Abstract
Bacillus thuringiensis is a soil‐dwelling Gram positive bacterium that has been utilized as a biopesticide for well over 60 years. It is known to contain flagella that are important for motility. One of the proteins found in flagella is flagellin, which is post‐translationally modified by O‐glycosylation with derivatives of pseudaminic acid. The biosynthetic pathway for the production of CMP‐pseudaminic acid in B. thuringiensis, starting with UDP‐N‐acetyl‐d‐glucosamine (UDP‐GlcNAc), requires seven enzymes. Here, we report the three‐dimensional structures of Pen and Pal, which catalyze the first and second steps, respectively. Pen contains a tightly bound NADP(H) cofactor whereas Pal is isolated with bound NAD(H). For the X‐ray analysis of Pen, the site‐directed D128N/K129A mutant variant was prepared in order to trap its substrate, UDP‐GlcNAc, into the active site. Pen adopts a hexameric quaternary structure with each subunit showing the bilobal architecture observed for members of the short‐chain dehydrogenase/reductase superfamily. The hexameric quaternary structure is atypical for most members of the superfamily. The structure of Pal was determined in the presence of UDP. Pal adopts the more typical dimeric quaternary structure. Taken together, Pen and Pal catalyze the conversion of UDP‐GlcNAc to UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine. Strikingly, in Gram negative bacteria such as Campylobacter jejuni and Helicobacter pylori, only a single enzyme (FlaA1) is required for the production of UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine. A comparison of Pen and Pal with FlaA1 reveals differences that may explain why FlaA1 is a bifunctional enzyme whereas Pen and Pal catalyze the individual steps leading to the formation of the UDP‐sugar product. This investigation represents the first structural analysis of the enzymes in B. thuringiensis that are required for CMP‐pseudaminic acid formation.
Keywords: Bacillus thuringiensis, CMP‐pseudaminic acid biosynthesis, flagellin glycosylation, Gram positive bacteria, UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine, X‐ray structure
Short abstract
Abbreviations
- HEPES
N‐2‐hydroxyethylpiperazine‐N′‐2‐ethanesulfonic acid
- MES
2‐(N‐morpholino)ethanesulfonic acid
- NAD+
nicotinamide adenine dinucleotide (oxidized)
- NADH
nicotinamide adenine dinucleotide (reduced)
- NADP+
nicotinamide adenine dinucleotide phosphate (oxidized)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced)
- Ni‐NTA
nickel nitrilotriacetic acid
- TEV
Tobacco Etch Virus
- Tris
tris‐(hydroxymethyl)aminomethane
- UDP
uridine diphosphate
- UDP‐GlcNAc
UDP‐N‐acetyl‐d‐glucosamine
Introduction
Bacillus thuringiensis is a fascinating soil‐dwelling Gram positive bacterium that has been used as a biopesticide in the United States since the 1950s.1 During growth and sporulation, it produces parasporal inclusions composed of one or more δ‐endotoxins, which are lethal to a wide range of agricultural pests.2 Depending upon the specific bacterial strain, B. thuringiensis has been used to control the populations of cabbage worms, tent caterpillars, mosquitoes, and blackflies.3
Like many Gram negative and Gram positive bacteria, B. thuringiensis is known to contain flagella, helically shaped appendages involved in motility and adhesion.4, 5 It is thought that flagella‐mediated motility plays a key role in the pathogenicity of some Gram negative bacteria including Escherichia coli, Salmonella typhimurium, Campylobacter spp., and Pseudomonas aeruginosa, amongst others.6 One of the proteins found in flagella is flagellin, which in many bacteria is post‐translationally modified by O‐glycosylation, often with derivatives of pseudaminic acid (Scheme 1).7 Whereas the biosynthetic pathways leading to the formation of pseudaminic acid and its derivatives have been investigated in Gram negative bacteria, less is known regarding their formation in Gram positive organisms.8, 9, 10, 11, 12, 13 A recent and elegant investigation from the Bar‐Peled laboratory at the University of Georgia has shed considerable insight into the manner in which pseudaminic acid is synthesized in B. thuringiensis.14 As indicated in Scheme 1, two enzymes, Pen and Pal, are required to first convert UDP‐N‐acetyl‐d‐glucosamine (UDP‐GlcNAc) to UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine. Interestingly, in Gram negative organisms, only one enzyme, FlaA1 (also referred to as PseB), is required.15, 16 Note that FlaA1 is a bifunctional 5‐inverting 4,6‐dehydratase.17
Scheme 1.

Reactions catalyzed by Pen and Pal.
