‐Factor Eating Questionnaire.
Figure 1.
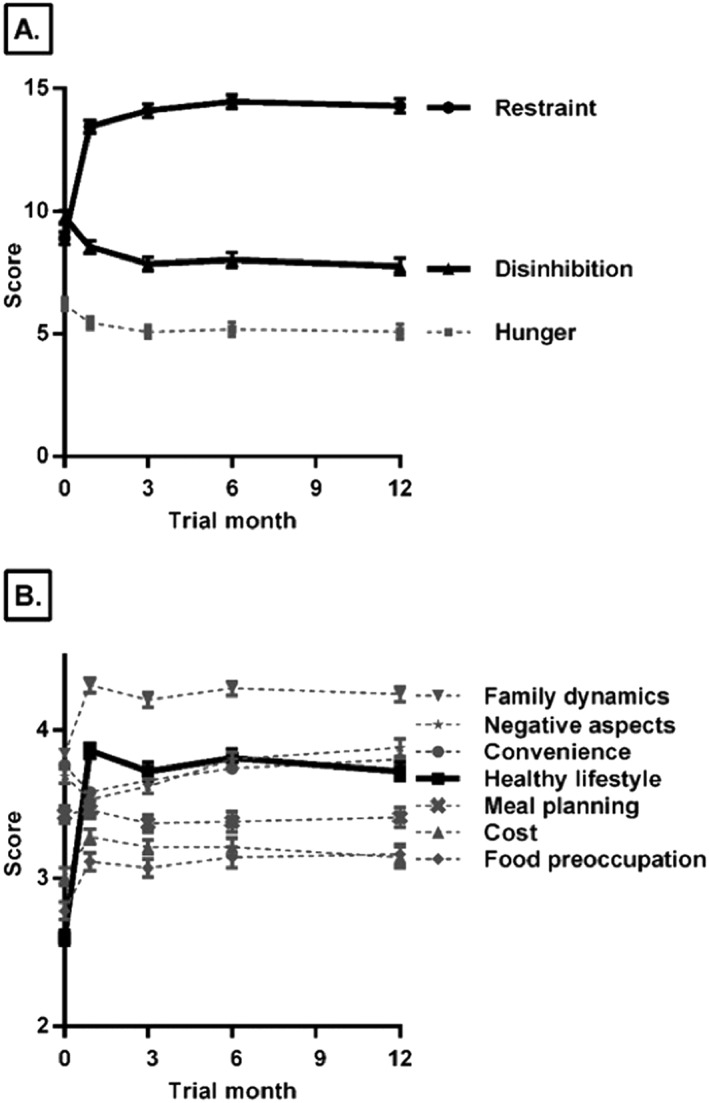
Mean (±standard error of the mean) scores over time for (a) the Three‐Factor Eating Questionnaire 19 and (b) the Diet Satisfaction Questionnaire 22 in 186 women in a weight‐loss trial. Relationships with weight loss and questionnaire scales that were included in the final model scales are shown in bold: the Dietary Restraint and Disinhibition scales of the Three‐Factor Eating Questionnaire and the Healthy Lifestyle scale of the Diet Satisfaction Questionnaire.
None of the baseline levels of the questionnaire scales were found to predict subsequent weight loss in the hierarchical model. The same was true for attendance in Month 1, which was not a significant predictor, likely due to the lack of variability in attendance rates across participants in the first month of treatment.
Month 3 weight loss
Initial change in multiple scales predicted the trajectory of weight loss during the first 3 months of the trial. As shown in Table 2, increases in TFEQ dietary restraint during the first month of intervention were associated with faster weight loss from baseline to Month 3 (P < 0.01). The Diet Satisfaction Questionnaire also showed associations with early weight loss. Specifically, increases in the healthy lifestyle scale predicted weight loss at Month 3 (P < 0.001).
When the flexible and rigid subscales of restraint were analyzed in the multivariate model, positive change in flexible restraint in the first month correlated with a greater rate of weight loss in the first 3 months (β = 0.03 ± 0.01, P = 0.01); in contrast, rigid restraint was not a significant predictor of weight loss (β = −0.004 ± 0.01, P = 0.75). Change in disinhibition in the first month was not related to subsequent weight loss nor was change in the internal and external disinhibition subscales when examined individually. The change in the susceptibility to hunger scale did not show associations with weight loss.
