Key Points
Question
Does postpartum prophylactic oral cephalexin and metronidazole decrease surgical site infection rates among obese women who receive standard preoperative antimicrobial prophylaxis and undergo cesarean delivery?
Findings
In this randomized clinical trial among 403 obese women, the rate of surgical site infection within 30 days following cesarean delivery was 6% with oral cephalexin and metronidazole and 15% with placebo, a significant difference.
Meaning
Among obese women who undergo cesarean delivery, use of prophylactic oral cephalexin and metronidazole may be warranted for prevention of surgical site infection.
Abstract
Importance
The rate of obesity among US women has been increasing, and obesity is associated with increased risk of surgical site infection (SSI) following cesarean delivery. The optimal perioperative antibiotic prophylactic regimen in this high-risk population undergoing cesarean delivery is unknown.
Objective
To determine rates of SSI among obese women who receive prophylactic oral cephalexin and metronidazole vs placebo for 48 hours following cesarean delivery.
Design, Setting, and Participants
Randomized, double-blind clinical trial comparing oral cephalexin and metronidazole vs placebo for 48 hours following cesarean delivery for the prevention of SSI in obese women (prepregnancy BMI ≥30) who had received standard intravenous preoperative cephalosporin prophylaxis. Randomization was stratified by intact vs rupture of membranes prior to delivery. The study was conducted at the University of Cincinnati Medical Center, Cincinnati, Ohio, an academic and urban setting, between October 2010 and December 2015, with final follow-up through February 2016.
Interventions
Participants were randomly assigned to receive oral cephalexin, 500 mg, and metronidazole, 500 mg (n = 202 participants), vs identical-appearing placebo (n = 201 participants) every 8 hours for a total of 48 hours following cesarean delivery.
Main Outcomes and Measures
The primary outcome was SSI, defined as any superficial incisional, deep incisional, or organ/space infections within 30 days after cesarean delivery.
Results
Among 403 randomized participants who were included (mean age, 28 [SD, 6] years; mean BMI, 39.7 [SD, 7.8]), 382 (94.6%) completed the trial. The overall rate of SSI was 10.9% (95% CI, 7.9%-14.0%). Surgical site infection was diagnosed in 13 women (6.4%) in the cephalexin-metronidazole group vs 31 women (15.4%) in the placebo group (difference, 9.0% [95% CI, 2.9%-15.0%]; relative risk, 0.41 [95% CI, 0.22-0.77]; P = .01). There were no serious adverse events, including allergic reaction, reported in either the antibiotic group or the placebo group.
Conclusions and Relevance
Among obese women undergoing cesarean delivery who received the standard preoperative cephalosporin prophylaxis, a postoperative 48-hour course of oral cephalexin and metronidazole, compared with placebo, reduced the rate of SSI within 30 days after delivery. For prevention of SSI among obese women after cesarean delivery, prophylactic oral cephalexin and metronidazole may be warranted.
Trial Registration
clinicaltrials.gov Identifier: NCT01194115
This randomized trial compares the effects of postoperative oral cephalexin-metronidazole vs placebo on frequency of surgical site infection (SSI) in obese women undergoing cesarean delivery.
Introduction
Health care–associated infections are the most common complications of hospital care and contribute to significant health care costs, patient morbidity, and mortality. These infections affected an estimated 2 million individuals in 2014 based on national data from acute care and long-term hospitals and rehabilitation facilities.1 Of the 51.4 million inpatient surgeries performed in the United States annually in 2010, cesarean deliveries are the most frequently performed surgery, accounting for 1.3 million cases.2 Studies from various populations totaling approximately 9000 cesarean deliveries from 2007 to 2011 demonstrated that an estimated 3% to 12% of all cesarean deliveries were complicated by surgical site infection (SSI), and the risk of SSI increased with increasing maternal weight.3,4,5,6 Wloch et al6 demonstrated that overweight women (body mass index [BMI] 25-30; calculated as weight in kilograms divided by height in meters squared) had an increased rate of SSI (odds ratio [OR], 1.6; 95% CI, 1.2-2.2) with a progressive risk associated with increasing BMI (30-35: OR, 2.4 [95% CI, 1.7-3.4]; >35: OR, 3.7 [95% CI, 2.6-5.2]).
Routine use of preoperative cephalosporin antibiotics has been shown to decrease the risk of SSI and is considered the standard of care.7,8 Obesity is an independent risk factor for SSI despite recommended preoperative antibiotic regimens.9 Limited studies have addressed optimal antibiotic regimens for the prevention of SSI among obese women. Preoperative cefazolin dose adjustments (2 g vs 3 g) have demonstrated higher antibiotic tissue concentrations but have not demonstrated clinical improvements in rates of SSI.10,11,12,13 Various postpartum antibiotic combinations and durations have been studied with conflicting benefit, but no studies have specifically addressed the obese population.14,15
The objective of this trial was to determine the effect of an additional 48-hour course of postoperative, prophylactic oral cephalexin plus metronidazole compared with the current standard preoperative intravenous cephalosporin alone on SSI among obese women after cesarean delivery.
Methods
Trial Design
This study was a single-center, double-blind, randomized clinical trial conducted to determine the efficacy of postoperative, prophylactic oral cephalexin plus metronidazole compared with placebo for 48 hours after cesarean delivery for the prevention of SSI among obese women who receive standard preoperative cefazolin prophylaxis. The study was approved by the University of Cincinnati Institutional Review Board. Written informed consent of study participants was obtained preoperatively or within 8 hours after cesarean delivery, followed by immediate randomization. Because the cesarean surgical approach was standardized for the study, the principal investigator set a predetermined study enrollment deadline to avoid potential changes in practice patterns that may occur with prolonged study duration. The complete trial protocol is available in Supplement 1.