Blast searches of the Pen and Pal amino acid sequences indicate that they both belong to the short‐chain dehydrogenase/reductase (SDR) superfamily. This large superfamily includes enzymes that function on a wide range of substrates as well as proteins serving as redox sensors.18 Biochemical analyses suggest that Pen contains a tightly bound NADP+ cofactor whereas Pal employs a tightly bound NAD+ for activity.14
Curious as to the molecular architectures of these two enzymes, we initiated an X‐ray crystallographic analysis of them as reported here. The structure of Pen, in complex with NADP(H) and UDP‐GlcNAc, was solved to 2.7 Å resolution. Unlike most members of the SDR superfamily, which typically function as dimers, Pen is a hexamer displaying 322 symmetry. This quaternary architecture is similar to that first described for FlaA1 from Helicobacter pylori, an inverting 4,6‐dehydratase. The model of Pal with bound NAD(H) was determined to 1.45 Å resolution. Two of the residues critical for catalysis in the 4,6‐dehydratases, an aspartate and a glutamate (or lysine in some cases), are replaced in Pal with a cysteine and a methionine, respectively. Taken together, the models of Pen and Pal, as described herein, add unique structural insight into the formation of pseudaminic acid in Gram positive bacteria.
Results and Discussion
Structure of Pen
The dTDP‐glucose 4,6‐dehydratases have been the topic of research investigation for nearly 20 years, with the first structure of one, namely RmlB from E. coli, being determined in this laboratory in 1998 (Protein Data Bank (PDB) accession no. 1BXK). The model of RmlB was determined to 1.9 Å resolution and fully refined to an R‐factor of 19.5%. Following this initial X‐ray analysis, the three‐dimensional architectures of other dTDP‐glucose 4,6‐dehydratases were determined and described in exquisite detail.19, 20, 21, 22 In addition, the GDP‐mannose 4,6‐dehydratases and the CDP‐glucose 4,6‐dehydratases have also been structurally characterized.23, 24, 25, 26 From these studies, it is now known that two amino acids, Asp 135 and Glu 136 (E. coli numbering) play key roles in catalysis by functioning as active site acids or bases, respectively. In some of these enzymes, such as the CDP‐glucose 4,6‐dehydratases, the glutamate is replaced with a lysine residue.25, 26 According to amino acid sequence alignments, the equivalent residues in Pen are Asp 128 and Lys 129 (Supplementary Material). Given that our repeated attempts to produce crystals of wild‐type Pen failed, a double site‐directed mutant protein was subsequently constructed whereby Asp 128 and Lys 129 were replaced with an asparagine and an alanine residue, respectively. This mutant variant crystallized as a ternary complex with NADP(H) and UDP‐GlcNAc. The following discussion refers specifically to the D128N/K129A mutant variant. It should be noted that Pen purifies with tightly bound nicotinamide adenine dinucleotide phosphate or NADP(H). No attempts were made to either oxidize or reduce it. Thus, given that the oxidation state of the cofactor is unknown, it will be referred to as NADP(H).
Previous size‐exclusion chromatography studies suggested that Pen functions as either a tetramer or a pentamer.14 In fact, the crystals utilized in this investigation contained a complete hexamer in the asymmetric unit (over 2000 amino acid residues). The model was refined to 2.7 Å resolution with an overall R‐factor of 20.4%. The electron density map displayed regions of disorder for all six subunits. In particular, many of the amino acid residues residing in the surface loops defined by Lys 247 to Ala 255 and Pro 294 to Ala 304 could not be built. Even though these surface loops were disordered, there was observed electron density for all bound ligands in the six subunits of the hexamer. Shown in Figure 1 are the electron densities corresponding to the UDP‐sugar ligand and the NADP(H) cofactor in subunit A. Given that the α‐carbons for the six subunits in the asymmetric unit superimpose with root‐mean‐square deviations of between 0.2 to 0.4 Å, the following discussion refers to the first subunit in the X‐ray coordinate file unless otherwise noted.
Figure 1.

Stereo view of the electron densities corresponding to the bound ligands in Pen. The electron density map was calculated with (F o–F c) coefficients and contoured at 3σ. The ligands were not included in the X‐ray coordinate file used to calculate the omit map, and thus there is no model bias. All figures were prepared with PyMOL.38
A ribbon representation of the complete hexamer, which has overall dimensions of ∼110 × 86 × 117 Å, is displayed in Figure 2(a). The total buried surface area for each subunit is ∼2900 Å2. The hexamer displays 322 symmetry and appears to be a trimer of dimers. The active sites within the dimers are separated by ∼24 Å. The dimers, however, pack in the hexamer such that the active sites of one abut closely to another as indicated by the black arrows in Figure 2(a). Indeed, the ribosyl phosphates groups of the NADH(P) cofactors between dimers are separated by only ∼8 Å [Fig. 2(a)].
Figure 2.

The structure of Pen. A ribbon representation of the Pen hexamer is shown in stereo in (a). The quaternary structure of the enzyme can be envisioned as a trimer of dimers. The dimers are colored in gold, light blue, and violet. The arrows indicate the positions of the interfaces between the dimers. A ribbon representation for a single subunit, depicted in stereo, is provided in (b). The β‐strands are highlighted in light blue whereas the α‐helices are shown in violet. A close up stereo view of the Pen active site is depicted in (c). Possible hydrogen bonding interactions are indicated by the dashed lines. The sole water molecule is represented as a red sphere. The green dashed line between the sugar C‐4′ carbon and the nicotinamide C‐4 carbon is meant to emphasize that the sugar lies in the proper orientation for hydride transfer.