Month 12 weight loss
In general, the same covariates that predicted Month 3 weight loss also predicted Month 12 weight loss (Table 3). Increases in restraint during Month 1 predicted both a greater rate of weight loss and a slower rate of regain for the full 12 months of the trial (both P < 0.05). Participants in the highest tertile of increase in restraint score (6 to 16 points) lost 6.1 ± 4.0% body weight at Month 3 and 7.9 ± 8.2% at Month 12, while those in the lowest tertile (−5 to 2 points) lost 3.4 ± 3.8% at Month 3 and 3.3 ± 5.3% at Month 12. The flexible and rigid restraint subscales did not significantly predict Month 12 weight loss.
Increases in the healthy lifestyle scale of the D‐Sat predicted both a greater rate of weight loss and a slower rate of regain for the full 12 months of the trial (both P < 0.001). Individuals in the highest tertile of increase (1.63 to 3.25 points) lost 6.2 ± 4.2% body weight at Month 3 and 7.1 ± 8.2% at Month 12 compared with those in the lowest tertile (−1.0 to 0.75 points) who lost 2.8 ± 3.0% at Month 3 and 3.4 ± 4.5% at Month 12.
Neither early change nor baseline levels of the six other scales on the D‐Sat were significant predictors of weight loss.
Early weight loss
As expected, the amount of weight loss in the first month predicted the rate of weight loss from baseline to Month 3 and the rate of loss and slower regain from baseline to Month 12 (all P < 0.001). Participants in the highest tertile of initial weight loss (2.9 to 7.3 kg) lost 8.1 ± 3.4% at Month 3 and 10.4 ± 8.2% at Month 12 compared with those in the lowest tertile (from a 1.4‐kg gain to a 1.5‐kg loss) who lost 1.4 ± 2.4% at Month 3 and 1.6 ± 4.2% at Month 12. Neither TFEQ restraint nor D‐Sat healthy lifestyle remained significant once early weight loss was included in the models.
Discussion
In this 1‐year weight‐loss trial among women, changes over the first month in several psychological factors and eating behaviours predicted weight loss in the first 3 months and across the year. Participants who exhibited greater initial improvements in dietary restraint and healthy lifestyle rating had a greater rate of weight loss.
Frequent weight measurement in this trial facilitated the modelling of the associations between these factors and weight loss during intervention. The relationship between dietary restraint and weight loss was consistent with associations previously reported in the literature 14; furthermore, these results extend those findings by quantifying early improvement in these scores and demonstrating their predictive ability. The finding that weight loss was associated with an early increase in restraint suggests a beneficial effect of adopting eating behaviours such as consciously limiting the amount of food served or increasing awareness of the kind and amount of food eaten. These findings parallel changes seen in the bariatric surgery field where increased restraint shortly following surgery was associated with greater long‐term weight loss 25, as well as other long‐term weight‐loss trials where lifestyle modification corresponds to both increases in restraint and greater weight loss 26. Therefore, overall findings with restraint support evidence that it represents positive behaviours that reflect self‐regulation and promote weight loss 14.
Analysis of the subscales of the TFEQ showed that an increase in flexible restraint was advantageous for short‐term weight loss in this trial. Flexible restraint is characterized by a tendency to allow “forbidden” foods to be eaten in small amounts with adjustment of subsequent intake, in contrast to rigid restraint, which represents an approach to eating characterized by strict dieting and avoidance of high‐calorie foods 20. These results agree with other weight‐loss trials showing that flexible restraint has benefits for weight loss 27. Differences in these two types of restraint may explain the conflicting results of previous studies, which have found both positive and negative associations with weight loss when the overall restraint scale was examined 14. Thus, individuals who show an increase in restraint, particularly flexible restraint, early in weight loss treatment are more likely to experience long‐term success.