Cesarean surgical approach was standardized at the study institution prior to the start of this trial according to evidence-based practice at the time of study conception.16 All participants received chlorhexidine skin preparation, unless there was a documented allergy in which case povidone-iodine would be used. Standard sterile draping of participants was performed. Intravenous cefazolin (2 g) was administered prior to skin incision. A Pfannenstiel incision was the preferred skin incision. However, decisions regarding the type of skin incision were left to the discretion of primary surgeons and individualized to participants.
The cesarean delivery was performed in standard accepted practice and the fascia was closed with a monofilament suture in a running-stitch fashion.17 The subcutaneous layer was reapproximated with 3-0 polyglactin in either running or interrupted sutures if the depth measured 2 cm or more. Skin incisions were closed using 4-0 polyglactin or poliglecaprone in a subcuticular fashion unless a primary surgeon believed skin staples were superior for an individual case. Deviations from the outlined standardized surgical protocol were recorded and analyzed. Surgical dressings were removed 24 to 36 hours postoperatively. Incisions were examined daily in the hospital by labor and delivery physician teams.
Patient Selection and Randomization
Women with a prepregnancy BMI of 30 or higher, with a final plan for cesarean delivery, and aged at least 13 years who were admitted to the labor unit for delivery were approached for study participation. Study enrollment occurred between October 2010 and December 2015 and participants were followed up through February 2016. Exclusion criteria included known immunodeficiency syndromes, non–English speaking, known allergies to cephalosporin or metronidazole, or planned administration of antibiotics in the postpartum period for any medical or obstetric indication.
Investigators, physicians, labor and postpartum staff, and participants remained blinded to treatment allocation through the entire duration of the study. All study participants received standard intravenous cefazolin (2 g) prior to surgical incision. After providing informed consent to participate in the trial, women were randomized to receive either a prophylactic 48-hour course of oral cephalexin, 500 mg, and oral metronidazole, 500 mg, or identical-appearing placebo, every 8 hours for a total of 6 doses of each antibiotic. The first dose of study medications was scheduled to be given 8 hours after intravenous cefazolin was administered. Study drug deviations were reported, including study drugs initiated more than 12 hours after administration of preoperative intravenous antibiotics, missed dose(s) of study drug, administration of study drugs more than 1 hour after the scheduled time of administration, and patient voluntary withdrawal from the study prior to the last scheduled dose.
Randomization scheme was stratified by membrane status prior to delivery (intact membranes vs rupture of membranes [ROM]). The University of Cincinnati Medical Center, Office of Clinical Research, Investigational Pharmacy prepared separate randomization schemes for study participants with intact membranes and ROM using the Wichmann-Hill random number generator with blocks of 10.18 Investigational medications and placebo preparations were blinded by overencapsulation in size 00 dark green opaque capsules. A small amount of lactose powder was added to capsules to minimize any movement within the capsule.
Study Outcomes
The primary study outcome was development of an SSI within 30 days of delivery. Surgical site infection was defined according to the National Healthcare Safety Network of the Centers for Disease Control and Prevention and comprised superficial incisional, deep incisional, or organ/space infections (see eTable 1 in Supplement 2 for outcome definitions).19 Prespecified secondary outcomes included any incisional morbidity, defined as any defect in the incisional integrity with or without the presence of an infection, including cellulitis, endometritis, and wound separation. Additional secondary outcomes included endometritis, cellulitis, fever of unknown etiology (any temperature greater than 38.3°C without an attributable source), and wound separation. Cellulitis was defined as an infection of the skin, underlying soft tissue, or both requiring antibiotics for treatment. Wound separation included any defect in the skin incision of at least 1 cm.
Participants returned for postoperative evaluations at 2 and 6 weeks postpartum. Postpartum diagnoses of SSI were made by treating physicians and verified by chart review or discussion with diagnosing physicians by principal investigators or research registered nurses , who were unaware of the study group assignments. Follow-up examination visits included a detailed history and review of systems to screen for infection and medication adverse effects following delivery. Examination of patients’ wounds determined evidence of wound separation, erythema, induration, or signs of wound abscess. Participants’ vital signs were measured and a point-of-care urinalysis performed to assess for presence of urinary tract infection. Individuals who did not present for either the 2- or 6-week postpartum follow-up examination were called by a research registered nurse or investigator for a minimum telephone assessment, rescheduled for a follow-up visit, or both. Participants were questioned regarding pain at incision site, drainage from incision, fever symptoms, separation of incision, antibiotic administration after hospital discharge, and additional visits to any medical facility for incisional problems or potential medication-related concerns.
Data Collection and Blinding
Research registered nurses and study investigators obtained demographic and medical history and follow-up outcomes at the time of enrollment, following delivery, and postpartum. Study data were collected by 3 coinvestigators blinded to study group designation (A.M.V., S.R., and C.R.W.) and were managed using the Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the Cincinnati Children’s Hospital Medical Center.20 REDCap is a secure web-based application designed to support data capture for research studies.
Data pertinent to other potential risk factors for SSI were collected, including race/ethnicity, chronic hypertension, and diabetes. Maternal race/ethnicity was self-reported as an open-ended question on collection of the demographic information at the time of enrollment and confirmed upon data abstracted from the electronic medical record. Analysis of race/ethnicity was included because prior studies have demonstrated higher rates of SSI among black women.21 The data were reviewed and cross-checked for accuracy by C.R.W. prior to data analysis. Statistical analyses were performed by E.D., who was blinded to study group designation and not involved in study recruitment or data collection. The data continued to be blinded until all statistical analyses were complete.