Shown in Figure 2(b) is a stereo ribbon representation of an individual subunit. The subunit adopts a bilobal appearance with the larger N‐terminal domain dominated by an eight‐stranded mixed β‐sheet flanked on one side by five α‐helices and on the other side by three α‐helices. The C‐terminal motif contains one two‐stranded parallel β‐sheet, one two‐stranded antiparallel β‐sheet, and three α‐helices. As is typical for members of the SDR superfamily, the active site is wedged between the two domains.
A close‐up stereo view of the active site is presented in Figure 2(c). The uracil ring of the UDP‐GlcNAc ligand is anchored into the active site by interactions with the side chain of Thr 194 and the backbone carbonyl oxygen of Thr 192. The ribose 2‐hydroxyl lies within 3.2 Å of the side chains of Thr 194 and Glu 267. The side chains of Asn 169 and Arg 200, the backbone amide of Val 177, and an ordered water molecule interact with the pyrophosphoryl group of the UDP‐sugar. Ser 127, Tyr 137, and Asn 169 serve to position the pyranosyl group of the substrate into the active site. Tyr 137 belongs to the YXXXK motif, which is a characteristic signature sequence for members of the SDR superfamily. The mostly conserved tyrosine in the YXXXK motif was first shown to function as an active site base in UDP‐galactose 4‐epimerase.27 The pyranosyl group of the UDP‐sugar adopts the 4C1 pucker.
With respect to the NADP(H) cofactor, the nicotinamide ring lies within 3.2 Å of the backbone amide group of Val 170 and the side chain of Ser 173. The ribose interacts with the side chains of Tyr 137 and Lys 141 and the backbone carbonyl of Val 83. The backbone amide groups of Thr 16 and Ile 17 and the guanidinium moiety of Arg 174 interact with the pyrophosphoryl group of the dinucleotide cofactor. Arg 38 plays a key role in cofactor binding by participating in both an electrostatic interaction with the ribose phosphate as well as in a cation‐π stacking interaction with the adenine ring.28 The adenine ring also interacts with the side chain of Asp 63 and the backbone amide of Ile 64. Besides the interaction with the side chain of Arg 38, the ribose phosphate group also is positioned within 3.2 Å of the side chain of Thr 14 and the backbone amide group of Ser 39. Finally, the distance between the C‐4′ carbon of the UDP‐sugar and the C‐4 carbon of the NADP(H) is 3.8 Å.
Comparison of Pen with FlaA1
As noted in the Introduction, Gram negative bacteria contain only one enzyme required for the conversion of UDP‐GlcNAc to UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine. Depending upon the organism, the enzyme has been referred to in the literature as either PseB or FlaA1. In 2006, a model for FlaA1 from H. pylori was reported, and the enzyme was shown to have the same hexameric quaternary structure as that observed for Pen.29 A subsequent detailed enzymological investigation of PseB from C. jejuni provided substantial mechanistic insight into the activity of this bifunctional inverting 4,6‐dehydratase.16 Accordingly, the reaction for PseB from C. jejuni can be described in terms of three steps as presented in Scheme 2. In the first step, a hydride is transferred from the sugar C‐4′ carbon to the nicotinamide ring C‐4 carbon of the cofactor, and Tyr 135 abstracts the C‐4′ hydroxyl proton leading to a C‐4′ keto intermediate. During the second step, the proton on the sugar C‐5′ carbon is removed by Lys 127 and, most likely, Asp 126 aids in the departure of the sugar C‐6′ hydroxyl group by donating a proton. Departure of the sugar C‐6′ hydroxyl group as a water leads to an enone intermediate. Finally, the hydride that was removed from the sugar C‐4′ carbon is transferred via NADPH to the sugar C‐6′ carbon, and a proton is donated back to the sugar C‐5′ carbon on the opposite side of the pyranosyl ring, thereby resulting in inversion of configuration. The source of the proton is not known, however. Importantly, the site‐directed mutant proteins, namely D126N, K127A, and Y135F, demonstrated severely compromised activities.16
Scheme 2.

Proposed mechanism for an inverting 4,6‐dehydratase.
