Key Points
Question
Does robotic-assisted laparoscopic surgery, as compared with conventional laparoscopic surgery, reduce the risk of conversion to laparotomy among patients undergoing surgery for rectal cancer?
Findings
In this randomized clinical trial that included 471 patients undergoing surgery for rectal cancer, the conversion rate was 8.1% for robotic surgery and 12.2% for laparoscopic surgery, not a statistically significant difference.
Meaning
Among patients undergoing resection for rectal cancer, robotic-assisted laparoscopic surgery performed by surgeons with varying experience with robotic surgery did not confer an advantage compared with laparoscopic surgery for reducing the odds of conversion to laparotomy.
This randomized clinical trial compares the effects of robotic-assisted vs conventional laparoscopic surgery on the risk of conversion to open laparotomy among patients undergoing resection for rectal cancer.
Abstract
Importance
Robotic rectal cancer surgery is gaining popularity, but limited data are available regarding safety and efficacy.
Objective
To compare robotic-assisted vs conventional laparoscopic surgery for risk of conversion to open laparotomy among patients undergoing resection for rectal cancer.
Design, Setting, and Participants
Randomized clinical trial comparing robotic-assisted vs conventional laparoscopic surgery among 471 patients with rectal adenocarcinoma suitable for curative resection conducted at 29 sites across 10 countries, including 40 surgeons. Recruitment of patients was from January 7, 2011, to September 30, 2014, follow-up was conducted at 30 days and 6 months, and final follow-up was on June 16, 2015.
Interventions
Patients were randomized to robotic-assisted (n = 237) or conventional (n = 234) laparoscopic rectal cancer resection, performed by either high (upper rectum) or low (total rectum) anterior resection or abdominoperineal resection (rectum and perineum).
Main Outcomes and Measures
The primary outcome was conversion to open laparotomy. Secondary end points included intraoperative and postoperative complications, circumferential resection margin positivity (CRM+) and other pathological outcomes, quality of life (36-Item Short Form Survey and 20-item Multidimensional Fatigue Inventory), bladder and sexual dysfunction (International Prostate Symptom Score, International Index of Erectile Function, and Female Sexual Function Index), and oncological outcomes.
Results
Among 471 randomized patients (mean [SD] age, 64.9 [11.0] years; 320 [67.9%] men), 466 (98.9%) completed the study. The overall rate of conversion to open laparotomy was 10.1%: 19 of 236 patients (8.1%) in the robotic-assisted laparoscopic group and 28 of 230 patients (12.2%) in the conventional laparoscopic group (unadjusted risk difference = 4.1% [95% CI, −1.4% to 9.6%]; adjusted odds ratio = 0.61 [95% CI, 0.31 to 1.21]; P = .16). The overall CRM+ rate was 5.7%; CRM+ occurred in 14 (6.3%) of 224 patients in the conventional laparoscopic group and 12 (5.1%) of 235 patients in the robotic-assisted laparoscopic group (unadjusted risk difference = 1.1% [95% CI, −3.1% to 5.4%]; adjusted odds ratio = 0.78 [95% CI, 0.35 to 1.76]; P = .56). Of the other 8 reported prespecified secondary end points, including intraoperative complications, postoperative complications, plane of surgery, 30-day mortality, bladder dysfunction, and sexual dysfunction, none showed a statistically significant difference between groups.
Results
Among 471 randomized patients (mean [SD] age, 64.9 [11.0] years; 320 [67.9%] men), 466 (98.9%) completed the study. The overall rate of conversion to open laparotomy was 10.1%. The overall CRM+ rate was 5.7%. Of the other 8 reported prespecified secondary end points, including intraoperative complications, postoperative complications, plane of surgery, 30-day mortality, bladder dysfunction, and sexual dysfunction, none showed a statistically significant difference between groups.
| End Point | No. With Outcome/Total No. (%) | Unadjusted Risk Difference (95% CI), % | Adjusted Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|
| Conventional Laparoscopy | Robotic-Assisted Laparoscopy | ||||
| Conversion to open laparotomy | 28/230 (12.2) | 19/236 (8.1) | 4.1 (−1.4 to 9.6) | 0.61 (0.31-1.21) | .16 |
| CRM+ | 14/224 (6.3) | 12/235 (5.1) | 1.1 (−3.1 to 5.4) | 0.78 (0.35-1.76) | .56 |
Conclusions and Relevance
Among patients with rectal adenocarcinoma suitable for curative resection, robotic-assisted laparoscopic surgery, as compared with conventional laparoscopic surgery, did not significantly reduce the risk of conversion to open laparotomy. These findings suggest that robotic-assisted laparoscopic surgery, when performed by surgeons with varying experience with robotic surgery, does not confer an advantage in rectal cancer resection.
