Key Points
Question
Is exposure to thiopurines and anti–tumor necrosis factor (TNF) agents, used alone or in combination, associated with an increased risk of lymphoma in patients with inflammatory bowel disease (IBD)?
Findings
In this cohort study of 189 289 patients with IBD, the risk of incident lymphoma was significantly higher in patients exposed to thiopurine monotherapy, anti-TNF monotherapy, or combination therapy compared with those who were unexposed. The risk was higher in patients exposed to combination therapy compared with thiopurine or anti-TNF monotherapy.
Meaning
The use of thiopurine monotherapy or anti-TNF monotherapy in patients with IBD was associated with a small but statistically significant increased risk of lymphoma, and this risk was higher with combination therapy than with each of these treatments used alone.
Abstract
Importance
An increased risk of lymphoma has been reported among patients receiving thiopurines for inflammatory bowel disease (IBD). The risk of lymphoma associated with anti–tumor necrosis factor (TNF) agents either alone or in combination with thiopurines is uncertain.
Objective
To assess the risk of lymphoma associated with thiopurines and anti-TNF agents, used alone or in combination, for the management of IBD.
Design, Setting, and Participants
Nationwide cohort study based on French National Health Insurance databases. Patients aged 18 years or older identified with IBD were included from January 1, 2009, through December 31, 2013, and followed up until December 31, 2015.
Exposures
At each time of the follow-up, patients were categorized as being exposed to thiopurine monotherapy, anti-TNF monotherapy, or combination therapy, or being unexposed.
Main Outcomes and Measures
The primary outcome was incident lymphoma.
Results
Among the 189 289 patients included (54% women; median age, 43 years [interquartile range, 32-56 years]) and followed up for a median of 6.7 years, 123 069 were never exposed during follow-up, 50 405 were exposed to thiopurine monotherapy, 30 294 to anti-TNF monotherapy, and 14 229 to combination therapy. Overall, 336 lymphoma cases occurred: 220 in unexposed patients (incidence rate [IR] per 1000 person-years, 0.26; 95% CI, 0.23-0.29), 70 in patients exposed to thiopurine monotherapy (IR, 0.54; 95% CI, 0.41-0.67), 32 in patients exposed to anti-TNF monotherapy (IR, 0.41; 95% CI, 0.27-0.55), and 14 in patients exposed to combination therapy (IR, 0.95; 95% CI, 0.45-1.45). In a multivariable Cox model, compared with unexposed patients, the risk of lymphoma was higher among those exposed to thiopurine monotherapy (adjusted hazard ratio [aHR], 2.60; 95% CI, 1.96-3.44; P < .001), anti-TNF monotherapy (aHR, 2.41; 95% CI, 1.60-3.64; P < .001), or combination therapy (aHR, 6.11; 95% CI, 3.46-10.8; P < .001). The risk was higher in patients exposed to combination therapy vs those exposed to thiopurine monotherapy (aHR, 2.35; 95% CI, 1.31-4.22; P < .001) or anti-TNF monotherapy (aHR, 2.53; 95% CI, 1.35-4.77; P < .001).
Conclusions and Relevance
Among adults with IBD, the use of thiopurine monotherapy or anti-TNF monotherapy was associated with a small but statistically significant increased risk of lymphoma compared with exposure to neither medication, and this risk was higher with combination therapy than with each of these treatments used alone. These findings may inform decisions regarding the benefits and risks of treatment.
This pharmacoepidemiology study conducted in France assesses the risk of lymphoma associated with thiopurines and anti–tumor necrosis factor agents, used alone or in combination, for treatment of inflammatory bowel disease.
Introduction
The management of inflammatory bowel diseases (IBDs) has been rapidly evolving in the past 2 decades. The efficacy of thiopurines and tumor necrosis factor antagonists (anti-TNFs) has been assessed by numerous trials, most of which have demonstrated clinical benefits. Thiopurines are effective in maintaining the remission of both Crohn disease and ulcerative colitis. Anti-TNF agents are superior to azathioprine in patients with Crohn disease not previously treated with thiopurines and are approved in patients with moderate to severe Crohn disease or ulcerative colitis for whom thiopurines have not resulted in disease control. Furthermore, combination therapy with anti-TNF agents and thiopurines seems to be more effective than monotherapy in patients with IBD who have not been treated previously with either thiopurines or anti-TNF agents. It is less clear whether combination therapy is superior to anti-TNF monotherapy in patients exposed to thiopurines.
These drugs have potential severe adverse effects. In particular, thiopurines are associated with an increased risk of lymphoma. However, it is unclear whether anti-TNF agents are associated with an increased risk of lymphoma or whether the increased risk reported in patients treated with anti-TNF agents is explained by past or concomitant exposure to thiopurines. In addition, the risk of lymphoma associated with combination therapy remains largely unknown, although anti-TNF agents and thiopurines are increasingly prescribed concomitantly. A recent study estimated that 18.3% of patients diagnosed as having Crohn disease in the past 5 years in France had received combination therapy.
The aim of the current study was to assess the association of thiopurines and anti-TNF agents, when used alone or in combination, with subsequent risk of lymphoma, using data from a large nationwide observational cohort study of French patients with IBD treated with thiopurines and/or anti-TNF agents or neither between 2009 and 2015.