Shown in Figure 3(a) is a superposition of the ribbon representations for the subunits of Pen and H. pylori FlaA1. These two enzymes demonstrate 40 and 61% amino acid sequence identities and similarities, respectively. Not surprisingly, the α‐carbons for the two models superimpose with a root‐mean‐square deviation of 1.2 Å, and as can be seen their overall architectures are remarkably similar. Importantly, the three catalytically important residues in FlaA1 are structurally conserved in Pen. The question thus arises as to why Pen stops after the first two steps leading to the formation of a UDP‐6‐deoxy‐d‐GlcNA‐5,6‐ene product? A superposition of the active sites for Pen and FlaA1 is presented in Figure 3(b). Strikingly, the active sites are similar except for the replacement of Ile 17 in Pen with Phe 22 in FlaA1. These residues are, however, ∼5 Å from the nicotinamide rings of the tightly bound cofactors. It should be kept in mind that the structure of Pen is that of a double site‐directed mutant protein so caution must be applied in any interpretation. However, there is one trend that may be of importance. In FlaA1, the C‐4 carbon of the nicotinamide ring is positioned at an equal distance from both the sugar C‐4′ carbon (3.9 Å) where the hydride is removed and the sugar C‐6′ carbon (3.9 Å) where the hydride is transferred. In the Pen model, the average distance in the six subunits of the hexamer between the C‐4 carbon of the nicotinamide ring and the sugar C‐4′ carbon is 3.7 Å whereas that between the nicotinamide C‐4 carbon and the sugar C‐6′ carbon is 4.2 Å. Thus, one hypothesis might be that the reaction stops in Pen at the enone intermediate because the NADPH is positioned too far from the sugar C‐6′ carbon to transfer its hydride. Clearly there are subtle factors in the Pen active site that cannot be teased out from static X‐ray structures. Importantly, however, the structure of Pen once again emphasizes the need for caution when annotating amino acid sequences and the importance of biochemical verifications. On the basis of the Pen amino acid sequence alone, it might have been argued that it functions as a bona fide inverting 4,6‐dehydratase, which is not correct.
Figure 3.

Comparison of Pen with FlaA1 from H. pylori. A superposition of the subunit ribbon drawings for Pen and FlaA1 is presented in stereo in (a). Pen and FlaA1 are highlighted in violet and gray, respectively. Shown in (b) is a close‐up stereo view of their active sites superimposed. Those residues and ligands belonging to Pen are depicted in violet whereas those corresponding to FlaA1 are colored in green. The labels correspond to those amino acid residues found in Pen.
Structure of Pal
The model of Pal presented here was determined to 1.45 Å resolution and refined to an overall R‐factor of 13.1%. A ribbon representation of the Pal dimer, with overall dimensions of approximately 51 × 64 × 84 Å, is displayed in Figure 4(a). As is typical for the dimeric members of the SDR superfamily, the subunit:subunit is formed by the fourth and fifth α‐helices from each subunit resulting in a four α‐helical bundle.30 These α‐helices, defined Ala 92—Asn 112 and Pro 146—Ala 164, provide most of the 2600 Å2 buried surface area per dimer. The observed electron densities for the bound ligands, UDP and NAD(H), are presented in stereo in Figure 4(b). Due to the instability of the Pal enone substrate, it was not possible to obtain a model for it in the presence of a nucleotide‐linked sugar. As in Pen, the overall subunit of Pal is distinctly bilobal with the N‐terminal region dominated by a seven‐stranded mixed β‐sheet and the smaller C‐terminal region containing two small β‐sheets (two strands each) and three α‐helices.
Figure 4.

The structure of Pal. A ribbon representation of Pal is shown in (a). The observed electron densities corresponding to the bound ligands in Pal are presented in stereo in (b). The map was calculated as described in in Figure 1 and contoured at 3σ. A close‐up stereo view of the Pal active site is displayed in (c) with possible hydrogen bonds indicated by the dashed lines and the positions of the water molecules represented by the red spheres.
Shown in Figure 4c is a stereo close‐up view of the Pal active site. The UDP ligand is anchored into the active site by the side chains of Asn 176, Gln 212, Arg 214, and Glu 275. The backbone amide nitrogen of Val 190 and the carbonyl oxygen of Asn 205 also lie within 3.2 Å of the UDP moiety. Ten water molecules surround the UDP ligand. The NAD(H) cofactor is positioned into the active site by eight water molecules as well as by the side chains of Asp 31, Ser 36, Asp 57, Asn 98, Tyr 147, and Lys 151 and the backbone amides of Phe 11, Ile 12, Asn 35, Ile 58, and Thr 177. Note that Tyr 147 and Lys 151 belong to the YXXXK motif of the SDR superfamily.
Pen and Pal display 23 and 41% amino acid sequence identities and similarities, respectively. Their α‐carbons correspond with a root‐mean‐square deviation of 1.5 Å. Pen is NADP+ dependent whereas Pal requires NAD+. Shown in Figure 5(a) is a superposition of the ribbon traces for Pen and Pal along with their respective bound ligands. Overall, their three‐dimensional architectures are decidedly similar. In Pen, however, there is a tight connection formed by Arg 38 and Ser 39 between β‐strand 2 and α‐helix 2 [Fig. 5(a)]. As previously discussed, the side chain of Arg 38 is intricately involved in positioning the cofactor into the active site by both electrostatic and cation‐π interactions. In Pal, the region connecting β‐strand 2 and α‐helix 2 adopts a more random coil character, and as a consequence projects inward toward the active site thereby precluding the binding of NADP(H) as highlighted in Figure 4(a).
Figure 5.