Statistical Analyses
A sample size was calculated to reflect a clinically and statistically meaningful difference in SSI rates between all obese women who received postcesarean antibiotics to those who received placebo. Prior to the designing of the study, the rate of SSI was 20% in the population of obese women undergoing cesarean delivery at our institution. The following assumptions were considered in the power analysis: 50% relative SSI reduction, baseline SSI rate of 20%, 10% rate of postpartum loss to follow-up, 2-sided α=.05, 80% power, and 1:1 ratio of exposed to unexposed. A relative risk reduction of 50% was chosen given studies available during study design that had demonstrated similar reductions with antibiotic prophylaxis, a reduction that was deemed to be clinically meaningful, and one that could potentially outweigh risks of expanded antibiotic use.22,23 The total sample size to detect a clinically relevant and moderately large effect size in SSI reduction was 438 participants.
The primary outcome was reported as the difference in proportion of SSI between the cephalexin-metronidazole group and the placebo group. To quantify the effect of antibiotics on the dichotomous outcomes of SSI and other wound morbidities, a log-binomial model, generalized linear model, with log link was used. The analysis was performed with an intention-to-treat principle, including participants lost to follow-up postpartum or with any study deviations. The number needed to treat to prevent 1 SSI was calculated.
Approximately 5% of patients had missing follow-up information on the primary outcome of SSI. To account for the potential influence of missing outcome data on risk estimates, a multiple imputation sensitivity analysis was performed. Multiple imputation using multivariate normal distribution with 10 imputations was used, achieving 99% relative efficiency and ensuring in-range values. A regression analysis was conducted on each imputed data set, which combines the results using a weighted average approach that accounts for the variation among imputed data sets.
Secondary outcomes were analyzed on an exploratory basis comparing the rates and differences in proportion of outcomes between the cephalexin-metronidazole and placebo groups with associated 95% confidence intervals.
Although randomization was stratified by membrane status, sample size calculations were not predetermined to represent subgroup comparisons. Therefore, post hoc analyses comparing SSI rates between women who received cephalexin-metronidazole vs those who received placebo in each subgroup (intact membranes or ROM) were performed to calculate the frequencies, differences, and 95% confidence intervals of primary and secondary outcomes among participants in the cephalexin-metronidazole group compared with placebo participants. A test of interaction was performed to analyze interaction between membrane status and the primary outcome.
Comparisons with a 2-sided P<.05 or 95% confidence interval without inclusion of the null were considered statistically significant. Statistical analysis was performed using Stata software, release 12 (Stata Corp).
Results
Study Population
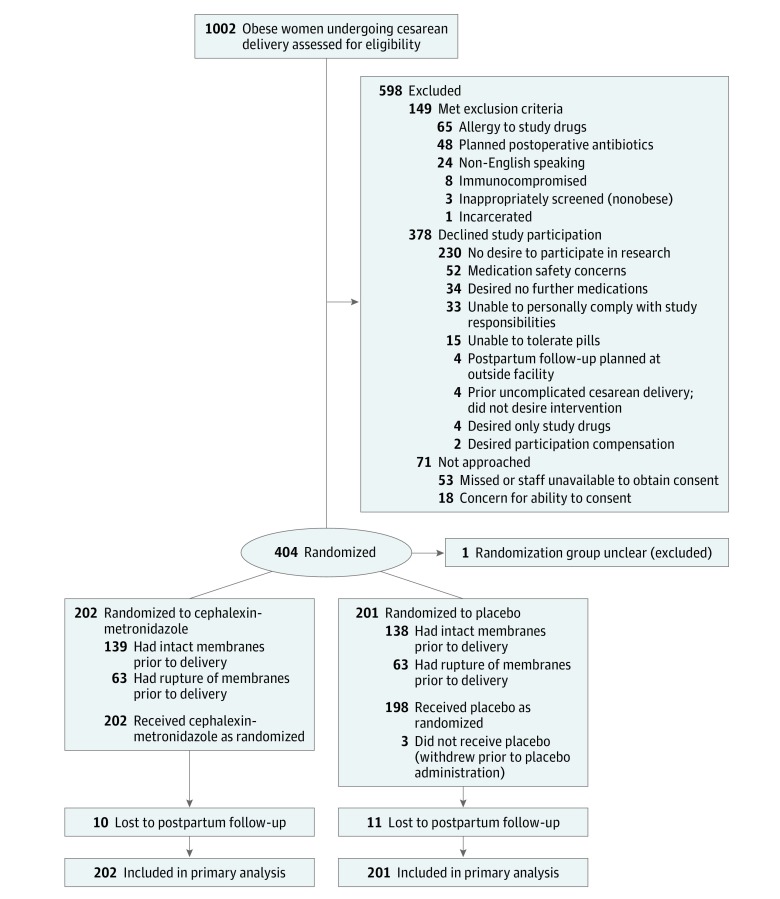
Of the 1002 women who met BMI eligibility for the trial, 149 (14.9%) met at least 1 exclusion criteria, 378 (37.7%) declined study participation, and 71 (7.1%) were not approached, secondary to unavailable study staff to enroll the patient and obtain informed consent or concern about the patient’s ability to provide informed consent or follow-up for postpartum care (Figure).
Figure. Flow of Participants in a Trial of Prophylactic Oral Cephalexin-Metronidazole vs Placebo for Surgical Site Infection in Obese Women Undergoing Cesarean Delivery.
A total of 404 study participants were enrolled, and 1 participant was excluded from analysis because her study group designation could not be determined. Enrollment was discontinued prior to achieving the targeted number of participants after reaching the predetermined study deadline that had been set by the principal investigator and approved by the institutional review board. The planned sample size was not achieved largely because of a higher-than-anticipated need to continue antibiotics for medical indications such as chorioamnionitis among participants with ROM.
Study participants were randomized to receiving postoperative cephalexin-metronidazole (n = 202 participants) or identical placebo (n = 201 participants). Maternal demographic, medical, and surgical characteristics of the study population are summarized in Table 1.
