Abstract
Naturally split inteins mediate a traceless protein ligation process known as protein trans‐splicing (PTS). Although frequently used in protein engineering applications, the efficiency of PTS can be reduced by the tendency of some split intein fusion constructs to aggregate; a consequence of the fragmented nature of the split intein itself or the polypeptide to which it is fused (the extein). Here, we report a strategy to help address this liability. This involves embedding the split intein within a protein sequence designed to stabilize either the intein fragment itself or the appended extein. We expect this approach to increase the scope of PTS‐based protein engineering efforts.
Keywords: chemical biology, protein engineering, intein splicing, protein semisynthesis
Introduction
Inteins are intervening protein domains found inserted within the sequence of what would otherwise be a fully functioning external protein (extein).1, 2 The intein undergoes a spontaneous posttranslational autoprocessing event, protein splicing, in which it self‐excises while simultaneously ligating the flanking extein segments. Most identified inteins exist as a contiguous domain, and the splicing reaction occurs in cis [Fig. 1(A) and Fig. S1(A)]. However, some inteins are fragmented into two separately expressed genes. Following expression, the naturally split inteins undergo association and splicing in trans [Fig. 1(B) and Fig. S1(B)]. All naturally split inteins characterized to date are asymmetric in nature;3, 4, 5, 6 typically the N‐terminal fragment (IntN) is around 100 amino acid residues long, while the smaller C‐terminal fragment (IntC) contains ∼35 residues. Interestingly, most of these “canonical” naturally split inteins exhibit extremely fast splicing kinetics in the context of their native extein residues.4, 5, 6, 7 For example, many members of the cyanobacterial DnaE split intein family support PTS with half‐lives on the order of a few minutes, and in some cases tens of seconds.5, 7 This facile reactivity, combined with the tight association of the IntN and IntC fragments, makes canonically split inteins powerful tools in chemical biology and protein engineering.1
Figure 1.
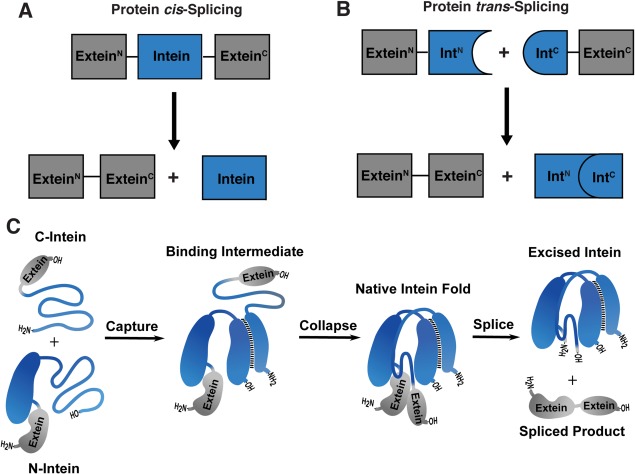
Protein cis and trans‐splicing. (A) Schematic depicting cis‐splicing of a contiguous intein (blue) to generate the spliced extein product (gray). (B) Schematic depicting trans‐splicing of a naturally split intein (blue) to generate the spliced extein product (gray). (C) Schematic depicting the two‐step mechanism of association and folding of a naturally split intein (blue) that enables splicing of the exteins (gray).
The association of canonically split DnaE inteins involves a disorder‐to‐order transition, coupling binding to formation of the folded intein domain [Fig. 1(C)].8, 9 A consequence of this assembly mechanism is that the IntN fragments are partially disordered prior to association with the complementary IntC partner, causing them to be prone to aggregation. Expression and purification of recombinant IntN fusion proteins can therefore be challenging under native conditions, limiting their application in intein‐based technologies. Strategies that lead to improvements in the physical properties of IntN fragments are thus badly needed. By contrast, IntC containing fusions are generally much better behaved proteins, likely due to the smaller size of the intein fragment and high density of positively charged residues therein.5
We recently applied a consensus protein design approach to the DnaE split intein family.10 This led to a split intein pair, CfaN and CfaC, which is able to carry out efficient PTS under a diverse set of reaction conditions, including high temperature and in the presence of chaotropes. Notably, extein fusions to the CfaN fragment exhibited increased expression yields in both bacterial and mammalian cells, compared to natural DnaE IntN inteins. The success of this consensus engineering strategy relied heavily on a deep understanding of the DnaE system, gained over many years, as well as the availability of a large number of family members from which to construct the final consensus sequence.3, 4, 5, 7, 11 In general, this type of information is not available for other naturally split inteins, which continue to be identified from metagenomic data.6, 12 More general strategies to enhance stability across both existing and yet to be discovered families of inteins would thus be beneficial. Herein, we offer two related approaches to this problem, both of which involve embedding the split intein within a protein sequence designed to stabilize either the intein fragment itself or the appended extein. Collectively, these strategies are expected to aid with the generation of engineered proteins using PTS.