Methods
Data Sources
This study was conducted using the French National Health Insurance claim database known as SNIIRAM (Système National d’Information Interrégimes de l’Assurance Maladie). This system covers the entire French population with various health insurance plans based on individuals’ employment status. The general health insurance plan covers employees in the industry, business, and service sectors; public service employees; and students, accounting for approximately 88% of the French population. Only claims reimbursed from the general health insurance plan were considered because of their availability and quality. This database contains individual, anonymous data on sociodemographic characteristics (eg, sex, age, and date of death) and all medical claims since 2008, including dispensed drugs with date of delivery, laboratory tests, and outpatient medical care. The database also contains medical information on the presence of any serious and costly long-term disease giving entitlement to 100% health insurance coverage based on the French National Health Insurance list of 30 eligible chronic conditions, with information on diagnosis encoded in the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) and date of disease onset. Information on affiliation to the Complementary Universal Health Insurance scheme, a system providing free access to health care for people whose annual income is lower than 50% of the poverty threshold in France, is also available.
An anonymous, unique identifier for each individual links SNIIRAM information to the national hospital discharge database (Programme Médicalisé Des Systèmes D’information [PMSI]). The PMSI includes information about every admission at a public hospital or in a private clinic in France since 2006, either for an inpatient stay or ambulatory care. Diagnoses (recorded using their ICD-10 codes) and treatments, either medical or surgical, provided during hospital stays are available. The SNIIRAM and PMSI databases have been described and used for epidemiological research previously.
This study was approved by the French Data Protection Supervisory Authority (Commission Nationale de l’Informatique et des Libertés). Given that data are anonymous, no informed consent is required for studies based on French medicoadministrative databases.
Study Population
All patients aged 18 years or older and diagnosed as having IBD before December 31, 2013, were included. Based on information from SNIIRAM and PMSI, an individual was considered diagnosed as having IBD either if he or she was eligible for 100% health insurance coverage for serious and costly long-term diseases with a diagnosis of Crohn disease (ICD-10 code K50) or ulcerative colitis (ICD-10 code K51), or if he or she had at least 2 hospital discharge diagnoses including ICD-10 codes K50 or K51 or 1 hospital discharge diagnosis including ICD-10 codes K50 or K51 along with a pharmacy claim for an IBD medication including aminosalicylates, enteral budesonide, thiopurines, or anti-TNF agents. When ICD-10 codes of IBD subtype differed between SNIIRAM and PMSI or across various hospital discharge diagnoses, the most recent code was considered.
The date of IBD diagnosis was defined as the earliest date between the first hospital discharge diagnosis of IBD available in the PMSI and the date of IBD onset as registered for eligibility for full reimbursement of care in the SNIIRAM. Prevalent cases were defined as an IBD diagnosis provided before January 1, 2009, and incident cases as an IBD diagnosis issued between January 1, 2009, and December 31, 2013. This cohort has been extensively described in a previous study.
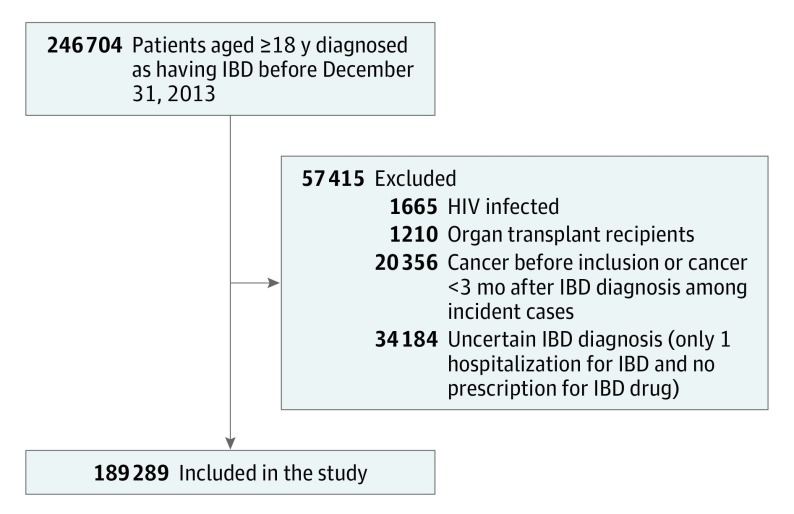
To avoid potentially confounding factors that may have increased the risk of lymphoma, organ transplant recipients, HIV-infected patients, and patients with a history of cancer including lymphoma were excluded (Figure). Individuals with a diagnosis of cancer or lymphoma within 3 months following their IBD diagnosis were also excluded to minimize the possible inclusion of nonincident cases. In addition, these patients follow different therapeutic management strategies. Furthermore, patients with a single hospital discharge diagnosis of IBD and no pharmacy claim for IBD medication, who were considered to have an uncertain IBD diagnosis, were excluded. Patients were followed up from January 1, 2009, or the date of their IBD diagnosis, whichever occurred first, until the first of the following events: death, any cancer diagnosis, or December 31, 2015.
Figure. Flowchart of the Study Population With Inflammatory Bowel Disease (IBD) Taken From the French National Health Insurance Databases.
Exposure Definition
Anti-TNF agents included infliximab and adalimumab. Infliximab is administered at hospitals or private clinics, whereas adalimumab is dispensed by pharmacies. Thiopurines (azathioprine and 6-mercaptopurine) are dispensed by pharmacies, which are authorized to deliver 1 month supply of treatment. Drug exposures were assumed to start the day of the drug infusion or delivery. Patients who received infliximab were considered as being exposed during the 2 months following the infusion, whereas those who received adalimumab or thiopurines were considered as being exposed for 1 month following the delivery.
At each time of the follow-up, patients were categorized as being exposed to thiopurine monotherapy, anti-TNF monotherapy, or combination therapy, or being unexposed. During follow-up, patients could be exposed successively to different lines of IBD treatment and could therefore contribute to more than 1 group of exposure. Combination therapy corresponded to a concomitant exposure to thiopurines and anti-TNF agents.