Table 1. Baseline Participant Characteristicsa.
| Characteristics | Cephalexin-Metronidazole (n = 202) |
Placebo (n = 201) |
|---|---|---|
| Maternal age, mean (SD), y | 28.5 (5.6) | 28.1 (5.6) |
| Maternal race/ethnicityb | ||
| Non-Hispanic black | 112 (55.4) | 92 (45.8) |
| Non-Hispanic white | 79 (39.1) | 104 (51.7) |
| Hispanic/Latino | 6 (3.0) | 3 (1.5) |
| Other | 5 (2.5) | 2 (1.0) |
| Parity, median (IQR)c | 1 (0-3) | 1 (1-2) |
| Body mass index, mean (SD)d | 40.1 (7.3) | 39.4 (8.4) |
| 30-39.9 | 117 (58.2) | 117 (57.9) |
| 40-49.9 | 65 (32.2) | 66 (32.8) |
| ≥50 | 20 (9.9) | 18 (9.0) |
| Limited prenatal care (<5 prenatal visits) | 16 (8.4) | 16 (8.4) |
| Multifetal gestation | 11 (5.5) | 16 (7.9) |
| Primary cesarean deliverye | 83 (40.9) | 81 (40.3) |
| Medical history | ||
| Tobacco use | 54 (26.7) | 60 (29.8) |
| Pregestational diabetes | 16 (7.9) | 19 (9.4) |
| Chronic hypertension | 49 (24.3) | 51 (25.4) |
| Perinatal and intrapartum factors | ||
| Gestational age at delivery, mean (SD), wk | 37.3 (2.9) | 36.9 (4.0) |
| Gestational diabetes | 41 (20.2) | 29 (14.4) |
| Preeclampsia | 43 (21.3) | 45 (22.4) |
| Group B streptococcus colonized | 65 (32.2) | 68 (33.8) |
| Labor prior to delivery | 70 (34.2) | 76 (37.5) |
| Rupture of membranes | 64 (31.5) | 63 (31.3) |
| Duration of ROM to delivery, median (IQR), h | 11 (5-18) | 6 (4-15) |
| No. of cervical examinations throughout the labor course, median (IQR) | 6 (3-9) | 6 (2-8) |
| Fetal scalp electrode | 38 (18.7) | 36 (17.9) |
| Intrauterine pressure catheter | 39 (19.2) | 42 (20.9) |
| Amnioinfusion | 8 (3.9) | 9 (4.5) |
| Surgical factors | ||
| Scheduled cesarean delivery | 128 (66) | 118 (62) |
| Indication for cesarean delivery | ||
| Repeat | 77 (40) | 79 (42) |
| Labor arrest | 16 (8.3) | 23 (12) |
| Fetal status | 39 (20) | 35 (18) |
| Maternal factor | 16 (8.3) | 26 (14) |
| Breech presentation | 14 (7.3) | 17 (8.9) |
| Suspected macrosomia | 4 (2.1) | 1 (0.5) |
| Multiple gestation | 11 (5.7) | 4 (2.1) |
| Other indication | 16 (8.3) | 5 (2.6) |
| Subcutaneous adipose thickness, mean (SD), cm | 3.4 (1.5) | 3.4 (1.2) |
| Pfannenstiel incision | 191 (94.1) | 190 (94.5) |
| Low transverse hysterotomy | 187 (92.1) | 184 (91.5) |
| Regional anesthesia | 192 (94.6) | 182 (90.5) |
| Estimated blood loss, mean (SD), mL | 920 (258) | 894 (235) |
| Blood transfusion | 17 (8.4) | 11 (5.5) |
| Duration of surgery, mean (SD), min | 71.0 (22.1) | 71.9 (1.7) |
| Suture skin closure | 189 (93.1) | 194 (96.5) |
Data are expressed as number (%) of participants unless otherwise specified.
Race and ethnic group were self-reported.
Interquartile range (IQR) for nonnormally distributed data and mean (SD) for normally distributed data.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters using self-reported height and prepregnancy or first-trimester documented weight.
Primary cesarean delivery is first-time cesarean delivery in a pregnant woman.
Of the 404 participants randomized, 382 (94.6%) completed the trial, 357 (88.1%) completed the medication regimen without deviation, 12 (3.0%) voluntarily withdrew from the study before the first medication administration or prior to completion of the scheduled regimen, and 43 (10.6%) had 1 or more drug administration deviations. Of the medication administration deviations, 22 (51.2%) were attributable to participants missing the last dose of study drug secondary to hospital discharge. Twenty-one (5.2%) of 404 participants could be reached only by telephone and were not examined during follow-up. The cephalexin-metronidazole and placebo groups had similar rates of loss to follow-up (5.0% vs 5.9%, respectively; difference, 0.5%; 95% CI, −3.8% to 4.9%; P = .81).
Study Outcomes
The overall rate of SSI, the primary outcome, was 10.9% (95% CI, 7.9%-14.0%) in the study population, occurring in 13 (6.4%) of 202 women who received a postoperative course of cephalexin-metronidazole vs 31 (15.4%) of 201 women in the placebo group (difference, 9.0%; 95% CI, 2.9%-15.0%; P = .01). The relative risk (RR) of SSI in the antibiotic group compared with the placebo group was 0.41 (95% CI, 0.22-0.77) (Table 2). Considering the influence of missing data, multiple imputation analysis of the primary outcome estimated an absolute between-group difference in rates of SSI with antibiotics vs placebo of 10.9% (95% CI, 6.1%-15.7%) and an RR of 0.36 (95% CI, 0.23-0.57), which were consistent with the original analysis. The number needed to treat to prevent 1 SSI in all obese women undergoing cesarean delivery was 12 (95% CI, 6.7-33.8).