Trial Registration
isrctn.org Identifier: ISRCTN80500123
Introduction
Laparoscopic surgery is increasingly used for the treatment of colon cancer, but its use for rectal cancer is more controversial. Two recent, large, multicenter randomized clinical trials support laparoscopic surgery, and 2 other major trials report evidence that does not allow a designation of noninferior as compared with open surgery.
Robotic assistance has the potential to overcome some of the limitations of laparoscopic rectal cancer surgery, providing an immersive 3-dimensional depth of field, articulating instruments, and a stable camera platform. Several small, nonrandomized studies have supported its safety and efficacy in rectal cancer surgery. Meta-analyses have failed to show superiority for robotic-assisted laparoscopic surgery over conventional laparoscopic surgery in short-term patient and pathological outcomes and have consistently reported longer operating times, but they have also shown a reduced need to convert to open surgery with the robot. A few nonrandomized studies have suggested that the robot may offer better preservation of bladder and sexual function.
The main concern about robotic surgery is the cost, including the capital and ongoing maintenance charges. A few studies have analyzed the costs of robotic rectal cancer surgery, reporting higher total hospital costs than for conventional laparoscopic surgery. Despite this, robotic rectal cancer surgery has continued to gain global utilization.
In 2009, the UK Medical Research Council and National Institute of Health Research, through the Efficacy and Mechanism Evaluation Programme, funded the Robotic vs Laparoscopic Resection for Rectal Cancer (ROLARR) trial to undertake an evaluation of the safety, efficacy, and short- and long-term outcomes of robotic-assisted vs conventional laparoscopic rectal cancer surgery. This trial was designed as a multicenter, international randomized clinical trial to accommodate the limited adoption of the robotic system at that time. This article presents the short-term results to 6-month follow-up.
Methods
This is an international, multicenter, randomized, unblinded, parallel-group trial comparing robotic-assisted vs conventional laparoscopic surgery for the curative treatment of rectal adenocarcinoma (distal extent ≤15 cm of the anal margin) by high anterior resection, low anterior resection, or abdominoperineal resection. Participating surgeons had to perform at least 30 minimally invasive (conventional laparoscopic or robotic-assisted laparoscopic) rectal cancer resections before taking part in the trial, of which at least 10 had to be conventional laparoscopic resections and at least 10 had to be robotic-assisted laparoscopic resections. The trial received national ethical approval in the United Kingdom or either local ethical committee or institutional review board approval at international centers. An independent trial steering committee and data monitoring and ethics committee oversaw the trial conduct. All participants provided written informed consent.
The trial design has been reported previously, and the full trial protocol is included in Supplement 1. For patients to be included, they had to be fit for resectional surgery with a diagnosis of adenocarcinoma of the rectum. Patients with benign lesions of the rectum, cancers of the anal canal, locally advanced cancers not amenable to curative surgery or requiring en bloc multivisceral resection, or synchronous colorectal tumors requiring multisegment surgical resection were not eligible.
Randomization (minimization incorporating a random element) was on a 1:1 basis. The stratification factors were treating surgeon, patient, sex, preoperative radiotherapy or chemoradiotherapy, intended procedure, and body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) classified according to World Health Organization criteria.
The specifics of each operation were at the discretion of the operating surgeon. The only absolute requirement under robotic surgery was that the robot had to be used for mesorectal resection. Pathology reporting was according to internationally agreed criteria. Patient self-reported bladder function and sexual function were measured at baseline and 6 months following surgery with the International Prostate Symptom Score (I-PSS), International Index of Erectile Function (IIEF), and Female Sexual Function Index (FSFI). The I-PSS is a standardized, patient self-reported measure of the subjective problems that the patient experiences with urinating, with scores ranging from 0 to 35 and higher scores indicating more severe symptoms. The IIIEF is a patient self-reported measure developed for the assessment of erectile function, with scores ranging from 5 to 75 and lower scores indicating greater severity of dysfunction. The FSFI is a patient self-reported measure of sexual function in women, with scores ranging from 2 to 36 and higher scores indicating greater function. Patients underwent clinical review at 30 days and 6 months postoperatively. Annual follow-up is continuing.