Covariates
Two groups of covariates were considered. Time-fixed covariates were defined at cohort entry and included sex, age, IBD type and duration (≥10 years, 0-9 years, and incident patients), affiliation to Complementary Universal Health Insurance (free access to health care for people with low income), exposure to methotrexate and aminosalicylates in the preceding 6 months of cohort entry, and history of cardiovascular disease, cerebrovascular disease, chronic pulmonary disease, chronic kidney disease, diabetes, cirrhosis, obesity, alcohol use disorder, and smoking-related conditions defined based on ICD-10 codes listed in eTable 1 in the Supplement.
Time-dependent covariates included the following indicators of IBD activity: exposure to corticosteroids, IBD-related hospitalization, and IBD-related surgery. At any change in exposure group, status with respect to corticosteroid use and IBD-related hospitalization and surgery was updated according to information available within 6 months prior to the treatment change.
Outcomes
The main outcome was incident lymphoma as defined by the following ICD-10 codes: C81, C82, C83, C84, C85, and C86. Based on information from SNIIRAM and PMSI, lymphoma onset was defined by the presence of at least 2 items among the following: (1) hospitalization with a discharge diagnosis of lymphoma; (2) 100% health insurance coverage for serious and costly long-term disease with a diagnosis of lymphoma; (3) chemotherapy in conjunction with a lymphoma diagnosis; and (4) radiotherapy in conjunction with a lymphoma diagnosis. Patients who had only been hospitalized or received long-term disease status for lymphoma were classified as having an “uncertain lymphoma diagnosis.” The relevant variable was categorized into Hodgkin lymphoma (ICD-10 code C81) and non-Hodgkin lymphoma for other ICD codes attributable to lymphoma.
Statistical Analyses
Cox proportional hazard regression models were used to estimate crude and adjusted hazard ratios (aHRs) and their 95% CIs comparing the risk of lymphoma according to treatment exposure treated as a time-dependent covariate. HRs comparing patients of each exposure group (thiopurine monotherapy, anti-TNF monotherapy, or combination therapy) with unexposed patients (either never exposed or previously treated but no longer exposed) were first estimated. Then, HRs associated with (1) exposure to anti-TNF monotherapy vs thiopurine monotherapy; (2) exposure to combination therapy vs thiopurine monotherapy; and (3) exposure to combination therapy vs anti-TNF monotherapy were estimated. In multivariable analysis, models were adjusted for baseline and time-dependent covariates.
The main analysis included patients with either prevalent or incident IBD. Analyses were conducted overall and according to the type of lymphoma (Hodgkin/non-Hodgkin). Additional analyses included analyses restricted to patients with incident IBD and analyses stratified by sex, age (18-34, 35-64, and ≥65 years), and IBD type (Crohn disease or ulcerative colitis).
Various sensitivity analyses were conducted using alternative assessment criteria for exposure and outcome, especially in case of occurrence of IBD treatment switches or withdrawals during follow-up. First, to exclude incipient lymphoma events, a lag period of 3 or 6 months following the initiation of each line of thiopurines and/or anti-TNF agents was introduced. Follow-up during these lag periods did not account for person-years in the exposed nor the unexposed groups. Second, analysis was restricted to the first line of IBD treatment by censoring follow-up time after any treatment switch or withdrawal. Third, to account for a potential persisting risk of lymphoma after treatment discontinuation, exposure time was extended for 3 or 6 months following treatment switch or withdrawal. In addition, an analysis including patients with an uncertain diagnosis of lymphoma was also performed.
All analyses were performed with SAS software version 9.3 (SAS Institute Inc). Statistical significance was defined as P < .05; all alternative hypotheses were 2-sided.
Results
Between January 1, 2009, and December 31, 2013, 246 704 individuals were identified with IBD. Among them, 1665 were HIV positive, 1210 were organ transplant recipients, 20 356 had a history of cancer, and 34 184 had an uncertain IBD diagnosis (Figure). A total of 189 289 individuals were therefore included in the cohort, among whom 127 948 (68%) had a prevalent IBD. The annual number of IBD incident cases ranged between 11 411 and 13 212 from 2009 to 2013. The median follow-up time was 6.7 years (interquartile range, 4.5-6.9 years).
Fifty-four percent of patients were women, and the median age at cohort entry was 43 years (Table 1). During follow-up, 123 069 patients (65%) remained persistently unexposed, while 40 803 (22%) experienced both periods of nonexposure and exposure to IBD treatments. Among exposed patients, 50 405 (27%) were exposed at least once to thiopurine monotherapy (mean exposure time, 17 months), 30 294 (16%) to anti-TNF monotherapy (19 months), and 14 229 (7.5%) to combination therapy (8 months). At the time of treatment initiation, 6% of patients initiating thiopurine monotherapy had been previously exposed to anti-TNF monotherapy (during 8 months on average) and 35% of patients initiating anti-TNF monotherapy had been previously exposed to thiopurine monotherapy (12 months). At the time of initiation of combination therapy, 50% of patients had been previously exposed to thiopurine monotherapy (7.5 months) and 43% to anti-TNF monotherapy (7 months).
