This study examines whether an antimicrobial stewardship approach grounded in behavioral theory and focusing on preserving prescriber autonomy and participation is associated with appropriateness of antimicrobial prescribing in hospitals.
Key Points
Question
How effective is an antimicrobial stewardship approach grounded in behavioral theory and focused on preserving prescriber autonomy and participation in improving appropriateness of antimicrobial prescribing in hospitals?
Findings
In this multicenter intervention study examining 1121 patient cases with 700 antimicrobial prescriptions during the baseline period and 882 patient cases with 531 antimicrobial prescriptions during the intervention period, appropriateness of antimicrobial prescribing significantly increased by 13.3% during a follow-up period of 12 months after intervention start.
Meaning
Future antimicrobial stewardship efforts in hospitals should use behavioral theory to improve intervention effectiveness in various clinical settings.
Abstract
Importance
Inappropriate antimicrobial prescribing leads to antimicrobial resistance and suboptimal clinical outcomes. Changing antimicrobial prescribing is a complex behavioral process that is not often taken into account in antimicrobial stewardship programs.
Objective
To examine whether an antimicrobial stewardship approach grounded in behavioral theory and focusing on preserving prescriber autonomy and participation is effective in improving appropriateness of antimicrobial prescribing in hospitals.
Design, Setting, and Participants
The Dutch Unique Method for Antimicrobial Stewardship (DUMAS) study was a prospective, stepped-wedge, participatory intervention study performed from October 1, 2011, through December 31, 2015. Outcomes were measured during a baseline period of 16 months and an intervention period of 12 months. The study was performed at 7 clinical departments (2 medical, 3 surgical, and 2 pediatric) in a tertiary care medical center and a general teaching hospital in the Netherlands. Physicians prescribing systemic antimicrobial drugs for any indication for patients admitted to the participating departments during the study period were included in the study.
Interventions
We offered prescribers a free choice of how to improve their antimicrobial prescribing. Prescribers were stimulated to choose interventions with higher potential for success based on a root cause analysis of inappropriate prescribing.
Main Outcomes and Measures
Appropriateness of antimicrobial prescriptions was determined using a validated approach based on guideline adherence and motivated guideline deviation and measured with repeated point prevalence surveys (6 per year). Appropriateness judgment was masked for the study period. Antimicrobial consumption was extracted from pharmacy records and measured as days of therapy per admission. We used linear and logistic mixed-model regression analysis to model outcomes over time.
Results
A total of 1121 patient cases with 700 antimicrobial prescriptions were assessed during the baseline period and 882 patient cases with 531 antimicrobial prescriptions during the intervention period. The mean antimicrobial appropriateness increased from 64.1% at intervention start to 77.4% at 12-month follow-up (+13.3%; relative risk, 1.17; 95% CI, 1.04-1.27), without a change in slope. No decrease in antimicrobial consumption was found.
Conclusions and Relevance
Use of a behavioral approach preserving prescriber autonomy resulted in an increase in antimicrobial appropriateness sustained for at least 12 months. The approach is inexpensive and could be easily transferable to various health care environments.
Introduction
Appropriate antimicrobial prescribing has significant clinical benefits (ie, reduced mortality) and reduces development of antimicrobial resistance and health care costs. Antimicrobial stewardship programs aim to improve antimicrobial prescribing but sometimes fail to acknowledge that improving antimicrobial prescribing actually means changing human behavior. Human behavior is not based on a fully rational process but depends on a complex interplay between several behavioral determinants and social norms. Despite its rational theoretical foundation, stewardship programs are known to persistently encounter prescriber resistance. This resistance is generated by the tension between the governance of the stewardship team and the autonomy of individual prescribers. Behavioral and social theory seem underused in antimicrobial stewardship intervention programs, contrary to more common use in other scientific fields. Previous studies using interventions based on behavioral theory have found promising results in improving antibiotic prescribing. Most of these studies focused on antibiotic prescribing for respiratory tract infections in primary care.
We used behavioral theory to design and implement an intervention approach to improve appropriateness of hospital antimicrobial prescribing for all indications. Our approach was inspired by the participatory action research paradigm, which focuses on collaboration and empowerment of the stakeholders in the change process and is effective in other complex health care situations. In our approach, prescribers were invited to choose and codevelop 1 or more interventions to improve their own prescribing, whereby they were stimulated to base their choice on conclusions of a prior root cause analysis of their prescribing patterns. The approach is therefore designed to benefit from tailoring to local determinants and draws on 3 behavioral principles: (1) respect for the prescribers’ autonomy to avoid feelings of resistance; (2) the inclination of people to value a product higher and feel more ownership for it if they made it themselves, which is referred to as the IKEA effect; and (3) the tendency of people to follow up on an active and public commitment. We aimed to test the approach’s effectiveness in improving appropriateness of antimicrobial prescribing in hospitals.
Methods
Study Design
The Dutch Unique Method for Stewardship (DUMAS) study was a prospective, stepped-wedge, participatory intervention study aimed to improve antimicrobial prescribing. The institutional medical ethics review boards of the VU University Medical Center, Amsterdam, the Netherlands, and OLVG, Amsterdam, the Netherlands, approved all study procedures and waived informed consent for patients.