The primary end point was the rate of conversion to open surgery, defined as the use of a laparotomy wound for any part of the mesorectal dissection. The use of a small abdominal wound to facilitate a low, stapled anastomosis and/or specimen extraction was permissible and not defined as an open conversion. Secondary end points were all prespecified and included pathological circumferential resection margin positivity (CRM+; defined as tumor ≤1 mm), intraoperative complications, postoperative (30-day and 6-month) complications, 30-day operative mortality, patient-reported bladder and sexual function, and pathological assessment of the quality of the plane of surgery. Quality of the plane of surgery was judged according to the method of Quirke and Dixon, grading the pathology specimen in terms of completeness of surgical resection. For high and low anterior resection, this was defined as mesorectal (best), intramesorectal (intermediate), and muscularis propria (worst). For abdominoperineal excision, this was defined as levator (best), sphincteric (intermediate), and intrasphincteric (worst). Other prespecified secondary end points, not reported herein, include central pathology review with photographic documentation of resection specimens, and a full quality-of-life analysis. A full health economic evaluation was undertaken separately. Longer-term end points (local recurrence rates, disease-free survival, and overall survival) will be reported at 3 years after the last patient randomization.
The target sample size was 400 patients, which provides 80% power at the 5% (2-sided) level of significance to detect a reduction in the conversion rate from 25% in the conventional laparoscopic group to 12.5% in the robotic-assisted laparoscopic group, allowing for 16% attrition. The anticipated conversion rate in the conventional laparoscopic group was based on the MRC CLASICC Trial, which was the best available evidence at that time. The MRC CLASICC trial reported a conversion rate of 34% for conventional laparoscopic rectal cancer resection, which was reduced to 25% to account for advances in surgical technique. Sufficient funding was available to extend recruitment to 471 patients to take advantage of excellent patient recruitment and maximize the power of the study. This decision was made in consultation with the independent trial steering committee and data monitoring and ethics committee without review of data or an interim analysis being performed.
All analyses were prespecified and were conducted on the intention-to-treat population, ie, all randomized patients were accounted for in the analyses, and patients were categorized into treatment groups based on their randomization regardless of what treatment they subsequently received. Complete case analyses were performed for all prespecified end points. When the complete case analysis excluded more than 3% of patients due to missing data, exploratory analyses to investigate the effect of missing data were performed. Specifically, to explore the mechanism of the missing data and the validity of a complete case analysis for each end point, patient characteristics were compared between those with and without missing data and multilevel logistic regression models were used to identify any associations between prognostic variables and whether a patient had missing data, to inform whether data were missing at random. All hypothesis tests were 2-sided and conducted at the 5% level of significance. Estimates and their corresponding 95% confidence intervals and P values are presented for fixed effects. For the (random) surgeon effect, the intracluster correlation coefficient, estimated via the analysis-of-variance method, and bias-corrected bootstrapped 95% confidence intervals are reported. Analyses were carried out in SAS version 9.4 statistical software (SAS Institute Inc).
Multilevel logistic regression was used to estimate the odds ratios (ORs) for conversion to laparotomy, CRM+, intraoperative complications, and postoperative complications between treatment groups, adjusting for the stratification factors, where operating surgeon was modeled as a random effect. Generalized linear mixed models were used to compare 6-month bladder and sexual function scores, adjusting for baseline scores and the stratification factors.
Sensitivity analyses were performed to determine the robustness of the findings from the primary analysis, including extension of the primary analysis to account for potential learning effects by including interaction terms for the operating surgeon’s level of relevant robotic-assisted and conventional laparoscopic experience and the treatment effect. Subgroup analyses relating to the primary end point across sex, BMI class, and procedure received as well as relating to CRM+ across sex, BMI class, and T stage were performed. All subgroup analyses tested heterogeneity of the treatment effect across the subgroups and also estimated the treatment effect within each subgroup, via the inclusion of an appropriate interaction term. All sensitivity analyses and subgroup analyses were prespecified.
Cost analysis was undertaken from the perspective of a public (ie, UK National Health Service [NHS]) health care professional for all patients (eAppendix 1 and eTable 1 in Supplement 2). Resource utilization data for 190 UK and US patients were collected at baseline, intraoperatively, 30 days postoperatively, and 6 months postoperatively using study forms. Costs were computed in British pounds using a price year of 2015 and estimated using UK NHS unit costs from national data sources including the NHS Reference Costs database, Personal Social Services Research Unit costs of health and social care, and British National Formulary. For reporting purposes, costs are converted with 2015 Organisation for Economic Co-operation and Development purchasing power parity (0.866 British pound per 1 US dollar) and reported herein as 2015 US dollars. Multiple imputation methods were used for missing data. Sensitivity analysis was undertaken to account for uncertainty (eAppendix 2, eTables 2 and 3, and eFigure in Supplement 2). Given wide variation in costs due to contractual arrangements, acquisition and maintenance costs for the robotic-assisted and conventional laparoscopic systems were excluded.