An unexpected finding was the lack of relationship between early change in disinhibition and subsequent weight loss. Decreasing disinhibition, or reducing the tendency to overeat in response to emotional and environmental cues, was hypothesized to result in a greater rate of early weight loss. While these findings are unexpected, disinhibition changed more gradually than restraint in this trial, suggesting that looking at it as a predictor later on, such as the change in disinhibition from baseline to Month 3, might have shown a stronger effect on predicting long‐term weight loss. This theory is supported by other work showing that change in disinhibition over a longer period of treatment predicted later weight loss 28.
The influence of diet satisfaction deserves additional study, as it was shown here that weight loss was related to an initial increase in one rating of diet satisfaction, namely that the diet supports a healthy lifestyle. A recent analysis of weight‐loss trial data showed that a decrease in perceived barriers to healthy eating was associated with better response to treatment over 18 months 29. These Portion‐Control Strategies Trial findings also parallel more general findings for the effects of satisfaction on weight loss. Previous studies have found that initial satisfaction with the type of intervention predicted subsequent weight loss 30, 31, 32 and that overall satisfaction with initial weight loss predicted long‐term weight loss and maintenance 32. Satisfaction with a prescribed diet may affect the likelihood of adopting and maintaining dietary changes and thus could have a substantial impact on weight loss. In weight‐loss treatment, consideration should be given to emphasizing the quality of the prescribed diet and identifying personal and environmental barriers to changes in eating behaviour because a participant's agreement with statements such as, ‘I am satisfied with my diet’ and ‘I believe that I am reducing my risk for disease by the way that I eat’, was associated with better outcomes. These findings show that an individual's assessment of how well their diet supports a healthy lifestyle compared with their pre‐treatment diet bears a relationship to long‐term weight loss, more so than other facets of diet satisfaction.
In this trial, 1‐month weight loss was a strong predictor of subsequent weight loss, which confirms extensive prior research demonstrating that initial weight loss predicts longer‐term success in behavioural weight‐loss treatments 5, 6, 7, 8, 9. These findings make sense given the fact that a subset of the outcome data (early weight loss) is used to predict the outcome (overall weight loss). Even though initial weight loss largely overpowers the effects of other variables on longer‐term weight loss, the findings prior to entering early weight loss in the models are informative for identifying factors that might predict early weight loss and offer potential intervention targets for improving outcomes.
It should not be assumed based on these findings that treatment could be stopped after early weight loss was achieved and long‐term weight loss would still be equivalent to that resulting from longer‐term treatment. Even for individuals who respond well to treatment by losing weight in the initial months, there is little evidence that their response would continue should treatment end. Therefore, future work should focus on both enhancing treatment for individuals initially identified as less responsive and on reinforcing behaviours in those responding well. Early assessment of the factors related to weight loss could be useful in tailoring treatment to accomplish these goals.
Although early assessment of individual factors associated with weight loss could facilitate individualized intervention, only a few studies have examined the impact of tailored treatment on later outcomes. One study found that individuals allowed to choose their weight‐loss diet at the start of treatment did not differ in weight loss at 1 year compared with those given no choice 33. However, little is known about the benefit of allowing individuals to change programmes after an initial lack of response. A recent study provided extra support for individuals identified in the first month as unresponsive to treatment 34; the results showed improvement in weight loss at 12 weeks for those who received extra support compared with those who did not. Follow‐up work is needed to determine whether this benefit continues in the longer‐term.
These findings raise the question of whether individual characteristics should be assessed only to predict subsequent weight loss or if they should also be the focus of intervention. Improvements in restraint often result from behavioural weight loss treatments, but this factor could be targeted by building the necessary tools and support structure for change. It is often assumed that these factors are driving weight loss, but few interventions have focused on them directly in an effort to prove causality, and future work should do so.
Programme attendance is often shown to influence long‐term weight loss, as it did when examined across the year in this trial 16. However, attendance in the first month did not predict subsequent weight loss, likely due to little variability in the measure. The trial population consisted of women who were predominantly Caucasian and well‐educated, which limits generalizability but also creates a basis for these findings that can be tested in more diverse populations.