Setting
The study was performed from October 1, 2011, through December 31, 2015. Seven departments from 2 hospitals participated, of which 3 were surgical, 2 were medical, and 2 were pediatric departments. Hospital 1 was a 700-bed tertiary care medical center with salaried specialists, and hospital 2 was a 550-bed teaching general medical center with self-employed specialists, both located in Amsterdam, the Netherlands. During the study period, hospital 1 used a preexisting preauthorization system for broad-spectrum antimicrobials, whereas hospital 2 performed antimicrobial audit and feedback interventions but only in departments not participating in the study.
Enrollment
The local antibiotic formulary committee selected departments for study participation based on the need for change (low appropriateness and moderate to high antimicrobial consumption), for which the results of 12 months of baseline antimicrobial appropriateness and consumption measurements were available. We then approached department heads or the department’s infectious disease expert with a participation request. Participation was voluntary, and we offered no financial compensation. Seven of 8 approached medical departments agreed to participate; 1 department head refused for unspecified reasons. Timing of the start of the intervention phase for each department was not randomized because of expected availability issues of relevant department stakeholders, education schedules, and potential approval delays of ethical review boards. Intervention start sequence and timing are shown in eFigure 1 in the Supplement.
Outcome Measures
Our primary outcome was antimicrobial appropriateness, measured with a validated appropriateness assessment instrument. One of 3 infectious diseases specialists (including M.A.v.A. and E.J.G.P.) assessed the adult prescriptions, and 1 of 3 infectious diseases/immunology pediatricians (including M.v.d.K.) assessed the pediatric prescriptions for appropriateness. They were masked for clinical outcomes and study period (baseline or intervention). Data were collected prospectively, but assessments were performed retrospectively to enable masking. Each of the following antimicrobial prescription factors was assessed for appropriateness: indication, choice of antimicrobial, dosage, administration route, and duration. A prescription was only deemed to be appropriate if one of the following criteria applied for each of the above factors: complete guideline adherence or guideline deviation or no guideline but based on rational reasons, as judged by the assessing infectious diseases specialist, immunology specialist, or pediatrician. Rationality was defined as an effective antimicrobial regimen that covered relevant pathogens without being excessive (ie, unnecessary combination therapy or broad spectrum when a more narrow spectrum is available). If present, drug allergies, oral intake, and previous culture results were taken into account. Cases that could not be assessed because of missing information were excluded. We notified clinical staff of both hospitals by email before the start of the baseline measurements.
Antimicrobial consumption was a secondary outcome, reported in days of therapy per 100 admissions per month. Antimicrobial appropriateness and consumption measurements only included prescriptions with Anatomical Therapeutic Chemical codes beginning with J01, J02, J04AB02, and J05AB. Other outcomes were changes in specific appropriateness categories, intravenous antimicrobial consumption, consumption of specific antimicrobial subgroups, and length of hospital stay.
Data Collection
Antimicrobial appropriateness was measured through point prevalence surveys at a rate of 6 times per year. Local antimicrobial stewardship teams performed the surveys as part of standard quality measurements. All team members were trained and supervised by the coordinating investigator (J.J.S.) using standard operating procedure documents. An antimicrobial case was included in the survey if the patient was admitted to a clinical ward of a participating department and had a prescription for a systemic antimicrobial agent at 0.00 hours on the day of the survey. Relevant clinical data needed for assessment, including prescription indication and reasons for guideline deviations, were collected by contacting the responsible ward physician or were retrieved from medical files. Antimicrobials prescribed for prokinetic reasons (erythromycin) were excluded. Data were then coded and stripped from any identifying information. To prevent anticipatory behavior, we did not notify the clinical wards of the exact survey dates.
Data on antimicrobial consumption, admission rates, admission diagnoses, and length of stay were derived from pharmacy systems and administrative records. Only data on patients with a length of stay of at least 24 hours were included. Two pediatric critical care units were not included because of lack of electronic data. Baseline and intervention periods were at least 12 months, but more data were collected whenever possible.
Root Cause Analysis
An analysis of local root causes of inappropriate prescribing was performed after 12 months of baseline measurements for the baseline phase of each department separately. The analysis was based on interviews of a purposive sample of department members. Sample size depended on department size but included at least 2 medical specialists, 2 junior physicians, and 2 nurses per department. Interviews were audio recorded. The interviewer (J.J.S.) was a psychologist and physician trained in qualitative research. Interviewees supplied written informed consent before the interview start. The interviews were guided by a topic list that consisted of standard questions that focused on the cause categories of the Eindhoven Classification Model: technical, organizational, human, and patient (see eTable 1 in the Supplement for a translated topic list). The interviewer asked additional questions on potential causes for inappropriate prescribing using the 5 whys method, which entails repeatedly asking for a cause underlying each cause of a certain event as supplied by the interviewee. For additional validity, the conclusions of the analysis were discussed with department members during the intervention approach.