Results
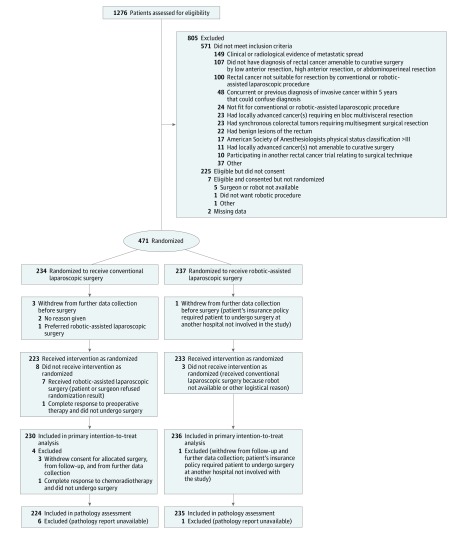
Between January 7, 2011, and September 30, 2014, 1276 patients were assessed for eligibility by 40 surgeons from 29 sites across 10 countries (United Kingdom, Italy, Denmark, United States, Finland, South Korea, Germany, France, Australia, and Singapore). Recruitment by country was 131 patients (6 sites) in the United Kingdom, 105 patients (5 sites) in Italy, 92 patients (3 sites) in Denmark, 59 patients (9 sites) in the United States, 35 patients (1 site) in Finland, 18 patients (1 site) in South Korea, 16 patients (1 site) in Germany, 11 patients (1 site) in France, 2 patients (1 site) in Australia, and 2 patients (1 site) in Singapore. A total of 471 patients (36.9%) were randomized: 234 to conventional laparoscopic surgery and 237 to robotic-assisted laparoscopic surgery (Figure 1). A total of 466 patients underwent an operation, with 456 (97.9%) undergoing the allocated treatment. Follow-up for analysis was at 30 days and 6 months, with a final follow-up date of June 16, 2015.
Figure 1. Diagram of the Flow of Participants.
The 2 treatment groups were well balanced with respect to baseline characteristics and operative procedures (Table 1). Of the 466 cases included in the primary intention-to-treat analysis, low anterior resection was performed in 317 (68.0%) and abdominoperineal resection was performed in 97 (20.8%). The mean operative time was 37.5 minutes longer in the robotic-assisted laparoscopic group than in the conventional laparoscopic group (mean [SD] operative time, 298.5 [88.71] vs 261.0 [83.24] minutes, respectively). The length of hospital stay was similar between groups.
Table 1. Patient Baseline Characteristics, Operative Details, and Pathology Outcomes.
| Variable | Conventional Laparoscopic Surgery | Robotic-Assisted Laparoscopic Surgery |
|---|---|---|
| Baseline | (n = 234) | (n = 237) |
| Age, mean (SD), y | 65.5 (11.93) | 64.4 (10.98) |
| ASA classification, No. (%) | ||
| I, Normal healthy patient | 52 (22.2) | 39 (16.5) |
| II, Patient with mild systemic disease | 124 (53.0) | 150 (63.3) |
| III, Patient with severe systemic disease | 52 (22.2) | 46 (19.4) |
| IV, Patient with severe systemic disease that is a constant threat to life | 1 (0.4) | 0 |
| Missing | 5 (2.1) | 2 (0.8) |
| Sex, No. (%) | ||
| Male | 159 (67.9) | 161 (67.9) |
| Female | 75 (32.1) | 76 (32.1) |
| BMI classification, No. (%)a | ||
| Underweight or normal, 0-24.9 | 87 (37.2) | 93 (39.2) |
| Overweight, 25.0-29.9 | 92 (39.3) | 90 (38.0) |
| Obese, ≥30.0 | 55 (23.5) | 54 (22.8) |
| Class I, 30.0-34.9 | 38 (16.2) | 41 (17.3) |
| Class II, 35.0-39.9 | 10 (4.3) | 9 (3.8) |
| Class III, ≥40.0 | 7 (3.0) | 4 (1.7) |
| Preoperative radiotherapy or chemoradiotherapy, No. (%) | ||
| Yes | 108 (46.2) | 111 (46.8) |
| No | 126 (53.8) | 126 (53.2) |
| Missing | 14 (5.9) | 6 (2.5) |
| Prior abdominal surgery, No. (%) | ||
| Yes | 67 (28.6) | 62 (26.2) |
| No | 162 (69.2) | 174 (73.4) |
| Missing | 5 (2.2) | 1 (0.4) |
| Intended operation, No. (%) | ||
| High anterior resection | 34 (14.5) | 35 (14.8) |
| Low anterior resection | 158 (67.5) | 159 (67.1) |
| Abdominoperineal resection | 42 (17.9) | 43 (18.1) |
| Operative details | (n = 230) | (n = 236) |
| Operation performed, No. (%) | ||
| High anterior resection | 19 (8.3) | 28 (11.9) |
| Low anterior resection | 165 (71.7) | 152 (64.4) |
| Abdominoperineal resection | 45 (19.6) | 52 (22.0) |