In summary, little research has investigated early changes in individual eating behaviours and psychological factors during intervention that are predictive of long‐term weight loss. By identifying early predictors beyond initial weight loss itself, these findings provide a more comprehensive picture of the many factors associated with weight loss. As such evidence emerges, so does the potential to identify unresponsive participants and to tailor treatment to each individual based on early levels of factors such as restraint and healthy lifestyle. This personalization based on early changes could benefit long‐term outcomes, but work remains to be done on the most effective methods for accomplishing this. Incorporating additional early measurements such as those identified in this trial will provide a more complete picture of each individual, determine which factors have the greatest impact, and identify what type of treatment could best promote and maintain long‐term weight loss.
Disclosures
B. J. R. receives royalties from the sale of the Volumetrics books. B. L. J., E. L., and L. S. R. declare no conflict of interest.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK059853), by the National Institute of Food and Agriculture, U.S. Department of Agriculture (2011‐67001‐30117), and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR000127). Food vouchers were provided by unrestricted gifts from ConAgra Foods, Inc. (Omaha, Nebraska, USA) and Nestlé USA (Glendale, California, USA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding bodies.
Acknowledgements
We thank the participants in the Portion‐Control Strategies Trial and the research team at Penn State, particularly interventionists Amy Ciccarella, Kitti Halverson, Cara Meehan, Jennifer Meengs, and Christine Sanchez.
James, B. L. , Roe, L. S. , Loken, E. , and Rolls, B. J. (2018) Early predictors of weight loss in a 1‐year behavioural weight‐loss programme. Obesity Science & Practice, 4: 20–28. doi: 10.1002/osp4.149.
The Portion‐Control Strategies Trial is registered at www.clinicaltrials.gov as NCT01474759.
References
- 1. Flegal KM, Kruszon‐Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 2016; 21: 2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alamuddin N, Wadden TA. Behavioral treatment of the patient with obesity. Endocrinol Metab Clin North Am 2016; 45: 565–580. [DOI] [PubMed] [Google Scholar]
- 3. MacLean PS, Wing RR, Davidson T, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015; 23: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teixeira PJ, Going SB, Sardinha LB, Lohman TG. A review of psychosocial pre‐treatment predictors of weight control. Obes Rev 2005; 6: 43–65. [DOI] [PubMed] [Google Scholar]
- 5. Unick JL, Neiberg RH, Hogan PE, et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015; 23: 1353–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casazza K, Brown A, Astrup A, et al. Weighing the evidence of common beliefs in obesity research. Crit Rev Food Sci Nutr 2015; 55: 2014–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller CK, Nagaraja HN, Weinhold KR. Early weight‐loss success identifies nonresponders after a lifestyle intervention in a worksite diabetes prevention trial. J Acad Nutr Diet 2015; 115: 1464–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nackers LM, Ross KM, Perri MG. The association between rate of initial weight loss and long‐term success in obesity treatment: does slow and steady win the race? Int J Behav Med 2010; 17: 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elfhag K, Rössner S. Initial weight loss is the best predictor for success in obesity treatment and sociodemographic liabilities increase risk for drop‐out. Patient Educ Couns 2010; 79: 361–366. [DOI] [PubMed] [Google Scholar]
- 10. Moroshko I, Brennan L, O'Brien P. Predictors of dropout in weight loss interventions: a systematic review of the literature. Obes Rev 2011; 12: 912–934. [DOI] [PubMed] [Google Scholar]
- 11. Carels RA, Cacciapaglia HM, Douglass OM, Rydin S, O'Brien WH. The early identification of poor treatment outcome in a women's weight loss program. Eat Behav 2003; 4: 265–282. [DOI] [PubMed] [Google Scholar]
- 12. Annesi JJ, Mareno N. Psychosocial changes as correlates of weight regain vs. continued loss within 2‐year trials of a self‐regulation‐focused community‐based intervention. Clin Obes 2017; 7: 22–23. [DOI] [PubMed] [Google Scholar]