Intervention Approach
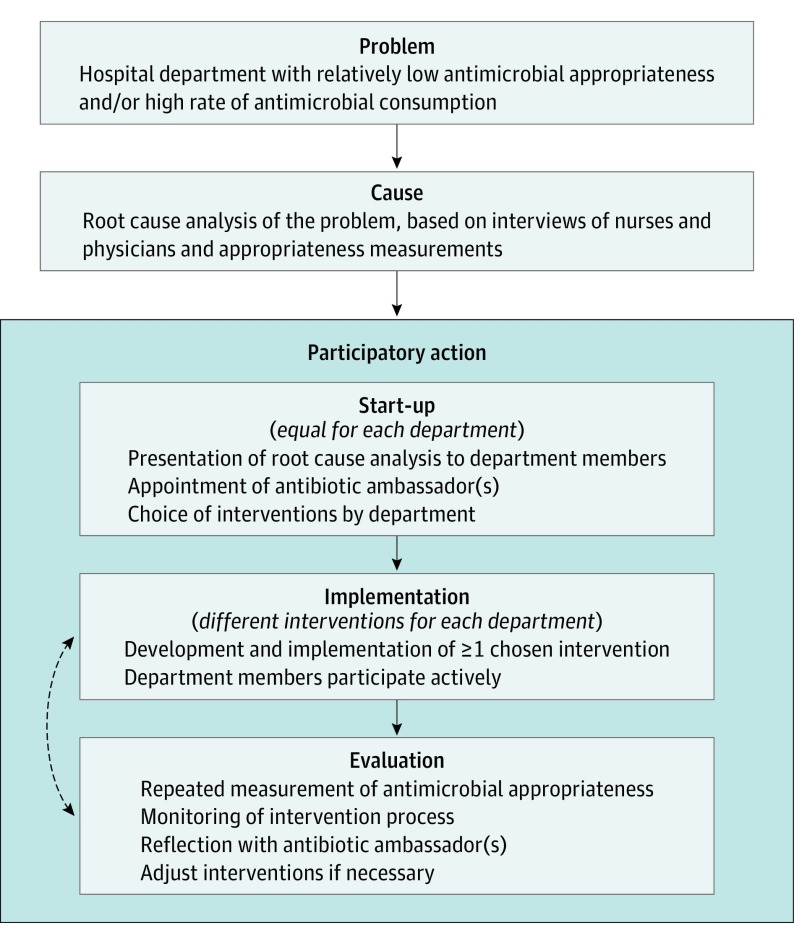
Figure 1 summarizes the intervention approach. The approach was performed for each department separately and started with a plenary introduction and discussion with department physicians. Participation was voluntary for each department and physician. Department members were stimulated to choose interventions with higher potential for success based on the root cause analysis, which would result in one set of interventions per department. Intervention choice was not predefined, was free, and was only restricted by practical feasibility. Essential to the approach was the appointment of 1 or more antibiotic ambassadors chosen by their peers, which defined the start of the intervention period. We also informed nurses from each department of the baseline results. The ambassador team contained at least 1 medical specialist per department, but participation of junior physicians, nurses, and quality-of-care personnel was encouraged. Department ambassadors were asked to represent their department during subsequent intervention discussions, to champion good antibiotic policy and the chosen interventions, and to help develop and implement the interventions. Support and involvement of study personnel with each department’s intervention approach were determined by the preferences of the antibiotic ambassador(s) and limited to a maximum of 12 months after the start of the intervention period.
Figure 1. Intervention Approach Used in the Current Study.
Statistical Analysis
We used logistic mixed regression analysis to model antimicrobial appropriateness time trajectories and linear mixed regression analysis to model monthly antimicrobial consumption and length-of-stay time trajectories. Each model contained the fixed-effects variables of time, study period, and the interaction term, which allowed the baseline period to function as control for the intervention period. The intervention period was considered to have started with the first plenary department meeting. Odds ratios were converted to relative risks for better interpretability. We included random effects for department and clinical ward in each model. Antimicrobial consumption analyses contained a random effect for month of the year to account for season effects. All continuous outcomes were log transformed before analysis. To be able to report outcomes on the original scale of measurement, we calculated predicted means per time point, which were then back-transformed in case of continuous outcomes. Regression coefficients from these models were back transformed and then transformed to change percentages for optimal interpretability.
The CIs were calculated with 10 000 bootstraps while accounting for the clustered nature of the data. Significance level was .05 (2-sided). Main analyses were limited to the period when data were available for all departments: 16 months before and 12 months after the start of the intervention period.
We performed a sensitivity analysis for both primary outcomes: a mixed-model analysis with only study period as the fixed effect, ignoring slopes. We performed the analyses of the antimicrobial appropriateness and consumption subgroups using the same single fixed-effect method because we assumed time trend estimations were more vulnerable to chance events in these small groups. We used R statistical software, version 3.2.3 with package lme4, version 1.1-11, for all analyses (R Development Core Team).
Results
Population and Point Prevalence Survey Characteristics
There were 21 306 clinical admissions during the baseline period and 15 394 clinical admission during the intervention period. The appropriateness surveys included 1121 patients during the baseline period and 882 patients during the intervention period. Detailed characteristics are given in Table 1.