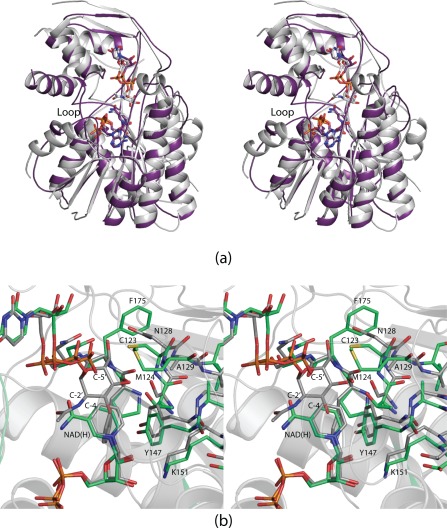
Comparison of Pen and Pal. A superposition of the ribbon representations for Pen and Pal is shown in stereo in (a). The models for Pen and Pal are depicted in white and purple, respectively. A superposition of their active sites is displayed in stereo in (b) with Pen and Pal colored in white and green bonds, respectively.
A superposition of the Pen and Pal active sites is presented in Figure 5(b). In the 4,6‐dehydratases, a conserved glutamate or lysine is required to abstract a proton from the pyranosyl C‐5′ carbon (Scheme 2) and an aspartate is needed to protonate the C‐6′ hydroxyl to aid in its departure. In order to trap a ternary complex of Pen with its substrate and the dinucleotide cofactor, these residues were changed to an asparagine (position 128) and an alanine (position 129), respectively. The reason why Pal cannot function as a 4,6‐dehydratase is due to the replacement of these residues with Cys 123 and Met 124, respectively [Fig. 5(b)]. Again, caution must be applied regarding annotations. Whereas Pal was suggested to be a dehydratase, it clearly does not have the appropriate amino acid residues required for such activity.
The biochemical role of Pal, as indicated in Scheme 1, is to oxidize the C‐4′ carbon, reduce the C‐6′ carbon, and protonate the C‐5′ carbon of UDP‐6‐deoxy‐d‐GlcNAc‐5,6‐ene thereby yielding the UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine product, which requires inversion of configuration about the C‐5′ carbon. Most likely Tyr 147 in Pal serves as the base to abstract the C‐4′ hydroxyl proton as the C‐4′ hydride is transferred to NAD+ to yield NADH and a keto moiety, similar to that outlined in Scheme 2 for PseB. The hydride on NADH can then be subsequently transferred to the sugar C‐6′ carbon. Like that reported for PseB from C. jejuni, however, the source of the proton to complete the reaction is unknown. There are no suitable side chains in the proper orientation to promote proton transfer to the opposite side of the pyranosyl ring, which would be required for inversion of configuration about the C‐5′ carbon.
In summary, three‐dimensional models for both Pen and Pal from B. thuringiensis have now been defined. Both structures, indeed, emphasize the need for caution when suggesting biochemical activities based solely on amino acid sequences. Without the detailed investigations from the Bar‐Peled laboratory and the structural analyses presented here, the predicted functions of Pen and Pal would remain incorrect. Additionally, as in the case of FlaA1 and PseB, the manner by which inversion of configuration about the C‐5′ carbon of the UDP‐sugar substrate is accomplished is still unclear. Why Gram positive bacteria evolved to utilize two separate enzymes for the conversion of UDP‐GlcNAc to UDP‐4‐keto‐6‐deoxy‐l‐N‐acetylaltrosamine rather than one as in Gram negative organisms is a fascinating but unanswered question.
Materials and Methods
Protein constructs, expression, and purification
The Pen and Pal genes were cloned from B. thuringiensis (ATCC 35646) genomic DNA. For protein expression, the gene encoding Pen was placed into the pET28T vector 31 whereas the gene encoding Pal was placed into the pET31 (Novagen) vector. The pET28T vector led to a protein with a TEV‐cleavable N‐terminal polyhistidine tag, whereas the pET31 vector generated a protein with a non‐cleavable C‐terminal polyhistidine tag. After repeated attempts, it was not possible to produce X‐ray quality crystals of wild‐type Pen. Hence, the D128N/K129A variant of Pen was constructed via the Stratagene QuikChange protocol, and the following discussion regarding purification and crystallization of Pen refers to the mutant variant.
Both Pen and Pal were expressed using Rosetta2(DE3) E. coli cells (Novagen). The cultures were grown in lysogeny broth supplemented with chloramphenicol (50 mg/L) and either 50 mg/L of kanamycin for Pen or 100 mg/L of ampicillin for Pal. The flasks were incubated at 37°C with shaking until an optical density of 0.8 was reached at 600 nm. The flasks were subsequently cooled in an ice bath, and protein expression was induced with 1 mM isopropyl β‐d‐1‐thiogalactopyranoside and allowed to continue at 16°C for 24 h. The cells were then harvested by centrifugation and frozen as pellets in liquid nitrogen.
Frozen cell pellets were disrupted by sonication on ice in a lysis buffer composed of 50 mM sodium phosphate, 20 mM imidazole, 10% glycerol, and 300 mM sodium chloride (pH 8.0). The lysates were cleared by centrifugation, and both Pen and Pal were purified at 4°C utilizing Ni‐NTA resin (Qiagen) according to the manufacturer′s instructions. For the purification of Pen, TEV protease was added in a 1:30 molar ratio to the pooled protein solution, which was subsequently dialyzed against 50 mM sodium phosphate, 300 mM sodium chloride, and 20 mM imidazole (pH 8.0) at 4°C for 24 h. The TEV protease and uncleaved protein were removed by passage over a Ni‐NTA column. Both the Pen and Pal solutions were ultimately dialyzed against 10 mM Tris‐HCl (pH 8.0) and 200 mM NaCl. Pen was concentrated to 13 mg/mL based on an extinction coefficient of 0.85 (mg/mL)−1cm−1 whereas Pal was concentrated to 25 mg/mL based on an extinction coefficient of 2.34 (mg/mL)−1cm−1.