Table 2. Study Outcomes.
| Outcomes | No. (%) [95% CI] With Outcome | Mean Between-Group Difference, % (95% CI) | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|---|
| Cephalexin-Metronidazole (n = 202) |
Placebo (n = 201) |
||||
| Primary outcome | |||||
| Surgical site infectiona | 13 (6.4) [3.0 to 9.8] | 31 (15.4) [10.4-20.4] | 9.0 (2.9 to 15.0) | 0.41 (0.22-0.77) | .01 |
| Secondary outcomes | |||||
| Incisional morbidityb | 20 (9.9) [5.8 to 14.1] | 32 (15.9) [10.8-21.0] | 6.0 (−0.5 to 13.0) | 0.61 (0.37-1.04) | .18 |
| Fever of unknown etiology | 9 (4.5) [1.6 to 7.3] | 10 (5.0) [2.0-8.0] | 0.5 (−3.6 to 4.6) | 0.89 (0.37-2.14) | .94 |
| Wound separation | 16 (7.9) [4.2 to 11.7] | 22 (10.9) [6.6-15.3] | 3.0 (−2.7 to 8.8) | 0.72 (0.39-1.33) | .56 |
| Cellulitis | 12 (5.9) [2.7 to 9.2] | 27 (13.4) [8.9-18.2] | 7.5 (1.7 to 13.0) | 0.44 (0.23-0.84) | .04 |
| Endometritis | 2 (1.0) [−0.4 to 2.4] | 8 (4.0) [1.3-6.7] | 3.0 (−0.05 to 6.0) | 0.24 (0.53-1.16) | .05 |
Defined as any superficial incisional, deep incisional, or organ/space infection.
Defined as any defect in the incisional integrity with or without the presence of an infection, including cellulitis, endometritis, and wound separation.
Prespecified secondary outcomes are also shown in Table 2. Cellulitis was the only secondary outcome with a significant reduction with prophylactic antibiotics, occurring in 12 of 202 (5.9%; 95% CI, 2.7%-9.2%) participants who received metronidazole-cephalexin vs 27 of 201 (13.4%; 95% CI, 8.9%=18.2%) participants who received placebo (mean difference, 7.5% [95% CI, 1.7%-13.0%]; RR, 0.44 [95% CI, 0.23-0.84]). There were no significant differences between cephalexin-metronidazole and placebo for the other prespecified secondary outcomes, including rates of incisional morbidity, fever of unknown etiology, wound separation, or endometritis.
There were no serious adverse events or allergic reactions reported for cephalexin or metronidazole. Twelve patients voluntarily withdrew from participation, 4 (2%) in the antibiotic group and 8 (4%) in the placebo group, and nausea was the most commonly reported reason for study discontinuation.
Post hoc subgroup analyses were conducted among patients with and without ROM (Table 3). Randomized participants who had ROM comprised 31.5% (63/203) of the cephalexin-metronidazole group and 31.3% (63/201) of the placebo group (difference, 0.2%; 95% CI, −8.9% to 9.3%; P = .97). Among participants with ROM prior to delivery, the cephalexin-metronidazole and placebo groups demonstrated no significant difference, respectively, in the duration of ROM (median, 11 [interquartile range, 5-18] hours vs 6 [interquartile range, 4-15] hours; P = .69) or in the frequency of internal monitoring (fetal scalp electrode: 59.4% vs 47.4%; difference, 3.2% [95% CI, −14.3% to 20.7%]; P = .80; intrauterine pressure catheter: 60.9% vs 66.7%; difference, 6.3% [95% CI, −10.7% to 23.4%]; P = .50; and amnio-infusion: 12.5% vs 14.3%; difference, 1.6% [95% CI, −10.6% to 13.7%]; P = .77).
Table 3. Post Hoc Study Outcomes Stratified by Membrane Statusa.
| Outcomes | No. (%) [95% CI] With Outcome | Mean Between-Group Difference, % (95% CI) | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|---|
| Cephalexin-Metronidazole | Placebo | ||||
| Ruptured Membranes (n = 126) | |||||
| Primary outcome | (n = 63) | (n = 63) | |||
| Surgical site infection | 6 (9.5) [2.1 to 16.9] | 19 (30.2) [18.6 to 41.7] | 20.6 (6.9 to 34.3) | 0.31(0.13-0.71) | .008 |
| Secondary outcomes | |||||
| Incisional morbidity | 10 (15.9) [6.7 to 25.1] | 19 (30.2) [18.6 to 41.7] | 14.3 (0.5 to 29.0) | 0.51 (0.26-0.99) | .10 |
| Fever of unknown etiology | 4 (6.3) [0.2 to 12.5] | 7 (11.1)) [3.2 to 19.0 | 4.8 (−5.2 to 14.8) | 0.55 (0.17-1.79) | .46 |
| Wound separation | 8 (12.7) [4.3 to 21.1] | 11 (17.5) [7.9 to 27.0] | 4.8 (−7.9 to 17.5) | 0.70 (0.30-1.62) | .54 |
| Cellulitis | 5 (7.9) [1.1 to 14.7] | 15 (23.8) [13.1 to 34.5] | 15.9 (3.2 to 28.6) | 0.32 (0.13-0.83) | .03 |
| Endometritis | 2 (3.2) [−1.2 to 7.6] | 8 (12.7) [4.3 to 21.1] | 9.5 (0.06 to 19.0) | 0.25 (0.06-1.13) | .048 |
| Intact Membranes (n = 277) | |||||
| Primary outcome | (n = 138) | (n = 139) | |||
| Surgical site infection | 7 (5.0) [1.4 to 8.7] | 12 (8.7) [4.0 to 13.4] | 3.7 (−2.3 to 9.6) | 0.58 (0.24-1.44) | .47 |
| Secondary outcomes | |||||
| Incisional morbidity | 10 (7.2) [(2.9 to 11.5] | 13 (9.4) [4.5 to 14.3] | 2.2 (−4.3 to 8.8) | 0.77 (0.35-1.69) | .78 |
| Fever of unknown etiology | 5 (3.6) [0.5 to 6.7] | 3 (2.2) [−0.3 to 4.6] | −1.4 (−5.4 to 2.5) | 1.67 (0.41-6.83) | .75 |
| Wound separation | 8 (5.8) [1.9 to 9.7] | 11 (8.0) [3.4 to 12.5] | 2.2 (−3.8 to 8.2) | 0.73 (0.30-1.75) | .75 |
| Cellulitis | 7 (5.0) [1.4 to 8.7] | 12 (8.7) [4.0 to 13.4] | 3.7 (−2.3 to 9.6) | 0.58 (0.24-1.44) | .47 |
| Endometritis | 0 | 0 | |||
Analyses examining subgroups according to intact or ruptured membranes are post hoc and should be considered exploratory.