Table 1. Patient and Point Prevalence Survey Characteristics During the Baseline (16 Months) and Intervention Periods (12 Months)a.
| Characteristic | Baseline Period | Intervention Period |
|---|---|---|
| No. of patients admitted to participating departments (range of total patients per department) | 21 306 (726-7501) | 15 934 (505-5741) |
| No. of patients included in point prevalence surveys | 1121 | 882 |
| Patients with at least 1 antimicrobial prescription | 459 (40.9) | 346 (39.2) |
| No. of prescriptions in point prevalence surveys | 700 | 531 |
| Exclusion because of incomplete information or used as prokinetic | 12 (1.7) | 7 (1.3) |
| Prophylactic indication | 114 (16.6) | 67 (12.8) |
| Medical | 84 (12.2) | 47 (9.0) |
| Surgical | 30 (4.4) | 20 (3.8) |
| Therapeutic indication | 574 (83.5) | 456 (87.2) |
| Respiratory tract infection | 143 (24.9) | 145 (31.8) |
| Urinary tract infection | 32 (5.6) | 35 (7.7) |
| Soft-tissue infection | 79 (13.8) | 59 (12.9) |
| Intra-abdominal infection | 48 (8.4) | 54 (11.8) |
| Intravascular infection | 19 (3.3) | 20 (4.4) |
| Sepsis due to other cause | 146 (25.4) | 76 (16.7) |
| Other indication | 107 (18.6) | 67 (14.7) |
Data are presented as number (percentage) of patients unless otherwise indicated.
Root Cause Analyses and Chosen Interventions
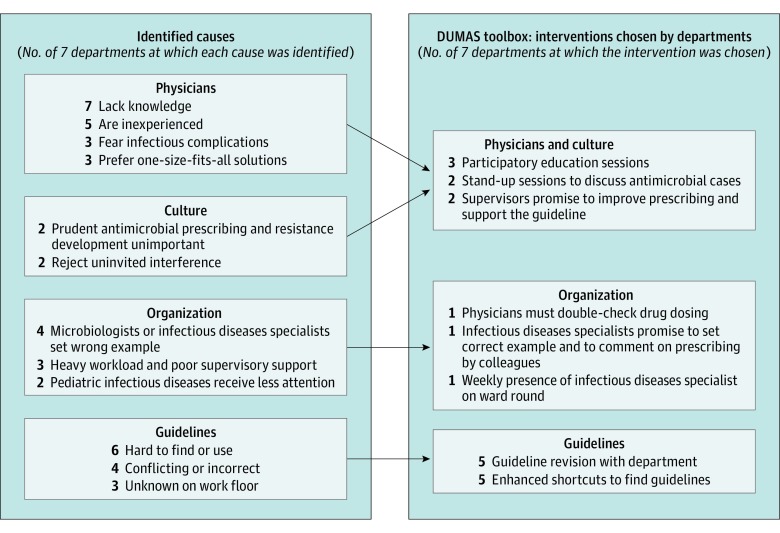
The root cause analyses identified causes in 4 themes: physician (eg, lack of knowledge), culture (eg, rejection of interference), organization (eg, infectious diseases experts set wrong example), and guidelines (eg, hard to find and use). Between 2 and 4 interventions per department were chosen, each connected to 1 or 2 of the above themes; for example, participatory education sessions (physician and culture), presence of infectious diseases physicians during ward round (organization), and guideline revision (guidelines). Detailed characteristics are given in Figure 2 and eTable 1 in the Supplement. Time from the first plenary meeting to the implementation of the first intervention varied between immediate (supervisors promise to improve) to 6 months for the first pediatrics department, where the antibiotic ambassadors team was formed 4 months after the plenary meeting because of logistical problems.
Figure 2. Summary of the Root Cause Analyses and Interventions Chosen by the Departments to Improve Their Prescribing.
DUMAS indicates Dutch Unique Method for Antimicrobial Stewardship.
Antimicrobial Appropriateness
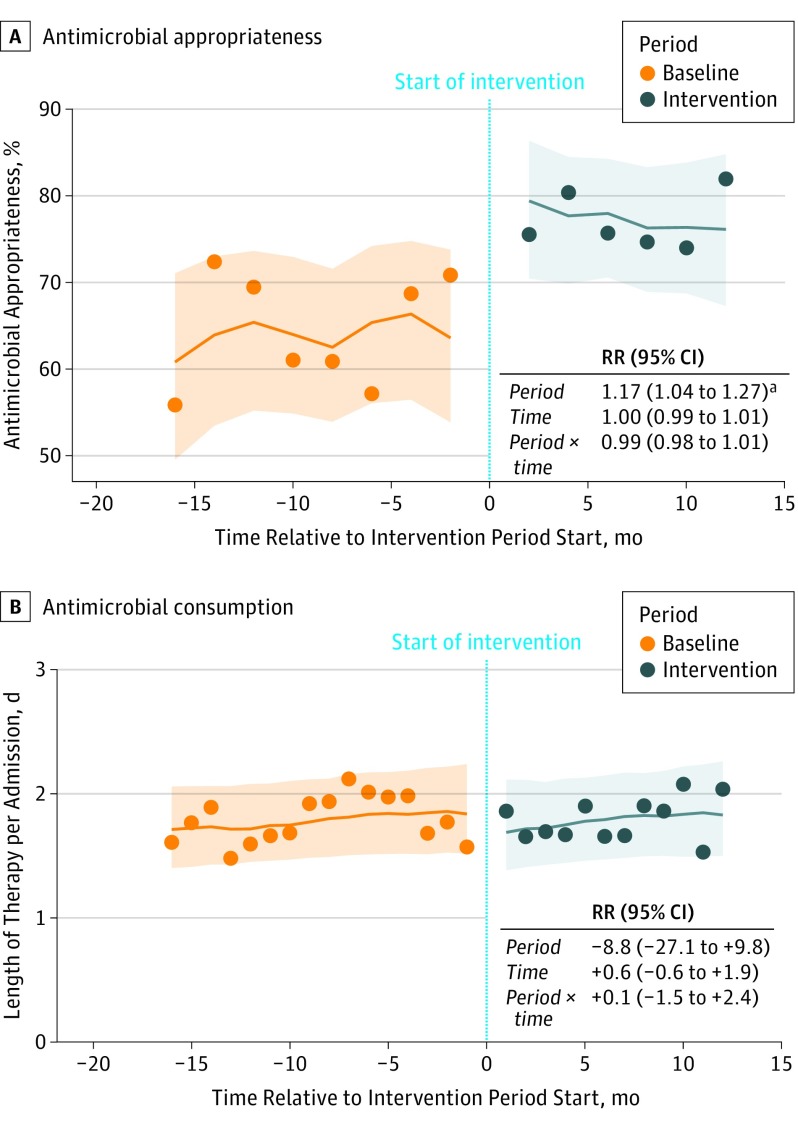
The intervention approach was associated with a significant 13.3% (95% CI, 64.1%-77.4%) increase in antimicrobial appropriateness (relative risk, 1.17; 95% CI, 1.04-1.27), without any significant changes in time trends (Figure 3A). Results of the analyses per appropriateness subgroup are given in Table 2 and per department in eFigure 2 and eTable 2 in the Supplement.