Table 1. Characteristics of Patients at Cohort Entry, Overall and by Exposure Group.
| Characteristic | Overall, No. (%) (N = 189 289) |
Exposure Group, No. (%) | |||
|---|---|---|---|---|---|
| Never Exposed to Thiopurines or Anti-TNF Agents During Follow-up (n = 123 069)a |
Exposed to Thiopurine Monotherapy (n = 50 405)a | Exposed to Anti-TNF Monotherapy (n = 30 294)a | Exposed to Combination Therapy (n = 14 229)a | ||
| Age, median (IQR), y | 43 (32-56) | 46 (35-59) | 37 (27-48) | 33 (25-44) | 31 (24-42) |
| Age groups, y | |||||
| 18-34 | 57 692 (31) | 28 631 (23) | 22 104 (44) | 15 095 (50) | 8139 (57) |
| 35-64 | 108 074 (57) | 74 740 (61) | 25 357 (50) | 14 027 (46) | 5749 (40) |
| ≥65 | 23 523 (12) | 19 838 (16) | 2944 (6) | 1172 (4) | 341 (2) |
| Sex | |||||
| Male | 86 506 (46) | 56 342 (46) | 23 167 (46) | 13 355 (44) | 6531 (46) |
| Female | 102 783 (54) | 68 867 (54) | 27 238 (54) | 16 939 (56) | 7698 (54) |
| Diagnosis of IBD | |||||
| Crohn disease | 95 537 (51) | 51 322 (42) | 32 488 (64) | 22 309 (74) | 10 199 (72) |
| Ulcerative colitis | 93 752 (49) | 71 887 (58) | 17 917 (36) | 7985 (26) | 4030 (28) |
| Disease duration, y | |||||
| ≥10 | 41 933 (23) | 29 699 (22) | 9098 (18) | 5153 (17) | 2032 (14) |
| 0-9 | 86 015 (45) | 55 076 (45) | 23 541 (47) | 14 087 (47) | 6443 (45) |
| Incident | 61 341 (32) | 38 434 (32) | 17 766 (35) | 11 054 (36) | 5754 (41) |
| Affiliation to Complementary Universal Health Insuranceb | 17 283 (9.0) | 9679 (7.9) | 5618 (11) | 4033 (13) | 1985 (14) |
| Comorbiditiesc | |||||
| Diabetes | 12 523 (6.6) | 9440 (7.7) | 2431 (4.8) | 1176 (3.9) | 468 (3.3) |
| Chronic pulmonary disease | 11 338 (6.0) | 7814 (6.3) | 2561 (5.1) | 1708 (5.6) | 670 (4.7) |
| Cardiovascular disease | 9659 (5.1) | 7233 (5.9) | 1862 (3.7) | 1043 (3.4) | 390 (2.7) |
| Smoking-related conditions | 5537 (2.9) | 3110 (2.5) | 1725 (3.4) | 1327 (4.4) | 554 (3.4) |
| Cerebrovascular disease | 3546 (1.9) | 2763 (2.2) | 627 (1.0) | 323 (1.0) | 139 (1.0) |
| Alcohol use disorder | 3066 (1.6) | 2153 (1.7) | 1216 (1.4) | 709 (1.5) | 285 (1.3) |
| Obesity | 2736 (1.4) | 1821 (1.5) | 1040 (1.2) | 720 (1.5) | 284 (1.3) |
| Chronic kidney disease | 1728 (0.9) | 1283 (1.0) | 317 (0.6) | 222 (0.7) | 66 (0.5) |
| Cirrhosis | 815 (0.4) | 567 (0.5) | 146 (0.3) | 141 (0.5) | 43 (0.3) |
| Other IBD medicationsd | |||||
| Aminosalicylates | 77 217 (41) | 55 259 (45) | 20 475 (41) | 11 116 (37) | 5310 (37) |
| Corticosteroids | 22 261 (11.8) | 12 036 (10) | 10 412 (21) | 7483 (25) | 4066 (29) |
| Methotrexate | 2609 (1.4) | 1121 (0.9) | 284 (0.5) | 1487 (4.9) | 183 (1.3) |
| IBD-related complicationsd | |||||
| Hospitalization for IBD | 6861 (3.6) | 1660 (1.3) | 7941 (16) | 5701 (19) | 4029 (28) |
| Surgery related to IBD | 354 (0.2) | 0 | 1443 (2.9) | 1499 (5.0) | 970 (6.8) |
Abbreviations: IBD, inflammatory bowel disease; IQR, interquartile range; TNF, tumor necrosis factor.
The total number is more than 189 289 as 1 patient could be included in more than 1 group of exposure.
Free access to health care for people with an annual income less than 50% of the poverty threshold.
Defined based on International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes listed in eTable 1 in the Supplement.
As registered within 6 mo before cohort entry.
Overall, 336 cases of lymphoma occurred: 220 in unexposed patients (incidence rate [IR] per 1000 person-years, 0.26; 95% CI, 0.23-0.29), 70 in patients exposed to thiopurine monotherapy (IR, 0.54; 95% CI, 0.41-0.67), 32 in patients exposed to anti-TNF monotherapy (IR, 0.41; 95% CI, 0.27-0.55), and 14 in patients exposed to combination therapy (IR, 0.95; 95% CI, 0.45-1.45) (Table 2). Lymphoma occurred predominantly in men (57%) at a median age of 60 years. Fifty four percent of patients with lymphoma had Crohn disease, and the median IBD duration at the time of lymphoma diagnosis was 8.5 years. The most common lymphoma subtypes were nonfollicular lymphoma (n = 130, 39%), including 83 patients with diffuse large B-cell lymphoma; Hodgkin lymphoma (n = 55, 16%); and follicular lymphoma (n = 41, 12%) (eTable 2 in the Supplement). Hodgkin lymphoma accounted for 14% of all lymphomas among unexposed patients, 19% among patients exposed to thiopurines, 19% among patients exposed to anti-TNF agents, and 43% among patients exposed to combination therapy (eTable 2 in the Supplement).