Crystallization of Pen and Pal
Crystallization conditions were surveyed by the hanging drop method of vapor diffusion using a laboratory‐based sparse matrix screen. Pen was screened in the absence of ligands or in the presence of either UDP or UDP‐GlcNAc. Pal was screened in the presence and absence of UDP.
Diffraction quality crystals of Pen were grown at 4°C with 5 mM UDP‐GlcNAc, 2.2–2.5 M ammonium sulfate, and 100 mM HEPES (pH 7.5). For X‐ray data collection, the crystals were transferred to a solution composed of 5 mM UDP‐GlcNAc, 2.8 M ammonium sulfate, 100 mM HEPES, and 15% ethylene glycol (pH 7.5). The required UDP‐GlcNAc was purchased from Sigma‐Aldrich.
Crystals of Pal were grown at room temperature in the presence of 5 mM UDP, 8–12% poly(ethylene) glycol 5000, 100 mM MES, and 2% 2‐methyl‐2,4‐pentanediol (pH 6.0). For X‐ray data collection, they were transferred to a cryoprotectant solution composed of 5 mM UDP, 22% poly(ethylene) glycol 5000, 2% 2‐methyl‐2,4‐pentanediol, 100 mM MES, and 16% ethylene glycol (pH 6.0).
Structural analyses of Pen and Pal
An X‐ray data set from a crystal of Pen was collected in house with a Bruker AXS Platinum‐135 CCD detector controlled with the PROTEUM software suite (Bruker AXS). The X‐ray source was Cu Kα radiation from a Rigaku RU200 X‐ray generator equipped with Montel optics and operated at 50 kV and 90 mA. The X‐ray data were processed with SAINT and scaled with SADABS (Bruker AXS).
An X‐ray data set from a crystal of Pal was collected at Argonne National Laboratory Beamline 19‐BM at a wavelength of 0.9794 Å. The data were processed with HKL3000.32 Relevant X‐ray data collection statistics for both Pen and Pal are listed in Table 1.
Table 1.
X‐ray Data Collection Statistics
| Pen | Pal | |
|---|---|---|
| Resolution limits (Å) | 50‐2.7 (2.8–2.7)b | 50–1.45 (1.5–1.45)b |
| Space group | P21 | C2221 |
| Unit cell dimensions | ||
| a | 92.5 | 96.4 |
| b | 141.9 | 98.3 |
| c | 111.7 | 98.6 |
| β | 93.2 | |
| Number of independent reflections | 55,673 (5327) | 82,030 (8031) |
| Completeness (%) | 90.7 (84.1) | 98.9 (97.9) |
| Redundancy | 5.0 (2.2) | 7.3 (4.4) |
| Avg I/avg σ(I) | 9.8 (2.4) | 53.7 (8.0) |
| R sym (%)a | 9.4 (30.1) | 4.1 (17.5) |
R sym = (∑| I – |/∑ I) × 100.
Statistics for the highest resolution bin.
Pen was solved by molecular replacement with the software package PHASER using as a search model PDB entry 2GN4.29, 33 Pal was solved by molecular replacement using as a search model PDB entry 2P5U. Iterative cycles of model‐building with Coot34, 35 and refinement with REFMAC36 were employed to produce the final X‐ray coordinate files for Pen and Pal.34, 35, 36 Model refinement statistics are provided in Table 2.
Table 2.
Model Refinement Statistics
| Pen | Pal | |
|---|---|---|
| Resolution limits (Å) | 50–2.7 | 50–1.45 |
| a R‐factor (overall)%/no. reflections | 20.4/55,673 | 13.1/82,030 |
| R‐factor (working)%/no. reflections | 20.1/52,775 | 12.9/78,049 |
| R‐factor (free)%/no. reflections | 25.1/2898 | 15.1/3981 |
| Number of protein atoms | 15,321 | 2486 |
| Number of heteroatoms | 681 | 646 |
| Average B values | ||
| Protein atoms (Å2) | 26.9 | 13.2 |
| Ligand (Å2) | 18.3 | 10.6 |
| Solvent (Å2) | 21.0 | 29.5 |
| Weighted RMS deviations from ideality | ||
| Bond lengths (Å) | 0.012 | 0.012 |
| Bond angles (°) | 1.7 | 1.8 |
| Planar groups (Å) | 0.006 | 0.009 |
| Ramachandran regions (%) b | ||
| Most favored | 95.0 | 98.7 |
| Additionally allowed | 4.5 | 1.3 |
| Generously allowed | 0.5 | 0.0 |
R‐factor = (Σ|F o – F c|/Σ|F o|) × 100 where F o is the observed structure‐factor amplitude and F c. is the calculated structure‐factor amplitude.