Figure 3. Antimicrobial Appropriateness and Consumption .
A, Antimicrobial appropriateness relative to the start of the intervention phase and logistic mixed-model regression analysis. Mean antimicrobial appropriateness increased 13.3%, from 64.1% at intervention start to 77.4% at 12-month follow-up. B, Antimicrobial consumption in days of therapy per admission relative to the start of the intervention phase and logistic mixed-model regression analysis. Points represent results from the point prevalence surveys; lines, predicted means from the regression analysis; and shaded area, 95% CIs around these predicted means. RR indicates relative risk.
aThe RR was significantly different from 1 at the .05 level.
Table 2. Point Prevalence Survey Outcomes During Baseline (16 Months) and Intervention Periods (12 Months).
| Outcome | Total Within Period, % | Absolute Difference | RR or Relative Difference (95% CI) | |
|---|---|---|---|---|
| Baseline Period | Intervention Period | |||
| Appropriate overall | 64.1 | 77.4 | 13.3 | 1.16 (1.11 to 1.23)a |
| Inappropriate, per category | ||||
| Antimicrobial unnecessary | 6.6 | 1.5 | −5.2 | 0.24 (0.08 to 0.45)a |
| Inappropriate choice | 11.2 | 8.0 | −3.2 | 0.62 (0.42 to 0.90)a |
| Inappropriate dose | 11.4 | 6.1 | −5.4 | 0.56 (0.35 to 0.80)a |
| Inappropriate administration | 1.8 | 2.0 | 0.2 | 1.19 (0.43 to 2.57)a |
| Excessive duration | 4.4 | 4.9 | 0.5 | 1.11 (0.64 to 1.83)a |
| Antimicrobial consumption in days of therapy or admission | ||||
| Overall | 2.00 | 2.02 | 0.03 | 1.2 (−14.7 to 19.9)b |
| Intravenous only | 1.21 | 1.28 | 0.07 | 5.8 (−8.4 to 22.7)b |
| By antimicrobial group | ||||
| Penicillin without BL inhibitor | 0.38 | 0.41 | 0.03 | 8.4 (−13.8 to 36.6)b |
| Penicillin with BL inhibitor | 0.50 | 0.37 | −0.13 | −26.3 (−41.1 to −8.4)b |
| Cephalosporin (first or second generation) | 0.01 | 0.01 | −0.00 | −15 (−56.5 to 66.5)b |
| Cephalosporin (third generation) | 0.25 | 0.31 | 0.06 | 22.7 (4.8 to 43.2)b |
| Carbapenem | 0.00 | 0.01 | 0.00 | 24.2 (−74.8 to 519.1)b |
| Quinolone | 0.09 | 0.07 | −0.02 | −22.8 (−49.3 to 17.3)b |
| Clindamycin | 0.01 | 0.01 | −0.00 | −11.8 (−51.8 to 62.4)b |
| Aminoglycoside | 0.03 | 0.03 | −0.00 | −12.3 (−49.7 to 51.1)b |
| Trimethoprim with or without sulfonamide | 0.02 | 0.02 | 0.00 | 11.2 (−47.8 to 137.0)b |
| Other antibiotic | 0.21 | 0.24 | 0.02 | 11.8 (−22.6 to 59.9)b |
| Antifungal or antiviral | 0.05 | 0.02 | −0.03 | −57.2 (−81.3 to −1.6)b |
Abbreviations: BL, β-lactamase; RR, relative risk.
Relative risk for appropriateness.
Relative difference.
Antimicrobial Consumption
Antimicrobial consumption did not decrease significantly during the intervention phase, and there were no changes in time trends (Figure 3B). Results of the analyses per antimicrobial drug group are given in Table 2 and per department in eTable 2 in the Supplement.
Other Results
Length of hospital stay did not change relative to the start of the intervention approach (eFigure 3 in the Supplement). The single fixed-effect sensitivity analysis supported the primary analysis showing similar results (Table 2).