Results
We recently reported a strategy to conditionally cage the activity of canonically split inteins.13 The approach exploits the mechanism by which the split intein pair associates and folds (Fig. S2). Each split intein fragment is fused to a truncated version of its cognate partner, an arrangement that engenders a highly favorable (but chemically unproductive) intramolecular interaction. Consequently, the normal split intein assembly pathway is blocked and no PTS takes place. Importantly, these caged split inteins are rapidly activated upon proteolytic release of the caging fragments—for obvious reasons, we refer to these as split intein zymogens.13 Beyond the ability to control splicing activity, we wondered whether use of this strategy would lead to a stabilization of the IntN fold and hence increase expression or splicing yield of corresponding extein fusions [Fig. 2(A)]. To explore this idea, we created a series of splicing constructs in which the model extein protein, SUMO, was fused to a set of wild‐type IntN sequences ( ), as well as caged versions thereof ( ). Each caged construct was designed as previously described13 and contains protease recognition sequence in the linker between IntN sequence and its cage. Expression at 37°C in E. coli followed by Ni‐NTA enrichment resulted in a poor yield for SUMO fusion constructs containing , Nrdj‐ , and gp41‐ compared to those with , IMPDH‐ , and gp41‐ [Fig. 2(B)]. Strikingly, introduction of the split intein cage increased expression yield for , Nrdj‐ , and gp41‐ to levels comparable to , IMPDH‐ , and gp41‐ [Fig. 2(B)]. Importantly, all of the caged IntN constructs exhibited efficient PTS when combined with the corresponding IntC fragment in the presence of the requisite protease trigger (Fig. S3).
Figure 2.
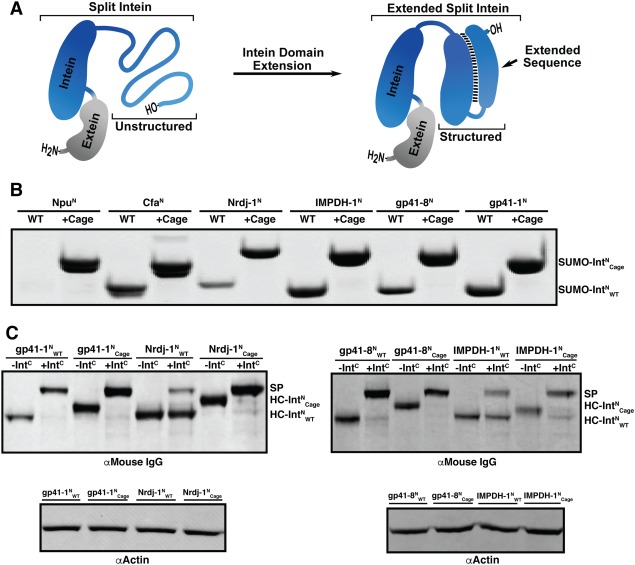
Stabilization of split inteins through intein extension. (A) Schematic depicting the extension strategy applied to stabilize a naturally split N‐intein, IntN (blue). (B) Coomassie‐stained SDS‐PAGE gel of SUMO fused to each IntN sequences (Npu, Cfa, Nrdj‐1, IMPDH‐1, gp41‐8, or gp41‐1) isolated from Ni‐NTA enrichment following expression at 37°C in E. coli. Each construct is shown either with (Cage) or without (WT) the intein cage. (C) Expression in HEK 293T cells and subsequent PTS activity of indicated wild type or caged IntN sequences fused to the C‐terminus of the heavy chain of a mouse αDec205 monoclonal antibody (HC‐ or HC‐ ). Top: Western blot analysis (αMouse IgG) of antibody present in medium after 96 hours. Samples are shown following incubation in the presence (+IntC) or absence (‐IntC) of the cognate IntC‐GFP and protease to generate the spliced product (SP). Bottom: Western blot analysis (αActin) of cell lysate as a loading control.