| Otherb | 1 (0.4) | 4 (1.7) |
| Height of tumor, cm from anal verge, No. (%)c | ||
| 11-15 | 69 (30.0) | 71 (30.10 |
| 6-10 | 99 (43.0) | 107 (45.3) |
| 0-5 | 61 (26.5) | 57 (24.2) |
| Missing | 1 (0.4) | 1 (0.4) |
| Operative time | ||
| Mean (SD), min | 261.0 (83.24) | 298.5 (88.71) |
| Missing, No. (%) | 4 (1.7) | 1 (0.4) |
| Stoma formation, No. (%) | ||
| Temporary | 157 (68.3) | 142 (60.2) |
| Permanent | 49 (21.3) | 53 (22.5) |
| No | 24 (10.4) | 41 (17.4) |
| Length of stay | ||
| Mean (SD), d | 8.2 (6.03) | 8.0 (5.85) |
| Missing, No. (%) | 13 (5.7) | 14 (5.9) |
| Pathology outcomes | (n = 230) | (n = 236) |
| T stage, No. (%) | ||
| 0 | 24 (10.4) | 22 (9.3) |
| 1 | 20 (8.7) | 24 (10.2) |
| 2 | 61 (26.5) | 64 (27.1) |
| 3 | 114 (49.6) | 117 (49.6) |
| 4 | 8 (3.5) | 5 (2.1) |
| Tx or missing | 3 (1.3) | 4 (1.7) |
| N stage, No. (%) | ||
| 0 | 150 (65.2) | 146 (61.9) |
| 1 | 58 (25.2) | 63 (26.7) |
| 2 | 21 (9.1) | 25 (10.6) |
| Missing | 1 (0.4) | 2 (0.8) |
| Lymph node yield | ||
| Mean (SD), No.d | 24.1 (12.91) | 23.2 (11.97) |
| Missing, No. (%) | 9 (3.9) | 1 (0.4) |
| Plane of surgery, No. (%)e | ||
| Mesorectal area, all specimens | ||
| Mesorectal | 173 (75.2) | 178 (75.4) |
| Intramesorectal | 38 (16.5) | 33 (14.0) |
| Muscularis propria | 12 (5.2) | 22 (9.3) |
| Missing | 7 (3.1) | 3 (1.3) |
| Sphincter area, abdominoperineal resections only | (n = 45) | (n = 52) |
| Levator | 18 (40.0) | 18 (34.6) |
| Sphincteric | 19 (42.2) | 22 (42.3) |
| Intrasphincteric or submucosal | 5 (11.1) | 9 (17.3) |
| Missing | 3 (6.7) | 3 (5.8) |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Derived from World Health Organization classification of obesity based on BMI, with a BMI of 25.0 to 29.9 indicating overweight and a BMI of 30.0 or greater indicating obese.
For the conventional laparoscopic group, this included laparoscopic biopsy of the peritoneum. For the robotic-assisted laparoscopic group, this included dorsal pelvic exenteration, ureter resection distally right-sided; Hartmann procedure (2 patients); and high anterior resection plus subtotal colectomy.
Height of tumor was determined by the lower border of the tumor from the anal verge at examination under anesthesia.
Lymph node yield refers to the number of lymph nodes retrieved from the specimen for histological analysis.
Plane of surgery was categorized according to the method of Quirke and Dixon by grading the pathological specimen for completeness of surgical resection. Mesorectal refers to an intact mesorectal envelope; intramesorectal has small defects in the mesorectal envelope; and muscularis propria has defects in the mesorectal envelope down to the muscular bowel wall. Levator plane refers to complete surgical resection without wasting of the specimen at the level of the levators; sphincteric plane incorporates the anal sphincter muscles but with wasting at the level of the levators; and intersphincteric or submucosal refers to inadequate extent of resection in terms of extrarectal tissue.
Participating surgeons had a wide range of previous experience with conventional and robotic-assisted laparoscopic surgery. On average, patients received an operation performed by a surgeon with experience of a median 91 (interquartile range, 45-180) previous conventional laparoscopic operations and a median 50 (interquartile range, 30-101) previous robotic-assisted laparoscopic operations.