Discussion
To our knowledge, this is the first hospital antimicrobial stewardship study grounded in behavioral science and allowing physicians a free choice in how to improve their own prescribing. In our multicenter study in 7 departments divided between 2 hospitals (a teaching and an academic hospital), we found that our approach was associated with a significant 13.3% increase in antimicrobial appropriateness during a period of 12 months after the intervention start. We found no reduction in antimicrobial consumption.
We believe the observed increase in antimicrobial appropriateness is clinically relevant because our definition of appropriateness specifically focused on unwanted prescriptions from a stewardship point of view. Attainment of underlying goals, such as empirical therapy, according to guidelines and de-escalation of therapy improves mortality and other clinical outcomes. The potential drawback of such a method is that it is based on expert opinion. However, in a recent validation study, the used appropriateness instrument had 80% agreement with a reference standard that consisted of the modal assessment of 15 medical specialists (infectious diseases specialists and clinical microbiologists). Of importance, the persistence of the effect during the relatively long follow-up period of 12 months suggests good sustainability. The trend back to baseline in Figure 3 is suggestive but too small and the CI is too wide to interpret this as such. The true effect of our approach can be estimated by extrapolating the results from our point prevalence surveys to all antimicrobial days of therapy prescribed at participating departments during the first 12 months of the intervention period (37 046 days). This would mean that the 13.3% increase in appropriateness equaled 4927 improved days of therapy.
Our study design incorporated an extensive number of repeated measurements, which allowed us to control intervention effects for baseline levels and trajectories. This way we could discern between the effects of our intervention approach and previous events or interventions. By starting the intervention approach at a different time for each department (stepped-wedge design), we minimized the chance that the overall effect was influenced by external events (eg, national campaigns for prudent antimicrobial use).
The effectiveness of our approach is explained by the advantages of using methods from behavioral science. We hypothesize that participating department members felt relatively nonthreatened by our approach because of their freedom in choosing a personal solution, which is an important theme in antimicrobial stewardship. Moreover, by committing to the project and choosing and developing their own intervention set, they may have felt more inclined to support the project and change their own prescribing behavior. This may have been an important intervention in itself. Finally, giving prescribers a free intervention choice could have led to them choosing an easy way out, for instance, choosing education as the only intervention. However, because our approach incorporated a root causes analysis of prescribing, a recommended strategy in stewardship, prescribers were gently nudged toward using interventions that were likely to be more effective. An approach similar to ours has been unsuccessful in improving antimicrobial prescribing in nursing homes. However, among other differences, that study used a predetermined list of possible interventions, which may have limited prescribers’ feeling of freedom and diminished support of the aforementioned IKEA effect.
We found no reduction of antimicrobial consumption in our study. This finding may reflect that overall antimicrobial use is a nonspecific measure without information on appropriateness of therapy. Moreover, an increase in antimicrobial prescribing quality can be reached without a reduction in days of therapy, for instance, by increasing streamlining, better dosing, and using more narrow-spectrum therapy empirically (Table 2). In line with this, we found a significant 26% reduction in the consumption of penicillins with β-lactamase inhibitors, which was the most prescribed type of antibiotic in our population. Alternatively, that finding could suggest that prescribers find it harder to stop or refrain from starting than to narrow antibiotic prescribing because these situations may be more dependent on individual clinical reasoning than on evidence-based guidelines.
The patient safety of our approach was based on the preserved full autonomy of prescribers at all times during the study, which would make a worsening of patient safety unlikely. Our focus on appropriateness had the advantage that it stimulated adherence to multidisciplinary and generally evidence-based guidelines, even when this would lead to more instead of fewer days of therapy. The absence of an increase in length of hospital stay can be seen as circumstantial evidence in this regard.
Limitations and Strengths
Our study has limitations. First, prescribers’ awareness of being monitored could have led to a change in behavior (Hawthorne effect). Because they were informed of the study before the start of the baseline measurements, this could have led to diminished intervention effects. Of importance, the department received even more attention from the research team during the start of the intervention phase; thus, the Hawthorne effect would then be even bigger. However, this behavioral phenomenon (ie, personal attention for commitment leads to behavioral change) is in fact a feature not a bug of the intervention approach mechanism.
Second, the stepped-wedge enrollment order was nonrandomized because the approach was dependent on practical circumstances, such as department preferences, room in the educational roster, or availability of department heads and opinion leaders. We believed that adapting to these circumstances superseded the advantages of randomization, especially because this adaption will also be necessary when implementing our approach in practice. Still, although we found no evidence of this, departments could have stalled their participation in the study until they improved their antibiotic prescribing on their own just before intervention start.
Third, the earlier validation study of the antimicrobial appropriateness method was limited to prescriptions for adult patients. However, there was no procedural difference with the method used for the assessment of pediatric prescriptions.
Fourth, execution of our approach in one pediatric department was less fluent, with delayed implementation of some interventions. This was caused by time constraints of the antibiotic ambassador and the department’s extensive size. The local effect of the approach on appropriateness mirrored this (eTable 2 in the Supplement), perhaps reflecting the importance of the ambassador on the effect.
Fifth, the Dutch health care system differs from other systems, which may limit generalizability. However, our results were achieved regardless of specialists’ payment structure because we included both salaried (hospital 1) and self-employed specialists (hospital 2).
Sixth, a potential weakness of a stepped-wedge design is contamination of the intervention; thus, information or effects of departments in the intervention period could have influenced departments still in the baseline period. Although this effect cannot be excluded, to our knowledge, there were no physicians who transferred between participating departments in this period.