One promising application of intein technology is the modification of the heavy chain (HC) of monoclonal antibodies (mAbs) with synthetic cargoes.14, 15 Intein‐based methods to modify mAbs have the potential to generate antibody drug conjugates (ADCs) with specific payloads of cytotoxic agents or synthetic antigens. Previous efforts have demonstrated that the identity of the intein directly impacts the yield of mAb HC IntN (HC‐IntN) fusion proteins.10, 16 With this in mind, we applied the intein zymogen strategy to the production of HC‐IntN fusion proteins in HEK 293T cells, focusing on the gp41‐1, gp41‐8, IMPDH‐1 and Nrdj‐1 systems which have yet to be investigated in the context of antibody conjugation. Gratifyingly, we observed an improvement in expression yield and/or PTS efficiency for all the caged HC‐ constructs relative to their uncaged counterparts [Fig. 2(C)]. Notably, the PTS reactions went essentially to completion for all the caged inteins, which, in the case of the IMPDH‐1 and Nrdj‐1 systems represents a substantial improvement in yield.
Many applications of PTS necessitate fission of a protein of interest within a folded domain, resulted in partially (or completely) unfolded extein fragments being fused to the IntN and IntC sequences. Clearly this has the potential to destabilize the constructs, lowering the final yield of spliced product. In contrast to the many efforts made to identify and design inteins with improved properties, there have been few attempts to engineer increased stability into extein sequences. A general solution to this problem is non‐trivial since it must accommodate the fact that extein sequences are different in every application. From our recent efforts to increase the incorporation of truncated histones into cellular chromatin,17 we conceived a general strategy in which the split intein is embedded between the requisite extein polypeptide and a tailored “chaperone” sequence designed to stabilize the extein [Fig. 3(A)]. In one version of this, the appended chaperone sequence could correspond to some missing residues from the protein from which the extein is derived such that a stable fold is restored through an intrasteric interaction. Following PTS, these chaperone residues would be replaced with the corresponding extein sequence attached to the other intein fragment, in effect resulting in a “metathesis” of the system.
Figure 3.
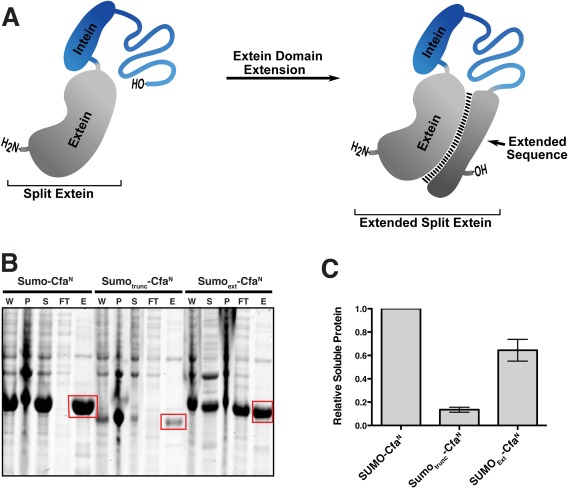
Stabilization of split intein fusions through extein extension. (A) Schematic depicting the extein extension strategy applied to stabilize a fragmented extein (gray). (B) Coomassie‐stained SDS‐PAGE gel of the Cfa N‐intein fused to full length SUMO (Sumo‐CfaN), truncated SUMO (Sumotrunc‐CfaN), or extended SUMO (Sumoext‐CfaN). Lanes corresponding to whole cell lysate (W), inclusion body pellet (P), soluble lysate (S), flow through (FT), and elution following Ni‐NTA enrichment (E) are shown. (C) Quantification of normalized protein signal from the Ni‐NTA enrichment lane highlighted in panel b (red box). Error = SD (n = 3).