Of the 471 patients who were randomized, 219 (46.5%) received preoperative radiotherapy or chemoradiotherapy, with no difference between the 2 treatment groups (Table 1). Also among these 471 patients, 222 (47.1%) received postoperative chemotherapy, with no difference between the 2 treatment groups.
Conversion Rate
Conversion to open surgery occurred in 47 of 466 patients (10.1%) overall: 28 of 230 patients (12.2%) in the conventional laparoscopic group and 19 of 236 patients (8.1%) in the robotic-assisted laparoscopic group (unadjusted difference in proportions, 4.1% [95% CI, −1.4% to 9.6%]). There was no statistically significant difference between robotic-assisted and conventional laparoscopic surgery with respect to odds of conversion (adjusted OR = 0.61 [95% CI, 0.31 to 1.21]; P = .16). Table 2 presents results from the multilevel logistic regression model and shows significantly increased odds of conversion in obese patients as compared with underweight or normal-weight patients (adjusted OR = 4.69 [95% CI, 2.08 to 10.58]; P < .001) and in men as compared with women (adjusted OR = 2.44 [95% CI, 1.05 to 5.71]; P = .04). Patients whose intended procedure was a low anterior resection had a significantly higher rate of conversion as compared with patients whose intended procedure was abdominoperineal resection (adjusted OR = 5.44 [95% CI, 1.60 to 18.52]; P = .007). Operating surgeon had a mild-to-moderate effect on odds of conversion, as reflected by the intracluster correlation coefficient estimate of 0.05 (95% CI, 0.01 to 0.06).
Table 2. Rates, Unadjusted Risk Differences, and Multilevel Logistic Regression Model of Odds of Conversion to Open Laparotomya.
| Effectb | No. With Conversion to Open Laparotomy/Total No. (%) | Unadjusted Risk Difference (95% CI), %c | Adjusted Odds Ratio (95% CI)d | P Value |
|---|---|---|---|---|
| Treatment | ||||
| Conventional laparoscopic surgery | 28/230 (12.2) | 4.1 (−1.4 to 9.6) | 1 [Reference] | .16 |
| Robotic-assisted laparoscopic surgery | 19/236 (8.1) | 0.61 (0.31-1.21) | ||
| Sex | ||||
| Male | 39/317 (12.3) | 6.9 (1.8 to 12.1) | 1 [Reference] | .04 |
| Female | 8/149 (5.4) | 2.44 (1.05-5.71) | ||
| BMI class, overweight vs underweight or normale | ||||
| Underweight or normal | 13/179 (7.3) | 2.3 (−2.7 to 7.2) | 1 [Reference] | .19 |
| Overweight | 9/180 (5.0) | 0.54 (0.21-1.37) | ||
| BMI class, obese vs underweight or normale | ||||
| Underweight or normal | 13/179 (7.3) | −16.1 (−25.0 to −7.2) | 1 [Reference] | <.001 |
| Obese | 25/107 (23.4) | 4.69 (2.08-10.58) | ||
| Previous radiotherapy or chemoradiotherapy | ||||
| No | 27/262 (10.3) | 0.5 (−5.0 to 6.0) | 1 [Reference] | .86 |
| Yes | 20/204 (9.8) | 1.07 (0.50-2.26) | ||
| Intended procedure, high vs low anterior resectionf | ||||
| Low anterior resection | 37/312 (11.9) | 3.0 (−4.6 to 10.7) | 1 [Reference] | .26 |
| High anterior resection | 6/68 (8.8) | 0.55 (0.19-1.56) | ||
| Intended procedure, abdominoperineal resection vs low anterior resectionf | ||||
| Low anterior resection | 37/312 (11.9) | 7.2 (1.5 to 12.9) | 1 [Reference] | .007 |
| Abdominoperineal resection | 4/86 (4.7) | 0.18 (0.05-0.63) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
The intracluster correlation coefficient (ICC) for operating surgeon as a random effect was 0.05 (95% CI, 0.01-0.06). The ICC is a measure of the proportion of variance in the outcome that is explained by the operating surgeon (eg, an ICC of 0 would indicate that the odds of conversion for a given patient would not be affected at all if he or she underwent surgery by a different operating surgeon). The ICCs for a range of outcomes across a number of surgical trials are reported in the ICC database.
The variables in this column were included in the model as fixed effects, and operating surgeon was included in the model as a random effect; these are all of the variables included in the model.
Risk differences are unadjusted estimates.
Odds ratios are adjusted estimates yielded by the model.