- 13. Nezami BT, Lang W, Lakicic JM, et al. The effect of self‐efficacy on behavior and weight in a behavioral weight‐loss intervention. Health Psychol 2016; 35: 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schaumberg K, Anderson DA, Anderson LM, Reilly EE, Gorrell S. Dietary restraint: what's the harm? A review of the relationship between dietary restraint, weight trajectory and the development of eating pathology. Clin Obes 2016; 6: 89–100. [DOI] [PubMed] [Google Scholar]
- 15. Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev 2008; 9: 409–419. [DOI] [PubMed] [Google Scholar]
- 16. Rolls BJ, Roe LS, James BL, Sanchez CE. Does the incorporation of portion‐control strategies in a behavioral program improve weight loss in a one‐year randomized controlled trial? Int J Obes (Lond) 2017; 41: 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. The Eating Attitudes Test: psychometric features and clinical correlates. Psychol Med 1982; 12: 871–878. [DOI] [PubMed] [Google Scholar]
- 18. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 19. Stunkard AJ, Messick S. The three‐factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 1985; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 20. Westenhoefer J. Dietary restraint and disinhibition: is restraint a homogeneous concept? Appetite 1991; 16: 45–55. [DOI] [PubMed] [Google Scholar]
- 21. Niemeier HM, Phelan S, Fava JL, Wing RR. Internal disinhibition predicts weight regain following weight loss and weight loss maintenance. Obesity (Silver Spring) 2007; 15: 2485–2494. [DOI] [PubMed] [Google Scholar]
- 22. Ello‐Martin JA. Reducing dietary energy density for the treatment of obesity: the long‐term effects on weight loss, hunger, and diet satisfaction. [dissertation]. University Park, PA: The Pennsylvania State University; 2006.
- 23. Stotland S, Zuroff DC. A new measure of weight locus of control: the Dieting Beliefs Scale. J Pers Assess 1990; 54: 191–203. [DOI] [PubMed] [Google Scholar]
- 24. Eaton CA, Rossi JS. Self‐efficacy in weight management. J Consult Clin Psychol 1991; 59: 739–744. [DOI] [PubMed] [Google Scholar]
- 25. Sarwer DB, Moore RH, Spitzer JC, et al. A pilot study investigating the efficacy of postoperative dietary counseling to improve outcomes after bariatric surgery. Surg Obes Relat Dis 2012; 8: 561–568. [DOI] [PubMed] [Google Scholar]
- 26. Volger S, Wadden TA, Sarwer DB, et al. Changes in eating, physical activity and related behaviors in a primary care‐based weight loss intervention. Int J Obes (Lond) 2013: S12–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Westenhoefer J, Engel D, Holst C, et al. Cognitive and weight‐related correlates of flexible and rigid restrained eating behaviour. Eat Behav 2013; 14: 69–72. [DOI] [PubMed] [Google Scholar]
- 28. JaKa MM, Sherwood NE, Flatt SW, et al. Mediation of weight loss and weight loss maintenance through dietary disinhibition and restraint. J Obes Weight Loss Ther 2015; 5: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng Y, Sereika SM, Danford CA, et al. Trajectories of weight change and predictors over 18‐month weight loss treatment. J Nurs Scholarsh 2017; 49: 177–184. [DOI] [PubMed] [Google Scholar]
- 30. Gupta S, Wang Z. Treatment satisfaction with different weight loss methods among respondents with obesity. Clin Obes 2016; 6: 161–170. [DOI] [PubMed] [Google Scholar]
- 31. Shapiro JR, Koro T, Doran N, et al. Text4Diet: a randomized controlled study using text messaging for weight loss behaviors. Prev Med 2012; 55: 412–417. [DOI] [PubMed] [Google Scholar]
- 32. Finch EA, Linde JA, Jeffery RW, Rothman AJ, King CM, Levy RL. The effects of outcome expectations and satisfaction on weight loss and maintenance: correlational and experimental analyses—a randomized trial. Health Psychol 2005; 24: 608–616. [DOI] [PubMed] [Google Scholar]
- 33. Coles LT, Fletcher EA, Galbraith CE, Clifton PM. Patient freedom to choose a weight loss diet in the treatment of overweight and obesity: a randomized dietary intervention in type 2 diabetes and pre‐diabetes. Int J Behav Nutr Phys Act 2014; 11: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Unick JL, Dorfman L, Leahey TM, Wing RR. A preliminary investigation into whether early intervention can improve weight loss among those initially non‐responsive to an internet‐based behavioral program. J Behav Med 2016; 39: 254–261. [DOI] [PubMed] [Google Scholar]