As a proof of concept, the intein embedding strategy was applied to the expression of the single domain protein, SUMO [Fig. 3(B)]. We designed three constructs; one in which full‐length SUMO was fused to CfaN (SUMO‐CfaN), one in which the first 74 residues of SUMO were fused to CfaN (SUMOTrunc‐CfaN), and one in which the split intein was embedded within full‐length SUMO intein at position 74 (SUMOExt‐CfaN). The split site was chosen based on its location within a looped region of the protein (Fig. S4). As expected, the SUMO‐CfaN construct was found to express well in E. coli at 37°C [Fig. 3(B)]. However, fusing the truncated (and presumably unfolded) version of SUMO to the split intein decreased the recovery of soluble protein by 7‐fold [Fig. 3(B,C)]. Consistent with the internal chaperone idea, extension of CfaN with the missing 25 C‐terminal residues of SUMO restored soluble expression levels to near those found for SUMO‐CfaN [Fig. 3(B,C)]. A similar improvement in expression yield was also observed following an overnight expression at 18°C in E. coli (Fig. S5). Importantly, purified SUMOExt‐CfaN possessed robust PTS activity, allowing generation of spliced product, in this case full length SUMO, after addition of the corresponding IntC‐extein fragment (Fig. S6). Lastly, ubiquitin‐like‐specific‐protease‐1 (Ulp1), which specifically recognizes the SUMO tertiary structure, was able to cleave a peptide tag from the C‐terminus of the embedded intein construct (Fig. S7). The activity of Ulp1 suggests that the SUMOC extension intrasterically stabilizes SumoN in a manner analogous to the folded full‐length SUMO protein.
Discussion
Naturally split inteins are widely employed in protein engineering studies. Applications include the segmental isotopic labeling of proteins, the generation of toxic proteins and the semisynthesis of proteins.18, 19 Despite the broad utility of PTS, the approach can suffer from the poor behavior of the reactive polypeptides. This is a particular problem for constructs containing the IntN fragment due to its partially disordered structure. In this study, we have shown that extension of IntN sequences with fragments of their cognate IntC binding partner can improve solubility and functionality of expressed constructs both in bacterial and mammalian cells. In E. coli, the intein extension strategy stabilized fusions of the Npu, Nrdj‐1, and gp41‐8 inteins. Extension of the gp41‐1, gp41‐8, IMPDH‐1, and Nrdj‐1 inteins improved solubility and/or PTS efficiency of mAb HC fusions expressed in HEK 293T cells. An analogous method was designed to combat insolubility caused by fragmentation of the extein and improved the expression yield of a split SUMO‐intein fusion in E. coli.
As with most protein engineering strategies, we imagine that the impact of our intein embedding strategies on PTS will be context dependent. Indeed, the IntN caging strategy led to negligible gains in soluble protein expression for Cfa and IMPDH‐1 in E. coli, whereas the other inteins tested benefited from the strategy. However, the caging strategy did lead to a major improvement in the efficiency of PTS for IMPDH‐1 and Nrdj‐1 when fused to the mAb‐HC, despite having little impact in expression yield. Additional studies are necessary to better elucidate the specific situations that are optimal for applying the intein embedding strategies described herein, as understanding the context dependence of each strategy would serve to inform future designs and applications. Further optimization of both methods could occur through screening different linker sequences, as the impact of the linker on association of fragmented inteins and exteins was not explored in this study. Moreover, in vivo activity screens of protein trans‐splicing have significantly accelerated the discovery of constructs with improved splicing activities.5, 12, 20, 21 A comparable screen for intein expression yield, such as applying a GFP reporter, may prove to be similarly beneficial.22 Given the improvements in expression yield and PTS efficiency reported in this study, and the potential for additional refinement, we expect the split intein embedding strategies will expand the utility of PTS‐based technologies.
Materials and Methods
A detailed description of all materials, equipment, and methods used in this study can be found in the Supporting Information.
Supporting information
Supporting Information
Acknowledgments
The authors thank Anahita Z. Mostafavi and Glen P. Liszczak for valuable discussions. This work was supported by the U.S. National Institutes of Health (NIH grant R37‐GM086868).
Thomas Muir is the winner of the 2017 Emil Thomas Kaiser Award.
Significance: Applications of protein trans‐splicing have been limited by the propensity of fragmented proteins to aggregate. We report a general strategy to improve protein trans‐splicing by appending a peptide sequence tailored to intrasterically stabilize fragmented intein or extein residues. This intein embedding strategy should expand the versatility split intein‐based technologies.