Derived from World Health Organization classification of obesity based on BMI, with a BMI of 25.0 to 29.9 indicating overweight and a BMI of 30.0 or greater indicating obese.
Intended procedure, rather than actual procedure, is included in the model. The intended procedure was collected at randomization and used for stratification. A sensitivity analysis adjusting for actual procedure instead of intended procedure showed no notable changes to the effect estimates, with the exception of the odds ratio comparing abdominoperineal resection vs low anterior resection, which was less pronounced (adjusted odds ratio = 0.43 [95% CI, 0.16-1.13]; P = .09).
Results from the sensitivity analysis that extended the primary analysis model to account for potential learning effects suggest that the benefit of robotic-assisted laparoscopic surgery compared with conventional laparoscopic surgery (with respect to conversion rate) is greater under surgeons who have more robotic-assisted laparoscopic experience, regardless of their level of conventional laparoscopic experience (eAppendix 2, eTables 2 and 3, and eFigure in Supplement 2).
No results from the prespecified subgroup analyses were statistically significant. Regarding the sex subgroup analysis, 39 of 317 men (12.3%) underwent conversion to laparotomy, 25 of 156 (16.0%) in the conventional laparoscopic group and 14 of 161 (8.7%) in the robotic-assisted laparoscopic group (unadjusted difference in proportions = 7.3% [95% CI, 0.1% to 14.6%]); 8 of 149 women (5.4%) underwent conversion to laparotomy, 3 of 74 (4.1%) in the conventional laparoscopic group and 5 of 75 (6.7%) in the robotic-assisted laparoscopic group (unadjusted difference in proportions = −2.6% [95% CI, −9.8% to 4.6%]). A Wald test of interaction between treatment effect and sex in the adjusted model yielded P = .09, and the estimated adjusted OR for conversion to laparotomy (robotic-assisted vs conventional laparoscopic surgery) in men given by the model was 0.46 (95% CI, 0.21 to 0.99; P = .04). Further details on all subgroup analyses are given in eAppendix 3 and eTables 4, 5, and 6 in Supplement 2.
Pathology Outcomes
The pathological outcomes are shown in Table 1. The majority of tumors, 356 of 466 (76.4%), were pT2 or pT3. The total number of lymph nodes retrieved at pathology (lymph node yield) was high in both the conventional and robotic-assisted laparoscopic groups (mean [SD], 24.1 [12.91] vs 23.2 [11.97] lymph nodes, respectively). Of 466 patients who had an operation, 459 (98.5%) had complete pathology data available. Of these 459 patients, 26 (5.7%) had CRM+: 14 of 224 (6.2%) in the conventional laparoscopic group and 12 of 235 (5.1%) in the robotic-assisted laparoscopic group (unadjusted difference in proportions = 1.1% [95% CI, −3.1% to 5.4%]). There was no statistically significant difference in the odds of CRM+ between the groups (adjusted OR = 0.78 [95% CI, 0.35 to 1.76]; P = .56) (Table 3). Subgroup analyses were largely uninformative owing to the low overall CRM+ rate. Proximal margin involvement was not observed in any patients, and distal margin involvement was observed in only 1 patient in the conventional laparoscopic group. Pathological assessment of the quality of the plane of surgery for the mesorectal area was captured for 456 of 466 patients (97.9%), and the quality of the plane was of the highest standard (mesorectal plane) in 351 of 456 cases (77.0%): 173 of 223 (77.6%) in the conventional laparoscopic group and 178 of 233 (76.4%) in the robotic-assisted laparoscopic group (unadjusted difference in proportions = 1.2% [95% CI, −6.5% to 8.9%]). There was no statistically significant difference in the odds of achieving the highest-standard plane of surgery (mesorectal plane) between the groups (adjusted OR = 0.94 [95% CI, 0.56 to 1.57]; P = .14) (Table 3).
Table 3. Secondary End Points by Treatment Group.
| End Point | No./Total No. (%) | Unadjusted Risk Difference (95% CI), % | Adjusted Odds Ratio (95% CI)a | P Value | |
|---|---|---|---|---|---|
| Conventional Laparoscopic Surgery | Robotic-Assisted Laparoscopic Surgery | ||||
| CRM+b | 14/224 (6.3) | 12/235 (5.1) | 1.2 (−3.1 to 5.4) | 0.78 (0.35 to 1.76) | .56 |
| Mesorectal area = mesorectal plane | 173/223 (77.6) | 178/233 (76.4) | 1.2 (−6.5 to 8.9) | 0.94 (0.56 to 1.57) | .14 |
| Intraoperative complication | 34/230 (14.8) | 36/236 (15.3) | −0.5 (−6.0 to 7.0) | 1.02 (0.60 to 1.74) | .94 |
| Postoperative complication within 30 d of operation | 73/230 (31.7) | 78/236 (33.1) | −1.3 (−9.8 to 7.2) | 1.04 (0.69 to 1.58) | .84 |
| Postoperative complication >30 d and ≤6 mo after operation | 38/230 (16.5) | 34/236 (14.4) | 2.1 (−4.5 to 8.7) | 0.72 (0.41 to 1.26) | .25 |
| Mortality within 30 d of operationc | 2/230 (0.9) | 2/236 (0.8) | 0.02 (−1.7 to 1.7) | NA | NA |
Abbreviations: CRM+, circumferential resection margin positivity; NA, not applicable.