Table 2. Incidence of Lymphoma According to Exposure Group.
| Lymphoma Type | Overall (1 060 336 PY) |
Unexposed to Thiopurines or Anti-TNF Agents (838 611 PY) |
Exposed to Thiopurine Monotherapy (129 743 PY) |
Exposed to Anti-TNF Monotherapy (77 229 PY) |
Exposed to Combination Therapy (14 753 PY) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Events | IR per 1000 PY (95% CI) | No. of Events | IR per 1000 PY (95% CI) | No. of Events | IR per 1000 PY (95% CI) | No. of Events | IR per 1000 PY (95% CI) | No. of Events | IR per 1000 PY (95% CI) | |
| All Patients | ||||||||||
| All lymphoma | 336 | 0.32 (0.28-0.35) |
220 | 0.26 (0.23-0.29) |
70 | 0.54 (0.41-0.67) |
32 | 0.41 (0.27-0.55) |
14 | 0.95 (0.45-1.45) |
| Hodgkin lymphoma | 55 | 0.05 (0.04-0.07) |
30 | 0.03 (0.02-0.04) |
13 | 0.10 (0.05-0.15) |
6 | 0.08 (0.02-0.14) |
6 | 0.41 (0.08-0.74) |
| Non-Hodgkin lymphoma | 281 | 0.27 (0.21 -0.33) |
190 | 0.23 (0.20-0.26) |
57 | 0.44 (0.33-0.55) |
26 | 0.34 (0.21-0.47) |
8 | 0.54 (0.16-0.92) |
| Patients With Incident IBDa | ||||||||||
| All lymphoma | 69 | 0.27 (0.21-0.33) |
48 | 0.24 (0.17-0.31) |
12 | 0.39 (0.17-0.61) |
5 | 0.24 (0.03-0.45) |
4 | 0.79 (0.01-1.57) |
| Hodgkin lymphoma | 13 | 0.05 (0.02-0.08) |
10 | 0.06 (0.03-0.09) |
1 | 0.04 (0.0-0.10) |
0 | 0 | 2 | 0.40 (0.0-0.95) |
| Non-Hodgkin lymphoma | 56 | 0.22 (0.16-0.28) |
38 | 0.19 (0.13-0.25) |
11 | 0.36 (0.15-0.57) |
5 | 0.24 (0.03-0.45) |
2 | 0.40 (0.0-0.95) |
Abbreviations: IBD, inflammatory bowel disease; IR, incidence rate; PY, person-years; TNF, tumor necrosis factor.
Patients with incident IBD account for 254 927 PY of follow-up overall: 198 555 as unexposed, 30 698 as exposed to thiopurine monotherapy, 20 637 as exposed to anti-TNF monotherapy, and 5037 as exposed to combination therapy.
As compared with unexposed patients, the incidence of lymphoma was higher among patients exposed to thiopurine monotherapy, anti-TNF monotherapy, or combination therapy, with absolute risk differences of 0.28, 0.15, and 0.69 per 1000 person-years, respectively (eFigure in the Supplement). In multivariable analysis, compared with unexposed patients, the risk of lymphoma was higher among those exposed to thiopurine monotherapy (aHR, 2.60; 95% CI, 1.96-3.44; P < .001), anti-TNF monotherapy (aHR, 2.41; 95% CI, 1.60-3.64; P < .001), or combination therapy (aHR, 6.11; 95% CI, 3.46-10.8; P < .001) (Table 3); the interaction across treatment groups was not statistically significant (P = .94). These increased risks associated with thiopurine monotherapy, anti-TNF monotherapy, and combination therapy were observed both for non-Hodgkin lymphoma and Hodgkin lymphoma (Table 3), and corresponding HRs were of similar magnitude when the analysis was restricted to the 61 341 patients with incident IBD (Table 3). Results were consistent regardless of age, sex, and IBD type (eTable 3 in the Supplement). Increasing age, male sex, Crohn disease diagnosis, and history of smoking-related conditions were also independently associated with a higher risk of lymphoma (eTable 4 in the Supplement).
Table 3. HRs Comparing the Risk of Lymphoma in Patients Exposed to Thiopurine Monotherapy, Anti-TNF Monotherapy, and Combination Therapy vs Unexposed Patients.
| Lymphoma Type | Exposed to Thiopurine Monotherapy vs Unexposed to Thiopurines or Anti-TNF Agents | Exposed to Anti-TNF Monotherapy vs Unexposed to Thiopurines or Anti-TNF Agents | Exposed to Combination Therapy vs Unexposed to Thiopurines or Anti-TNF Agents | |||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
|
| All Patients | ||||||
| All lymphoma | 2.06 (1.58-2.70) | 2.60 (1.96-3.44) | 1.57 (1.08-2.28) | 2.41 (1.60-3.64) | 3.60 (2.10-6.19) | 6.11 (3.46-10.8) |
| Hodgkin lymphoma | 2.78 (1.45-5.33) | 2.83 (1.37-5.84) | 2.21 (0.92-5.35) | 2.23 (0.81-6.13) | 11.4 (4.76-27.2) | 12.1 (4.46-33.1) |
| Non-Hodgkin lymphoma | 1.95 (1.45-2.62) | 2.57 (1.90-3.49) | 1.47 (0.97-2.22) | 2.48 (1.58-3.89) | 2.38 (1.17-4.84) | 4.48 (2.15-9.34) |
| Patients With Incident IBD | ||||||
| All lymphoma | 1.58 (0.84-3.00) | 2.35 (1.16-4.75) | 0.98 (0.39-2.48) | 1.49 (0.54-4.12) | 3.14 (1.13-8.71) | 5.90 (1.79-19.4) |
Abbreviations: HR, hazard ratio; IBD, inflammatory bowel disease; TNF, tumor necrosis factor.
Multivariable Cox model adjusted for baseline characteristics including sex, age, affiliation to Complementary Universal Health Insurance, IBD diagnosis and duration, exposure to methotrexate and aminosalicylates, comorbidities and time-dependent covariates including exposure to corticosteroids, and IBD-related hospitalizations and surgical procedures.