Our approach offers good potential for implementation in other hospitals, even in resource-challenged circumstances, because it adapts to local possibilities, requires no expensive investments, and is successful in surgical, medical, and pediatric settings. The root cause analysis method was relatively simple and pragmatic and was performed without help from quality improvement personnel. Our study was performed with a minimal budget, comprising the salary of 1 research physician and an estimated 3 hours per week of infectious diseases specialist efforts for 3 years. Of importance, for practical implementation without research objectives, many (but not all) of our time-consuming appropriateness measurements may then be omitted. On the other hand, a bigger financial budget may increase effectiveness because more expensive desired interventions, such as mobile applications, could then be implemented.
Conclusions
Use of a participatory approach based on behavioral theory with a central focus on prescriber autonomy resulted in an increase in antimicrobial appropriateness sustained for at least 12 months. The approach is unique, inexpensive, and suited to different types of hospital departments.
eFigure 1. Enrollment in the DUMAS Study
eFigure 2. Antimicrobial Appropriateness per Department
eFigure 3. Length of Hospital Stay
eTable 1. Root Cause Analysis Interview Topic List
eTable 2. Results of the Root Cause Analysis and Chosen Interventions
eTable 3. Antimicrobial Appropriateness and Consumption per Department
References
- 1.Bell BG, Schellevis F, Stobberingh E, Goossens H, Pringle M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis. 2014;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuts EC, Hulscher MEJL, Mouton JW, et al. . Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):847-856. [DOI] [PubMed] [Google Scholar]
- 3.Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin North Am. 2014;28(2):169-175. [DOI] [PubMed] [Google Scholar]
- 4.Tonkin-Crine S, Walker AS, Butler CC. Contribution of behavioural science to antibiotic stewardship. BMJ. 2015;350:h3413. [DOI] [PubMed] [Google Scholar]
- 5.Charani E, Edwards R, Sevdalis N, et al. . Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis. 2011;53(7):651-662. [DOI] [PubMed] [Google Scholar]
- 6.Charani E, Castro-Sanchez E, Sevdalis N, et al. . Understanding the determinants of antimicrobial prescribing within hospitals: the role of “prescribing etiquette”. Clin Infect Dis. 2013;57(2):188-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hulscher MEJL, Grol RPTM, van der Meer JWM. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10(3):167-175. [DOI] [PubMed] [Google Scholar]
- 8.Meeker D, Knight TK, Friedberg MW, et al. . Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. 2014;174(3):425-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cabana MD, Rand CS, Powe NR, et al. . Why don’t physicians follow clinical practice guidelines? a framework for improvement. JAMA. 1999;282(15):1458-1465. [DOI] [PubMed] [Google Scholar]
- 10.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298(20):2415-2417. [DOI] [PubMed] [Google Scholar]
- 11.Spellberg B, Srinivasan A, Chambers HF. New societal approaches to empowering antibiotic stewardship. JAMA. 2016;315(12):1229-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drew RH. Antimicrobial stewardship programs: how to start and steer a successful program. J Manag Care Pharm. 2009;15(2 suppl):S18-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannan A, Buono E, McLaws ML, Gottlieb T. A survey of medical staff attitudes to an antibiotic approval and stewardship programme. Intern Med J. 2009;39(10):662-668. [DOI] [PubMed] [Google Scholar]
- 14.Stach LM, Hedican EB, Herigon JC, Jackson MA, Newland JG. Clinicians’ attitudes towards an antimicrobial stewardship program at a children’s hospital. J Pediatric Infect Dis Soc. 2012;1(3):190-197. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg M, Dresser LD, Daneman N, et al. . A national survey of critical care physicians’ knowledge, attitudes, and perceptions of antimicrobial stewardship programs. J Intensive Care Med. 2016;31(1):61-65. [DOI] [PubMed] [Google Scholar]
- 16.Cotta MO, Robertson MS, Marshall C, Thursky KA, Liew D, Buising KL. Implementing antimicrobial stewardship in the Australian private hospital system: a qualitative study. Aust Health Rev. 2015;39(3):315-322. [DOI] [PubMed] [Google Scholar]
- 17.Parker HM, Mattick K. The determinants of antimicrobial prescribing among hospital doctors in England: a framework to inform tailored stewardship interventions. Br J Clin Pharmacol. 2016;82(2):431-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grayson ML, Macesic N, Huang GK, et al. . Use of an innovative personality-mindset profiling tool to guide culture-change strategies among different healthcare worker groups. PLoS One. 2015;10(10):e0140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke JP. Antibiotic resistance: squeezing the balloon? JAMA. 1998;280(14):1270-1271. [DOI] [PubMed] [Google Scholar]
- 20.Davey P, Brown E, Charani E, et al. . Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2013;4(4):CD003543. [DOI] [PubMed] [Google Scholar]
- 21.Davey P, Peden C, Charani E, Marwick C, Michie S. Time for action—improving the design and reporting of behaviour change interventions for antimicrobial stewardship in hospitals: early findings from a systematic review. Int J Antimicrob Agents. 2015;45(3):203-212. [DOI] [PubMed] [Google Scholar]