The rate of SSI was 19.8% among women with ROM and 6.9% among those with intact membranes [difference, 13.9%; 95% CI, 12.8%-26.9%; P < .001). Post hoc analysis revealed that among participants with ROM prior to cesarean delivery, SSI occurred in 6 (9.5%) of 60 in the cephalexin-metronidazole group and 19 (30.2%) of 58 in the placebo group (difference, 22.8%; 95% CI, 8.3%-37.2%; P = .008). Among participants with intact membranes, SSI occurred in 7 (5.0%) of 132 in the cephalexin-metronidazole group compared with 12 (8.7%) of 132 in the placebo group (difference, 3.8%; 95% CI, −2.5% to 10.1%; P = .47). Interaction testing was performed between study groups (cephalexin-metronidazole vs placebo) and by membrane status (intact vs ROM) (eTable 2 in Supplement 2). The rate of SSI was highest in those with ROM who received placebo (30.2%) and lowest in those with intact membranes who received antibiotics (5.0%), but the test for interaction did not show statistical significance at P = .30.
Discussion
In this clinical trial involving obese women undergoing cesarean delivery after receiving standard intravenous preoperative cefazolin prophylaxis, a 48-hour postoperative course of oral cephalexin-metronidazole, compared with placebo, significantly decreased the rate of SSI within 30 days after delivery. Among the secondary outcomes measured, patients treated with cephalexin-metronidazole had lower rates of cellulitis but no significant decrease in other secondary outcomes, including rates of incisional morbidity, fever of unknown etiology, wound separation, or endometritis.
Cephalexin-metronidazole therapy has broad-spectrum antibiotic coverage and has demonstrated efficacy in the treatment of intra-abdominal infections in nonobstetric patients as well as decreased postcesarean infections when given preoperatively.15,24 Obesity is associated with comorbidities, anatomical factors (ie, skin folds and increased tissue pressure), and bioactive adipose tissue that increase the risk of SSI.25 Adipose tissue is highly active and secretes a wide range of hormone factors, chemokines, and, importantly, cytokines, regulating inflammation and oxidative stress.26 In addition to greater anaerobic coverage, metronidazole has anti-inflammatory properties and the antimicrobial activity of metronidazole’s hydroxymetabolite against common isolates associated with postcesarean infections, including Mycoplasma, Ureaplasma, and Gardnerella species.14,27,28
Cephalexin and metronidazole have high oral bioavailability and pharmacoeconomic advantages and are generally well tolerated, supporting the drug combination as a choice for postpartum prophylactic coverage.29 A 48-hour time course for prophylaxis was chosen considering the biological stages of wound healing. After the initial 1- to 2-day hemostatic and inflammatory phases of healing, the proliferation period occurs and includes epithelization, angiogenesis, and granulation tissue formation. This period is critical for the proper deposition of fibroblasts, basement membrane organization, and collagen formation.30 Because regulation of this inflammatory phase is important for the remodeling periods of healing, we chose a 48-hour course of prophylactic antibiotics to provide coverage while reepithelialization occurs. This study was the first to our knowledge to evaluate the use of a prophylactic postcesarean course of cephalexin-metronidazole for prevention of SSI in an obese population.
Cellulitis was the most frequent incisional morbidity observed in the study and had improved rates of SSI among participants who received postpartum antibiotics. The secondary outcomes were not powered to determine a statistically significant difference between participants who received cephalexin-metronidazole vs those who received placebo. Therefore, this study cannot conclude that postpartum cephalexin-metronidazole reduces the other individual secondary outcomes. Rupture of membranes was associated with SSI. However, the tests for interaction between the intact membranes and ROM subgroups and postpartum cephalexin-metronidazole were not statistically different and should not be interpreted as showing a difference in significance or effect size among the subgroups with and without ROM.
Prior to the routine use of preoperative prophylactic antibiotics, Elliott and Flaherty31 demonstrated a prolonged course of cephalosporin decreased the risk of endometritis compared with no antibiotic prophylaxis. Previous studies comparing prolonged antibiotic administration with a short or preoperative course of antibiotics for prevention of postcesarean infectious morbidity have not shown an infectious reduction with postpartum antibiotic regimens.32,33 However, these studies included women of all BMI categories, took place in low-resource areas, had higher proportions of immunocompromised participants, were underpowered, and were confounded by significant nutritional challenges, which are not generalizable to our population.
The use of additional broad-spectrum antibiotics has been shown to reduce SSI in women in all BMI categories undergoing cesarean delivery. Tita et al5 demonstrated a decrease (RR, 0.51; 95% CI, 0.38-0.68; P < .001) in postcesarean SSI by expanding the preoperative antimicrobial spectrum with azithromycin among women in labor and those with ROM but not specifically among obese women. These study results were published in the final year of the current study enrollment period, and azithromycin was chosen to increase coverage of Mycoplasma isolates. Although this study targeted prepregnancy obesity and the C/SOAP Consortium study focused on women in labor as the high-risk cohort,5 there is considerable population overlap, and both studies demonstrated similar and significant reductions in SSI.