The risk of lymphoma did not differ between patients exposed to thiopurine monotherapy and those exposed to anti-TNF monotherapy (aHR, 0.93; 95% CI, 0.60-1.44; P = .93), but was higher in patients exposed to combination therapy vs those exposed either to thiopurine monotherapy (aHR, 2.35; 95% CI, 1.31-4.22; P < .001) or anti-TNF monotherapy (aHR, 2.53; 95% CI, 1.35-4.77; P < .001) (Table 4). Among patients exposed to anti-TNF agents, the risk of lymphoma did not statistically differ between adalimumab and infliximab (absolute risk difference, 0.04 per 1000 person-years; aHR, 0.95; 95% CI, 0.47-1.90; P = .97).
Table 4. HRs Comparing the Risk of Lymphoma in Patients Exposed to Anti-TNF Monotherapy or Combination Therapy vs Thiopurine Monotherapy and in Patients Exposed to Combination Therapy vs Anti-TNF Monotherapy.
| Lymphoma Type | Exposed to Anti-TNF Monotherapy vs Exposed to Thiopurine Monotherapy | Exposed to Combination Therapy vs Exposed to Thiopurine Monotherapy | Exposed to Combination Therapy vs Exposed to Anti-TNF Monotherapy | |||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Crude HR (95% CI) |
Adjusted HR (95% CI)a |
Crude HR (95% CI)a |
Adjusted HR (95% CI)a |
|
| All Patients | ||||||
| All lymphoma | 0.76 (0.50-1.16) | 0.93 (0.60-1.44) | 1.75 (0.98-3.10) | 2.35 (1.31-4.22) | 2.30 (1.23-4.31) | 2.53 (1.35-4.77) |
| Hodgkin lymphoma | 0.80 (0.30-2.14) | 0.79 (0.28-2.20) | 4.10 (1.55-10.8) | 4.29 (1.62-11.3) | 5.14 (1.65-16.0) | 5.44 (1.72-17.2) |
| Non-Hodgkin lymphoma | 0.75 (0.47-1.20) | 0.96 (0.59-1.56) | 1.22 (0.58-2.56) | 1.74 (0.82-3.69) | 1.62 (0.74-3.59) | 1.81 (0.81-4.00) |
| Patients With Incident IBD | ||||||
| All lymphoma | 0.62 (0.22-1.76) | 0.64 (0.22-1.87) | 1.98 (0.64-6.14) | 2.51 (0.78-8.13) | 3.20 (0.86-12.0) | 3.95 (1.01-15.5) |
Abbreviations: HR, hazard ratio; IBD, inflammatory bowel disease; TNF, tumor necrosis factor.
Multivariable Cox models adjusted for baseline characteristics including sex, age, affiliation to Complementary Universal Health Insurance, IBD diagnosis and duration, exposure to methotrexate and aminosalicylates, comorbidities and time-dependent covariates including exposure to corticosteroids, and IBD-related hospitalizations and surgical procedures.
Results regarding thiopurine monotherapy and anti-TNF monotherapy remained unchanged in the various sensitivity analyses, with aHR associated with thiopurine monotherapy ranging from 2.47 (95% CI, 1.89-3.24) to 2.70 (95% CI, 1.94-3.75) and aHR associated with anti-TNF monotherapy ranging from 2.48 (95% CI, 1.61-3.82) to 2.65 (95% CI, 1.56-4.49) (eTable 5 in the Supplement). The increased risk of lymphoma associated with combination therapy was consistent across all sensitivity analyses, with aHR ranging from 4.69 (95% CI, 2.04-10.74) to 6.70 (95% CI, 4.01-11.2), except in the analysis censoring time after any treatment switch or withdrawal, which provided an aHR of 2.54 (95% CI, 0.34-19.21) (P = .82) (eTable 5 in the Supplement).
Discussion
In this study based on a large nationwide cohort of patients with IBD, exposure to thiopurine monotherapy, anti-TNF monotherapy, or combination therapy was associated with an increased risk of lymphoma compared with exposure to neither of these treatments. The risk of lymphoma associated with anti-TNF monotherapy did not differ from that with thiopurine monotherapy, whereas this risk was higher with combination therapy than with thiopurine or anti-TNF monotherapy. Although these differences in the risk of lymphoma were significant in relative terms, their absolute magnitude of less than 1 case per 1000 person-years should be considered against the potential benefit of successful treatment of IBD.
Several studies have reported an increased risk of lymphoma associated with thiopurines. A meta-analysis estimated a standardized incidence rate of lymphoma associated with thiopurines of 2.80 (95% CI, 1.82-4.32) in population-based studies, which is consistent with the HR of 2.60 (95% CI, 1.96-3.44) found in the present study. As in previous studies, the most common lymphoma subtypes were diffuse large B-cell lymphoma, Hodgkin lymphoma, and follicular lymphoma.
The risk of lymphoma associated with anti-TNF agents remains unclear. Monotherapy with anti-TNF agents was not associated with an increased risk of malignancy, including lymphoma, in meta-analyses of randomized clinical trials nor in a nationwide Danish cohort study. In contrast, a study based on the Kaiser Permanente database reported that the use of anti-TNF agents was associated with an increased risk of lymphoma. However, most patients in the study had been previously exposed to thiopurines, therefore, no clear conclusion could be drawn. In the present study, restricting the analysis to the first line of IBD treatment, and thus considering only sequences of anti-TNF agents occurring in the absence of previous exposure to thiopurines, did not modify the HR of lymphoma associated with anti-TNF agents, thus providing further evidence for an association of anti-TNF agents with an increased risk of lymphoma. In addition, results were consistent for adalimumab and infliximab. Because anti-TNF agents impair the function of NK cells and negatively affect antilymphoma activity, there is a biological plausibility for the association of anti-TNF agents with an increased risk of lymphoma.