- 22.Meeker D, Linder JA, Fox CR, et al. . Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tannenbaum D, Doctor JN, Persell SD, et al. . Nudging physician prescription decisions by partitioning the order set: results of a vignette-based study. J Gen Intern Med. 2015;30(3):298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallsworth M, Chadborn T, Sallis A, et al. . Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet. 2016;387(10029):1743-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler CC, Simpson SA, Dunstan F, et al. . Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little P, Stuart B, Francis N, et al. ; GRACE Consortium . Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet. 2013;382(9899):1175-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yardley L, Douglas E, Anthierens S, et al. ; GRACE consortium . Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement Sci. 2013;8:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Buul LW, Sikkens JJ, van Agtmael MA, Kramer MHH, van der Steen JT, Hertogh CMPM. Participatory action research in antimicrobial stewardship: a novel approach to improving antimicrobial prescribing in hospitals and long-term care facilities. J Antimicrob Chemother. 2014;69(7):1734-1741. [DOI] [PubMed] [Google Scholar]
- 29.Curry LA, Spatz E, Cherlin E, et al. . What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? a qualitative study. Ann Intern Med. 2011;154(6):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charani E, Cooke J, Holmes A. Antibiotic stewardship programmes: what’s missing? J Antimicrob Chemother. 2010;65(11):2275-2277. [DOI] [PubMed] [Google Scholar]
- 31.Allerberger F, Gareis R, Jindrák V, Struelens MJ. Antibiotic stewardship implementation in the EU: the way forward. Expert Rev Anti Infect Ther. 2009;7(10):1175-1183. [DOI] [PubMed] [Google Scholar]
- 32.van Limburg M, Sinha B, Lo-Ten-Foe JR, van Gemert-Pijnen JE. Evaluation of early implementations of antibiotic stewardship program initiatives in nine Dutch hospitals. Antimicrob Resist Infect Control. 2014;3(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamilton KW, Gerber JS, Moehring R, et al. ; Centers for Disease Control and Prevention Epicenters Program . Point-of-prescription interventions to improve antimicrobial stewardship. Clin Infect Dis. 2015;60(8):1252-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norton MI, Mochon D, Ariely D. The IKEA effect: when labor leads to love. J Consum Psychol. 2012;22:453-460. [Google Scholar]
- 35.Wentzel J, van Velsen L, van Limburg M, et al. . Participatory eHealth development to support nurses in antimicrobial stewardship. BMC Med Inform Decis Mak. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thursky KA, Mahemoff M. User-centered design techniques for a computerised antibiotic decision support system in an intensive care unit. Int J Med Inform. 2007;76(10):760-768. [DOI] [PubMed] [Google Scholar]
- 37.Allison ST, Messick DM. The feature-positive effect, attitude strength, and degree of perceived consensus. J Pers Soc Psychol. 1988;14(2):231-241. [DOI] [PubMed] [Google Scholar]
- 38.Deutsch M, Gerard HB. A study of normative and informational social influences upon individual judgement. J Abnorm Psychol. 1955;51(3):629-636. [DOI] [PubMed] [Google Scholar]
- 39.Cialdini RB, Cacioppo JT, Bassett R. Low-ball procedure for producing compliance: commitment then cost. J Pers Soc Psychol. 1978;36(5):463-476. [Google Scholar]
- 40.Cioffi D, Garner R. On doing the decision: effects of active vs passive choice on commitment and self-perception. Pers Soc Psychol Bull. 1996;22:133-144. [Google Scholar]
- 41.Sikkens JJ, van Agtmael MA, Peters EJG, Vandenbroucke-Grauls CMJE, Kramer MHH, de Vet HCW. Assessment of appropriate antimicrobial prescribing: do experts agree? J Antimicrob Chemother. 2016;71(10):2980-2987. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization Collaborating Centre for Drug Statistics Methodology ATC/DDD Index 2017. https://www.whocc.no/atc_ddd_index/. Accessed March 28, 2017.
- 43.Van Vuuren W, Shea CE, Van der Schaaf TW. The Development of an Incident Analysis Tool for the Medical Field. Eindhoven, the Netherlands: Eindhoven University of Technology; 1997. [Google Scholar]
- 44.Smits M, Janssen J, de Vet R, et al. . Analysis of unintended events in hospitals: inter-rater reliability of constructing causal trees and classifying root causes. Int J Qual Health Care. 2009;21(4):292-300. [DOI] [PubMed] [Google Scholar]
- 45.Jones C, Medlen N, Merlo C, Robertson M, Shepherdson J. The lean enterprise. BT Technol J. 1999;17:15-22. [Google Scholar]
- 46.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. [DOI] [PubMed] [Google Scholar]
- 47.Gerber JS, Prasad PA, Fiks AG, et al. . Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312(23):2569-2570. [DOI] [PubMed] [Google Scholar]
- 48.Thaler RH, Sunstein CR. Nudge: improving decisions about health, wealth, and happiness. Const Polit Econ. May 2008:356-360. [Google Scholar]
- 49.van Buul LW, van der Steen JT, Achterberg WP, et al. . Effect of tailored antibiotic stewardship programmes on the appropriateness of antibiotic prescribing in nursing homes. J Antimicrob Chemother. 2015;70(7):2153-2162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Enrollment in the DUMAS Study
eFigure 2. Antimicrobial Appropriateness per Department
eFigure 3. Length of Hospital Stay
eTable 1. Root Cause Analysis Interview Topic List
eTable 2. Results of the Root Cause Analysis and Chosen Interventions
eTable 3. Antimicrobial Appropriateness and Consumption per Department