Obesity during pregnancy has been associated with numerous adverse maternal and fetal outcomes such as macrosomia, cesarean delivery, and cerebral palsy.34,35,36 In addition, obese women who have medical or perinatal complications are less likely to initiate or will prematurely discontinue breastfeeding.37 Although both cephalexin and metronidazole are detected in breast milk in low concentrations, previous studies have not demonstrated any serious adverse outcomes with cephalexin or metronidazole used as single or combination therapy.38,39,40 Long-term childhood or adverse neonatal outcomes specific to cephalexin-metronidazole exposure cannot be determined, as outcome measures were not evaluated for this study protocol. Recognizing the maternal and neonatal benefit of breastfeeding, the lack of known neonatal adverse effects, and maternal reduction in SSI, the benefit of this antibiotic regimen likely outweighs the theoretical risks of breast milk exposure in the obese population.
This study has several strengths. First, the study used a randomized, blinded design; included representation of a diverse, high-risk patient population; and included a relatively large cohort of obese participants. Second, the primary outcome of interest is a significant health care–associated infection that is common and preventable. Although prior studies have demonstrated undergoing labor prior to delivery significantly increased postoperative infections after cesarean deliveries among all BMI categories, these studies were not focused to the obese population nor stratified by membrane status prior to delivery.3,21 Third, the magnitude of the reduction of SSI with postcesarean delivery related to antibiotic prophylaxis in this population (RR, 0.41; 95% CI, 0.22-0.77) is both statistically significant and clinically meaningful.
This study also has several limitations. First, the trial was performed at a single site with a high prevalence of obesity, which may not be generalizable to all obstetric practices. Second, women diagnosed as having chorioamnionitis were excluded from study enrollment because they represent a separate risk population. The current study design was unable to assess efficacy among this population. Third, the analyses did not account for multiple comparisons and subgroup interactions, so all secondary outcomes and findings based on stratification of intact or ruptured membranes should be considered exploratory and hypothesis generating. Fourth, this study had insufficient power to determine a significant reduction in SSI in the subgroup of women with intact membranes. The majority of women with intact membranes had scheduled cesarean deliveries and did not have contractions resulting in cervical change, and only 7% of women in this study went into labor with intact membranes prior to cesarean delivery. Hence, the efficacy of prophylactic postpartum antibiotics in this subgroup cannot be determined from the current study, but the findings provide support to promote further study of this subset of participants. Fifth, this trial included a very specific population of women with prepregnancy obesity undergoing cesarean delivery, and the findings should not be extrapolated to other populations or other surgical procedures. Sixth, enrollment was terminated before target recruitment was met (targeted: n=438; actual: n=404) secondary to a predetermined stop date; however, a lower rate of attrition was experienced (10% projected; 5% actual). Seventh, adverse events were monitored per institutional review board protocol, but data regarding more specific minor adverse effects were not collected unless reported by participants.
Conclusions
Among obese women undergoing cesarean delivery who received standard preoperative cephalosporin prophylaxis, a postoperative 48-hour course of cephalexin-metronidazole, compared with placebo, reduced the rate of SSI within 30 days of delivery. For prevention of SSI among obese women after cesarean delivery, prophylactic oral cephalexin and metronidazole may be warranted.
Trial Protocol
eTable 1. Definitions
eTable 2. Tests of Interaction Among Study Subgroups Between Membrane Status and Intervention
References
- 1.Centers for Disease Control and Prevention Healthcare-associated infections. 2014. https://www.cdc.gov/hai/surveillance/. Accessed May 2, 2017.
- 2.Centers for Disease Control and Prevention Obstetrical procedures. 2014. https://www.cdc.gov/nchs/fastats/obstetrical-procedures.htm. Accessed May 2, 2017.
- 3.Alanis MC, Villers MS, Law TL, Steadman EM, Robinson CJ. Complications of cesarean delivery in the massively obese parturient. Am J Obstet Gynecol. 2010;203(3):271.e1-271.e7. [DOI] [PubMed] [Google Scholar]
- 4.Leth RA, Uldbjerg N, Nørgaard M, Møller JK, Thomsen RW. Obesity, diabetes, and the risk of infections diagnosed in hospital and post-discharge infections after cesarean section: a prospective cohort study. Acta Obstet Gynecol Scand. 2011;90(5):501-509. [DOI] [PubMed] [Google Scholar]
- 5.Tita AT, Szychowski JM, Boggess K, et al. ; C/SOAP Trial Consortium . Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375(13):1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wloch C, Wilson J, Lamagni T, Harrington P, Charlett A, Sheridan E. Risk factors for surgical site infection following caesarean section in England: results from a multicentre cohort study. BJOG. 2012;119(11):1324-1333. [DOI] [PubMed] [Google Scholar]
- 7.Mackeen AD, Packard RE, Ota E, Berghella V, Baxter JK. Timing of intravenous prophylactic antibiotics for preventing postpartum infectious morbidity in women undergoing cesarean delivery. Cochrane Database Syst Rev. 2014;(12):CD009516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witt A, Döner M, Petricevic L, et al. Antibiotic prophylaxis before surgery vs after cord clamping in elective cesarean delivery: a double-blind, prospective, randomized, placebo-controlled trial. Arch Surg. 2011;146(12):1404-1409. [DOI] [PubMed] [Google Scholar]
- 9.ACOG practice bulletin 156: obesity in pregnancy. Obstet Gynecol. 2015;126(6):e112-e126. [DOI] [PubMed] [Google Scholar]
- 10.Peppard WJ, Eberle DG, Kugler NW, Mabrey DM, Weigelt JA. Association between pre-operative cefazolin dose and surgical site infection in obese patients. Surg Infect (Larchmt). 2017;18(4):485-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmadzia HK, Patel EM, Joshi D, et al. Obstetric surgical site infections: 2 grams compared with 3 grams of cefazolin in morbidly obese women. Obstet Gynecol. 2015;126(4):708-715. [DOI] [PubMed] [Google Scholar]
- 12.Swank ML, Wing DA, Nicolau DP, McNulty JA. Increased 3-gram cefazolin dosing for cesarean delivery prophylaxis in obese women. Am J Obstet Gynecol. 2015;213(3):415.e1-415.e8. [DOI] [PubMed] [Google Scholar]
- 13.Maggio L, Nicolau DP, DaCosta M, Rouse DJ, Hughes BL. Cefazolin prophylaxis in obese women undergoing cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2015;125(5):1205-1210. [DOI] [PubMed] [Google Scholar]
- 14.Andrews WW, Hauth JC, Cliver SP, Savage K, Goldenberg RL. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101(6):1183-1189. [DOI] [PubMed] [Google Scholar]
- 15.Meyer NL, Hosier KV, Scott K, Lipscomb GH. Cefazolin vs cefazolin plus metronidazole for antibiotic prophylaxis at cesarean section. South Med J. 2003;96(10):992-995. [DOI] [PubMed] [Google Scholar]
- 16.Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery. Am J Obstet Gynecol. 2005;193(5):1607-1617. [DOI] [PubMed] [Google Scholar]
- 17.Dahlke JD, Mendez-Figueroa H, Rouse DJ, Berghella V, Baxter JK, Chauhan SP. Evidence-based surgery for cesarean delivery: an updated systematic review. Am J Obstet Gynecol. 2013;209(4):294-306. [DOI] [PubMed] [Google Scholar]
- 18.Dallal GE. Wichmann-Hill random number generator. http://randomization.com/. Accessed October 28, 2016.