References
- 1. Shah NH, Muir TW (2014) Inteins: nature's gift to protein chemists. Chem Sci 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Novikova O, Topilina N, Belfort M (2014) Enigmatic distribution, evolution, and function of inteins. J Biol Chem 289:14490–14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu H, Hu Z, Liu XQ (1998) Protein trans‐splicing by a split intein encoded in a split DnaE gene of Synechocystis sp. PCC6803. Proc Natl Acad Sci USA 95:9226–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iwai H, Zuger S, Jin J, Tam PH (2006) Highly efficient protein trans‐splicing by a naturally split DnaE intein from Nostoc punctiforme. FEBS Lett 580:1853–1858. [DOI] [PubMed] [Google Scholar]
- 5. Shah NH, Dann GP, Vila‐Perello M, Liu Z, Muir TW (2012) Ultrafast protein splicing is common among cyanobacterial split inteins: implications for protein engineering. J Am Chem Soc 134:11338–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carvajal‐Vallejos P, Pallisse R, Mootz HD, Schmidt SR (2012) Unprecedented rates and efficiencies revealed for new natural split inteins from metagenomic sources. J Biol Chem 287:28686–28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zettler J, Schutz V, Mootz HD (2009) The naturally split Npu DnaE intein exhibits an extraordinarily high rate in the protein trans‐splicing reaction. FEBS Lett 583:909–914. [DOI] [PubMed] [Google Scholar]
- 8. Zheng Y, Wu Q, Wang C, Xu MQ, Liu Y (2012) Mutual synergistic protein folding in split intein. Biosci Rep 32:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shah NH, Eryilmaz E, Cowburn D, Muir TW (2013) Naturally split inteins assemble through a “capture and collapse” mechanism. J Am Chem Soc 135:18673–18681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens AJ, Brown ZZ, Shah NH, Sekar G, Cowburn D, Muir TW (2016) Design of a split intein with exceptional protein splicing activity. J Am Chem Soc 138:2162–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oeemig JS, Aranko AS, Djupsjobacka J, Heinamaki K, Iwai H (2009) Solution structure of DnaE intein from Nostoc punctiforme: structural basis for the design of a new split intein suitable for site‐specific chemical modification. FEBS Lett 583:1451–1456. [DOI] [PubMed] [Google Scholar]
- 12. Thiel IV, Volkmann G, Pietrokovski S, Mootz HD (2014) An atypical naturally split intein engineered for highly efficient protein labeling. Angew Chem Int Ed 53:1306–1310. [DOI] [PubMed] [Google Scholar]
- 13. Gramespacher JA, Stevens AJ, Nguyen DP, Chin JW, Muir TW (2017) Intein zymogens: Conditional assembly and splicing of split inteins via targeted proteolysis. J Am Chem Soc 139:8074–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohlmann S, Bringmann P, Greven S, Harrenga A (2011) Site‐specific modification of ED‐B‐targeting antibody using intein‐fusion technology. BMC Biotechnol 11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbuto S, Idoyaga J, Vila‐Perello M, Longhi MP, Breton G, Steinman RM, Muir TW (2013) Induction of innate and adaptive immunity by delivery of poly dA:dT to dendritic cells. Nat Chem Biol 9:250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vila‐Perello M, Liu Z, Shah NH, Willis JA, Idoyaga J, Muir TW (2013) Streamlined expressed protein ligation using split inteins. J Am Chem Soc 135:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stevens AJ, Sekar G, Shah NH, Mostafavi AZ, Cowburn D, Muir TW (2017) A promiscuous split intein with expanded protein engineering applications. Proc Natl Acad Sci USA 114:8538–8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu D, Cowburn D (2017) Segmental isotopic labeling of proteins for NMR study using intein technology. Methods Mol Biol 1495:131–145. [DOI] [PubMed] [Google Scholar]
- 19. Shi C, Tarimala A, Meng Q, Wood DW (2014) A general purification platform for toxic proteins based on intein trans‐splicing. Appl Microbiol Biotechnol 98:9425–9435. [DOI] [PubMed] [Google Scholar]
- 20. Lockless SW, Muir TW (2009) Traceless protein splicing utilizing evolved split inteins. Proc Natl Acad Sci USA 106:10999–11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zettler J, Eppmann S, Busche A, Dikovskaya D, Dotsch V, Mootz HD, Sonntag T (2013) SPLICEFINDER: a fast and easy screening method for active protein trans‐splicing positions. PLoS One 8:e72925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waldo GS, Standish BM, Berendzen J, Terwilliger TC (1999) Rapid protein‐folding assay using green fluorescent protein. Nat Biotechnol 17:691–695. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