Distribution of Ramachandran angles according to PROCHECK.37
Conflict of Interest
The authors have no competing financial interests.
Supporting information
Supporting Information
Acknowledgments
A portion of the research described in this paper was performed at Argonne National Laboratory, Structural Biology Center at the Advanced Photon Source (U. S. Department of Energy, Office of Biological and Environmental Research, under Contract DE‐AC02–06CH11357). We gratefully acknowledge Dr. Norma Duke for assistance during the X‐ray data collection. X‐ray coordinates have been deposited in the Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, N. J. (accession nos. 6BWC and 6BWL).
Broader statement: Flagella are appendages that function in the motility of some Gram positive and Gram negative bacteria. These whip‐like structures may play roles in bacterial invasion. One protein component of flagella is flagellin, which is post‐translationally modified with pseudaminic acid. The presence of this sugar is important for proper flagella assembly. Here we report the structures of two enzymes, Pen and Pal, that catalyze the initial steps in the biosynthesis of pseudaminic acid in Bacillus thuringiensis.
References
- 1. Ibrahim MA, Griko N, Junker M, Bulla LA (2010) Bacillus thuringiensis: a genomics and proteomics perspective. Bioeng Bugs 1:31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Latham JR, Love M, Hilbeck A (2017) The distinct properties of natural and GM cry insecticidal proteins. Biotechnol Genet Eng Rev 33:62–96. [DOI] [PubMed] [Google Scholar]
- 3. Ruiu L (2015) Insect pathogenic bacteria in integrated pest management. Insects 6:352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gillis A, Dupres V, Mahillon J, Dufrene YF (2012) Atomic force microscopy: a powerful tool for studying bacterial swarming motility. Micron 43:1304–1311. [DOI] [PubMed] [Google Scholar]
- 5. Gillis A, Dupres V, Delestrait G, Mahillon J, Dufrene YF (2012) Nanoscale imaging of Bacillus thuringiensis flagella using atomic force microscopy. Nanoscale 4:1585–1591. [DOI] [PubMed] [Google Scholar]
- 6. Erhardt M (2016) Strategies to block bacterial pathogenesis by interference with motility and chemotaxis. Curr Top Microbiol Immunol 398:185–205. [DOI] [PubMed] [Google Scholar]
- 7. Merino S, Tomas JM (2014) Gram‐negative flagella glycosylation. Int J Mol Sci 15:2840–2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry P (2001) Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J Biol Chem 276:34862–34870. [DOI] [PubMed] [Google Scholar]
- 9. Schirm M, Soo EC, Aubry AJ, Austin J, Thibault P, Logan SM (2003) Structural, genetic and functional characterization of the flagellin glycosylation process in Helicobacter pylori . Mol Microbiol 48:1579–1592. [DOI] [PubMed] [Google Scholar]
- 10. Schirm M, Arora SK, Verma A, Vinogradov E, Thibault P, Ramphal R, Logan SM (2004) Structural and genetic characterization of glycosylation of type a flagellin in Pseudomonas aeruginosa . J Bacteriol 186:2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schirm M, Schoenhofen IC, Logan SM, Waldron KC, Thibault P (2005) Identification of unusual bacterial glycosylation by tandem mass spectrometry analyses of intact proteins. Anal Chem 77:7774–7782. [DOI] [PubMed] [Google Scholar]
- 12. Schoenhofen IC, McNally DJ, Brisson JR, Logan SM (2006) Elucidation of the CMP‐pseudaminic acid pathway in Helicobacter pylori: synthesis from UDP‐N‐acetylglucosamine by a single enzymatic reaction. Glycobiology 16:8C–14C. [DOI] [PubMed] [Google Scholar]
- 13. Parker JL, Day‐Williams MJ, Tomas JM, Stafford GP, Shaw JG (2012) Identification of a putative glycosyltransferase responsible for the transfer of pseudaminic acid onto the polar flagellin of Aeromonas caviae Sch3N. Microbiologyopen 1:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Z, Hwang S, Ericson J, Bowler K, Bar‐Peled M (2015) Pen and Pal are nucleotide‐sugar dehydratases that convert UDP‐GlcNAc to UDP‐6‐deoxy‐d‐GlcNAc‐5,6‐ene and then to UDP‐4‐keto‐6‐deoxy‐l‐AltNAc for CMP‐pseudaminic acid synthesis in Bacillus thuringiensis . J Biol Chem 290:691–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schoenhofen IC, McNally DJ, Vinogradov E, Whitfield D, Young NM, Dick S, Wakarchuk WW, Brisson JR, Logan SM (2006) Functional characterization of dehydratase/aminotransferase pairs from Helicobacter and Campylobacter: enzymes distinguishing the pseudaminic acid and bacillosamine biosynthetic pathways. J Biol Chem 281:723–732. [DOI] [PubMed] [Google Scholar]