- 19.Centers for Disease Control and Prevention Surveillance for surgical site infection (SSI) events; protocols. 2016. https://www.cdc.gov/nhsn/acute-care-hospital/ssi/. Accessed October 24, 2016.
- 20.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamilio DM, Scifres CM. Extreme obesity and postcesarean maternal complications. Obstet Gynecol. 2014;124(2 Pt 1):227-232. [DOI] [PubMed] [Google Scholar]
- 22.Kaimal AJ, Zlatnik MG, Cheng YW, et al. Effect of a change in policy regarding the timing of prophylactic antibiotics on the rate of postcesarean delivery surgical-site infections. Am J Obstet Gynecol. 2008;199(3):310.e1-310.e5. [DOI] [PubMed] [Google Scholar]
- 23.Smaill FM, Gyte GM. Antibiotic prophylaxis vs no prophylaxis for preventing infection after cesarean section. Cochrane Database Syst Rev. 2010;(1):CD007482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nicolau DP, Patel KB, Quintiliani R, Nightingale CH. Cephalosporin-metronidazole combinations in the management of intra-abdominal infections. Diagn Microbiol Infect Dis. 1995;22(1-2):189-194. [DOI] [PubMed] [Google Scholar]
- 25.Pierpont YN, Dinh TP, Salas RE, et al. Obesity and surgical wound healing: a current review. ISRN Obes. 2014;2014:638936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts S, Maccato M, Faro S, Pinell P. The microbiology of post-cesarean wound morbidity. Obstet Gynecol. 1993;81(3):383-386. [PubMed] [Google Scholar]
- 28.Austin MN, Beigi RH, Meyn LA, Hillier SL. Microbiologic response to treatment of bacterial vaginosis with topical clindamycin or metronidazole. J Clin Microbiol. 2005;43(9):4492-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendesky A, Menéndez D, Ostrosky-Wegman P. Is metronidazole carcinogenic? Mutat Res. 2002;511(2):133-144. [DOI] [PubMed] [Google Scholar]
- 30.Janis JE, Harrison B. Wound healing, I: basic science. Plast Reconstr Surg. 2016;138(3)(suppl):9S-17S. [DOI] [PubMed] [Google Scholar]
- 31.Elliott JP, Flaherty JF. Comparison of lavage or intravenous antibiotics at cesarean section. Obstet Gynecol. 1986;67(1):29-32. [PubMed] [Google Scholar]
- 32.Westen EH, Kolk PR, van Velzen CL, et al. Single-dose compared with multiple day antibiotic prophylaxis for cesarean section in low-resource settings: a randomized controlled, noninferiority trial. Acta Obstet Gynecol Scand. 2015;94(1):43-49. [DOI] [PubMed] [Google Scholar]
- 33.Lyimo FM, Massinde AN, Kidenya BR, Konje E, Mshana SE. Efficacy of single dose of gentamicin in combination with metronidazole vs multiple doses for prevention of post-caesarean infection: study protocol for a randomized controlled trial. Trials. 2012;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyrrell J, Richmond RC, Palmer TM, et al. ; Early Growth Genetics Consortium . Genetic evidence for causal relationships between maternal obesity-related traits and birth weight. JAMA. 2016;315(11):1129-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villamor E, Tedroff K, Peterson M, et al. Association between maternal body mass index in early pregnancy and incidence of cerebral palsy. JAMA. 2017;317(9):925-936. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. 2017;317(21):2207-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitsantas P, Pawloski LR. Maternal obesity, health status during pregnancy, and breastfeeding initiation and duration. J Matern Fetal Neonatal Med. 2010;23(2):135-141. [DOI] [PubMed] [Google Scholar]
- 38.Passmore CM, McElnay JC, Rainey EA, D’Arcy PF. Metronidazole excretion in human milk and its effect on the suckling neonate. Br J Clin Pharmacol. 1988;26(1):45-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda S. Transfer of antibiotics into maternal milk. Biol Res Pregnancy Perinatol. 1984;5(2):57-60. [PubMed] [Google Scholar]
- 40.Benyamini L, Merlob P, Stahl B, et al. The safety of amoxicillin/clavulanic acid and cefuroxime during lactation. Ther Drug Monit. 2005;27(4):499-502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Definitions
eTable 2. Tests of Interaction Among Study Subgroups Between Membrane Status and Intervention