The higher risk of lymphoma found in patients exposed to combination therapy compared with either thiopurine or anti-TNF monotherapy suggests that the increased risks associated with each of these treatments used alone may accumulate when they are combined. Additional evidence for such a cumulative phenomenon was provided in the analysis considering nonexposed patients as the comparison group: the value of the HR estimate for combination therapy (ie, 6.11), which was very close to the product of the corresponding HR estimates for thiopurine monotherapy and anti-TNF monotherapy (2.60 × 2.41, ie, 6.27), and the absence of interaction between thiopurines and anti-TNF agents, which suggests that the association of each of these treatments with the risk of lymphoma remains of the same magnitude whether they are used alone or in combination. Of note, combination therapy was generally initiated in patients previously exposed to thiopurine or anti-TNF monotherapy, suggesting that the increased risk found in patients with combination therapy may result from past exposure rather than from the combination therapy itself. However, results of the various sensitivity analyses intended to distinguish between current and past exposures were largely similar to those of the main analysis. There was one exception in the sensitivity analysis restricted to the first line of IBD treatment, which did not show an increased risk of lymphoma in patients exposed to combination therapy, although this analysis was limited because it was based on only 2524 person-years of follow-up and a single case of lymphoma.
Strengths
This study had several strengths. First, the database is comprehensive in that it includes all medical prescriptions and hospital stays for IBD in France. Second, by examining a national database of patients with IBD over a 5.5-year period, this study included a large number of patients treated with anti-TNF therapy, either alone or in combination with thiopurines. This allowed study of the association of each drug individually and the combination of the drugs with lymphoma incidence. A total of 336 patients with lymphoma was identified, including 70 in patients exposed to thiopurines. By comparison, a meta-analysis included 93 patients with lymphoma, including 24 exposed to thiopurines.
Limitations
This study had several limitations. First, including patients with prevalent IBD at cohort entry, who had possibly been previously exposed to IBD treatment but had no history of cancer, may have selected the most resistant individuals and thus resulted in an underestimation of the association between exposure to IBD treatments and the risk of lymphoma. However, analysis restricted to patients with incident IBD provided similar results, suggesting that such a selection bias, if any, was limited. Second, there was no clinical validation of the SNIIRAM and PMSI data for the diagnosis of IBD and lymphoma. However, IBD incidence rates in this cohort were similar to those reported in previous studies. In addition, the identification of lymphoma cases included a combination of criteria based on diagnoses and indicators of therapeutic management recorded in the SNIIRAM and PMSI databases, and results were consistent when using an alternative definition of lymphoma. Misclassification of lymphoma subtype between Hodgkin and non-Hodgkin lymphoma could not be ruled out, particularly for Hodgkin-like disease wrongly encoded as Hodgkin lymphoma.
Third, because combination therapy is often used in severe forms of IBD, the association of combination therapy with an increased risk of lymphoma may reflect a role of the burden of inflammation rather than of treatment. This hypothesis cannot be excluded, although available data did not provide evidence of an independent role of IBD on the risk of lymphoma. Fourth, as this study was based on administrative databases, clinical information, such as disease activity, smoking history, or Montreal phenotype, were not available. Fifth, the duration of follow-up under combination therapy in the study was relatively short (8 months on average) and the absolute number of events was small (14 events), resulting in wide confidence intervals. Future studies will be necessary to assess the risks of lymphoma associated with these therapies over longer follow-up.
Conclusions
Among adults with IBD, the use of thiopurine monotherapy or anti-TNF monotherapy was associated with a small but statistically significant increased risk of lymphoma compared with exposure to neither medication, and this risk was higher with combination therapy than with each of these treatments used alone. These findings may inform decisions regarding benefits and risks of treatment.
eTable 1. Codes Used to Define Exclusion Criteria and Covariates.
eTable 2. Distribution of Lymphoma Subtypes, Overall and by Treatment Group.
eTable 3. Risk of Lymphoma in Patients Exposed to Thiopurine Monotherapy, Anti-TNF Monotherapy, and Combination Therapy Vs Unexposed Patients According to Age Group, Sex and IBD Diagnosis.
eTable 4. Associations Between Exposure Group, Covariates, and the Risk of Lymphoma in the Full Cox Multivariable Model.
eTable 5. Risk of Lymphoma in Patients Exposed to thiopurine monotherapy, anti-TNF monotherapy, and combination therapy Vs Unexposed Patients: Sensitivity Analyses.
eFigure. Cumulative Incidence of Lymphoma by Exposure Group.