- 16. Morrison JP, Schoenhofen IC, Tanner ME (2008) Mechanistic studies on PseB of pseudaminic acid biosynthesis: a UDP‐N‐acetylglucosamine 5‐inverting 4,6‐dehydratase. Bioorg Chem 36:312–320. [DOI] [PubMed] [Google Scholar]
- 17. Samuel J, Tanner ME (2002) Mechanistic aspects of enzymatic carbohydrate epimerization. Nat Prod Rep 19:261–277. [DOI] [PubMed] [Google Scholar]
- 18. Kavanagh KL, Jornvall H, Persson B, Oppermann U (2008) Medium‐ and short‐chain dehydrogenase/reductase gene and protein families: the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65:3895–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allard ST, Giraud MF, Whitfield C, Graninger M, Messner P, Naismith JH (2001) The crystal structure of dTDP‐d‐glucose 4,6‐dehydratase (RmlB) from Salmonella enterica serovar Typhimurium, the second enzyme in the dTDP‐l‐rhamnose pathway. J Mol Biol 307:283–295. [DOI] [PubMed] [Google Scholar]
- 20. Allard ST, Beis K, Giraud MF, Hegeman AD, Gross JW, Wilmouth RC, Whitfield C, Graninger M, Messner P, Allen AG, Maskell DJ, Naismith JH (2002) Toward a structural understanding of the dehydratase mechanism. Structure 10:81–92. [DOI] [PubMed] [Google Scholar]
- 21. Beis K, Allard ST, Hegeman AD, Murshudov G, Philp D, Naismith JH (2003) The structure of NADH in the enzyme dTDP‐d‐glucose dehydratase (RmlB). J Am Chem Soc 125:11872–11878. [DOI] [PubMed] [Google Scholar]
- 22. Allard ST, Cleland WW, Holden HM (2004) High resolution X‐ray structure of dTDP‐glucose 4,6‐dehydratase from Streptomyces venezuelae . J Biol Chem 279:2211–2220. [DOI] [PubMed] [Google Scholar]
- 23. Webb NA, Mulichak AM, Lam JS, Rocchetta HL, Garavito RM (2004) Crystal structure of a tetrameric GDP‐d‐mannose 4,6‐dehydratase from a bacterial GDP‐d‐rhamnose biosynthetic pathway. Protein Sci 13:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mulichak AM, Bonin CP, Reiter WD, Garavito RM (2002) Structure of the MUR1 GDP‐mannose 4,6‐dehydratase from Arabidopsis thaliana: implications for ligand binding and specificity. Biochemistry 41:15578–15589. [DOI] [PubMed] [Google Scholar]
- 25. Vogan EM, Bellamacina C, He X, Liu HW, Ringe D, Petsko GA (2004) Crystal structure at 1.8 A resolution of CDP‐d‐glucose 4,6‐dehydratase from Yersinia pseudotuberculosis . Biochemistry 43:3057–3067. [DOI] [PubMed] [Google Scholar]
- 26. Koropatkin NM, Holden HM (2005) Structure of CDP‐d‐glucose 4,6‐dehydratase from Salmonella typhi complexed with CDP‐d‐xylose. Acta Crystallogr D Biol Crystallogr 61:365–373. [DOI] [PubMed] [Google Scholar]
- 27. Thoden JB, Wohlers TM, Fridovich‐Keil JL, Holden HM (2000) Crystallographic evidence for Tyr 157 functioning as the active site base in human UDP‐galactose 4‐epimerase. Biochemistry 39:5691–5701. [DOI] [PubMed] [Google Scholar]
- 28. Gallivan JP, Dougherty DA (1999) Cation‐pi interactions in structural biology. Proc Natl Acad Sci USA 96:9459–9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishiyama N, Creuzenet C, Miller WL, Demendi M, Anderson EM, Harauz G, Lam JS, Berghuis AM (2006) Structural studies of FlaA1 from Helicobacter pylori reveal the mechanism for inverting 4,6‐dehydratase activity. J Biol Chem 281:24489–24495. [DOI] [PubMed] [Google Scholar]
- 30. Allard ST, Giraud MF, Naismith JH (2001) Epimerases: structure, function and mechanism. Cell Mol Life Sci 58:1650–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thoden JB, Holden HM (2005) The molecular architecture of human N‐acetylgalactosamine kinase. J Biol Chem 280:32784–32791. [DOI] [PubMed] [Google Scholar]
- 32. Otwinowski Z, Minor W (1997) Processing of X‐ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326. [DOI] [PubMed] [Google Scholar]
- 33. McCoy AJ, Grosse‐Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2007) Phaser crystallographic software. J Appl Cryst 40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Emsley P, Cowtan K (2004) Coot: model‐building tools for molecular graphics. Acta Cryst 60:2126–2132. [DOI] [PubMed] [Google Scholar]
- 35. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Cryst 66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murshudov GN, Vagin AA, Dodson EJ (1997) Refinement of macromolecular structures by the maximum‐likelihood method. Acta Cryst 53:240–255. [DOI] [PubMed] [Google Scholar]
- 37. Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Cryst 26:283–291. [Google Scholar]
- 38. DeLano WL (2002) The PyMOL molecular graphics system. San Carlos, CA: DeLano Scientific.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