References
- 1.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50(4):485-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383-1395. [DOI] [PubMed] [Google Scholar]
- 3.Reinisch W, Sandborn WJ, Hommes DW, et al. . Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780-787. [DOI] [PubMed] [Google Scholar]
- 4.Rutgeerts P, Van Assche G, Sandborn WJ, et al. ; EXTEND Investigators . Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142(5):1102-1111.e2. [DOI] [PubMed] [Google Scholar]
- 5.D’Haens G, Baert F, van Assche G, et al. ; Belgian Inflammatory Bowel Disease Research Group; North-Holland Gut Club . Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet. 2008;371(9613):660-667. [DOI] [PubMed] [Google Scholar]
- 6.Jones JL, Kaplan GG, Peyrin-Biroulet L, et al. . Effects of concomitant immunomodulator therapy on efficacy and safety of anti-tumor necrosis factor therapy for Crohn’s disease: a meta-analysis of placebo-controlled trials. Clin Gastroenterol Hepatol. 2015;13(13):2233-2240.e1. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak B, Svanström H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. 2013;177(11):1296-1305. [DOI] [PubMed] [Google Scholar]
- 8.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54(8):1121-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaugerie L, Brousse N, Bouvier AM, et al. ; CESAME Study Group . Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374(9701):1617-1625. [DOI] [PubMed] [Google Scholar]
- 10.Nyboe Andersen N, Pasternak B, Basit S, et al. . Association between tumor necrosis factor-α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA. 2014;311(23):2406-2413. [DOI] [PubMed] [Google Scholar]
- 11.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7(8):874-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrinton LJ, Liu L, Weng X, Lewis JD, Hutfless S, Allison JE. Role of thiopurine and anti-TNF therapy in lymphoma in inflammatory bowel disease. Am J Gastroenterol. 2011;106(12):2146-2153. [DOI] [PubMed] [Google Scholar]
- 13.Osterman MT, Sandborn WJ, Colombel J-F, et al. . Increased risk of malignancy with adalimumab combination therapy, compared with monotherapy, for Crohn’s disease. Gastroenterology. 2014;146(4):941-949. [DOI] [PubMed] [Google Scholar]
- 14.Kirchgesner J, Lemaitre M, Rudnichi A, et al. . Therapeutic management of inflammatory bowel disease in real-life practice in the current era of anti-TNF agents: analysis of the French administrative health databases 2009-2014. Aliment Pharmacol Ther. 2017;45(1):37-49. doi: 10.1111/apt.13835 [DOI] [PubMed] [Google Scholar]
- 15.Weill A, Vallier N, Salanave B, et al. . Frequency of thirty long-term illnesses for beneficiaries of the French general health insurance scheme in 2004. Pratiques et Organisation des Soins. 2006;37:173-188. [Google Scholar]
- 16.Bouillon K, Bertrand M, Maura G, Blotière P-O, Ricordeau P, Zureik M. Risk of bleeding and arterial thromboembolism in patients with non-valvular atrial fibrillation either maintained on a vitamin K antagonist or switched to a non-vitamin K-antagonist oral anticoagulant: a retrospective, matched-cohort study. Lancet Haematol. 2015;2(4):e150-e159. [DOI] [PubMed] [Google Scholar]
- 17.Colas S, Collin C, Piriou P, Zureik M. Association between total hip replacement characteristics and 3-year prosthetic survivorship: a population-based study. JAMA Surg. 2015;150(10):979-988. [DOI] [PubMed] [Google Scholar]
- 18.Weill A, Dalichampt M, Raguideau F, et al. . Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raguideau F, Lemaitre M, Dray-Spira R, Zureik M. Association between oral fluoroquinolone use and retinal detachment. JAMA Ophthalmol. 2016;134(4):415-421. [DOI] [PubMed] [Google Scholar]
- 20.Miranda S, Chaignot C, Collin C, Dray-Spira R, Weill A, Zureik M. Human papillomavirus vaccination and risk of autoimmune diseases: a large cohort study of over 2 million young girls in France. Vaccine. 2017;35(36):4761-4768. [DOI] [PubMed] [Google Scholar]
- 21.Swoger JM, Regueiro M. Stopping, continuing, or restarting immunomodulators and biologics when an infection or malignancy develops. Inflamm Bowel Dis. 2014;20(5):926-935. [DOI] [PubMed] [Google Scholar]
- 22.Annese V, Beaugerie L, Egan L, et al. ; ECCO . European evidence-based consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015;9(11):945-965. [DOI] [PubMed] [Google Scholar]
- 23.Kotlyar DS, Lewis JD, Beaugerie L, et al. . Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13(5):847-58.e4. [DOI] [PubMed] [Google Scholar]
- 24.Dulai PS, Siegel CA. The risk of malignancy associated with the use of biological agents in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 2014;43(3):525-541. [DOI] [PubMed] [Google Scholar]
- 25.Williams CJM, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-α therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39(5):447-458. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein GR, Rutgeerts P, Sandborn WJ, et al. . A pooled analysis of infections, malignancy, and mortality in infliximab- and immunomodulator-treated adult patients with inflammatory bowel disease. Am J Gastroenterol. 2012;107(7):1051-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nocturne G, Boudaoud S, Ly B, Pascaud J, Paoletti A, Mariette X. Impact of anti-TNF therapy on NK cells function and on immunosurveillance against B-cell lymphomas. J Autoimmun. 2017;80(February):56-64. [DOI] [PubMed] [Google Scholar]
- 28.Harris NL, Jaffe ES, Diebold J, et al. . World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835-3849. [DOI] [PubMed] [Google Scholar]
- 29.Baecklund E, Iliadou A, Askling J, et al. . Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54(3):692-701. [DOI] [PubMed] [Google Scholar]
- 30.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372(15):1441-1452. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JD, Bilker WB, Brensinger C, Deren JJ, Vaughn DJ, Strom BL. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology. 2001;121(5):1080-1087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Codes Used to Define Exclusion Criteria and Covariates.
eTable 2. Distribution of Lymphoma Subtypes, Overall and by Treatment Group.
eTable 3. Risk of Lymphoma in Patients Exposed to Thiopurine Monotherapy, Anti-TNF Monotherapy, and Combination Therapy Vs Unexposed Patients According to Age Group, Sex and IBD Diagnosis.
eTable 4. Associations Between Exposure Group, Covariates, and the Risk of Lymphoma in the Full Cox Multivariable Model.
eTable 5. Risk of Lymphoma in Patients Exposed to thiopurine monotherapy, anti-TNF monotherapy, and combination therapy Vs Unexposed Patients: Sensitivity Analyses.
eFigure. Cumulative Incidence of Lymphoma by Exposure Group.