Adjusted for sex, body mass index class, preoperative radiotherapy, intended procedure, and operating surgeon.
Defined as tumor cells within 1 mm of the circumferential resection margin on histological analysis.
Adjusted analysis was not performed for mortality within 30 days of operation due to the small number of events.
Complications
Table 4 shows the complication rates up to 6 months postoperatively. Of 466 patients, 70 (15.0%) had an intraoperative complication: 34 of 230 (14.8%) in the conventional laparoscopic group and 36 of 236 (15.3%) in the robotic-assisted laparoscopic group (unadjusted risk difference = −0.5% [95% CI, −6.0% to 7.0%]). There was no significant difference between the groups (adjusted OR = 1.02 [95% CI, 0.60 to 1.74]; P = .94). The most common intraoperative complications were damage to an organ or structure, significant hemorrhage, and surgical equipment failure. Overall, 151 patients (32.4%) reported a complication within 30 days: 73 of 230 patients (31.7%) in the conventional laparoscopic group and 78 of 236 (33.1%) in the robotic-assisted laparoscopic group (unadjusted risk difference = −1.3% [95% CI, −9.8% to 7.2%]). There was no significant difference between the groups (adjusted OR = 1.04 [95% CI, 0.69 to 1.58]; P = .84). Seventy-two patients (15.5%) reported a complication after 30 days and within 6 months: 38 of 230 patients (16.5%) in the conventional laparoscopic group and 34 of 236 (14.4%) in the robotic-assisted laparoscopic group (unadjusted risk difference = 2.1% [95% CI, −4.5% to 8.7%]). There was no significant difference between the groups (adjusted OR = 0.72 [95% CI, 0.41 to 1.26]; P = .25). The occurrence of anastomotic leak was determined by the surgeon and reported as gastrointestinal complication. Of the 361 patients with an anastomosis, 40 (11.1%) reported an anastomotic leak within 6 months: 18 of 181 patients (9.9%) in the conventional laparoscopic group and 22 of 180 (12.2%) in the robotic-assisted laparoscopic group.
Table 4. Number of Patients With Intraoperative and Postoperative Complications.
| Complicationa | No. (%) | |
|---|---|---|
| Conventional Laparoscopic Surgery (n = 230) |
Robotic-Assisted Laparoscopic Surgery (n = 236) |
|
| Intraoperative | ||
| Overall | 34 (14.8) | 36 (15.3) |
| Damage to organ or structure | 5 (2.2) | 11 (4.7) |
| Significant hemorrhage | 11 (4.8) | 4 (1.7) |
| Equipment failure | 6 (2.6) | 8 (3.4) |
| Fecal contamination | 6 (2.6) | 7 (3.0) |
| Anastomotic complication | 6 (2.6) | 7 (3.0) |
| Iatrogenic tumor perforation | 3 (1.3) | 2 (0.8) |
| Inadequate tumor localization or clearance | 2 (0.9) | 2 (0.8) |
| Respiratory event | 2 (0.9) | 1 (0.4) |
| Cardiac event | 1 (0.4) | 1 (0.4) |
| Within 30 d postoperatively | ||
| Overall | 73 (31.7) | 78 (33.1) |
| Gastrointestinal complication | 40 (17.4) | 35 (14.8) |
| Surgical site infection | 19 (8.3) | 21 (8.9) |
| Urinary complication | 14 (6.1) | 17 (7.2) |
| Respiratory complication | 6 (2.6) | 4 (1.7) |
| Cardiac complication | 6 (2.6) | 3 (1.3) |
| Other | 12 (5.2) | 17 (7.2) |
| >30 d and ≤6 mo postoperatively | ||
| Overall | 38 (16.5) | 34 (14.4) |
| Gastrointestinal complication | 18 (7.8) | 20 (8.5) |
| Urinary complication | 6 (2.6) | 7 (3.0) |
| Surgical site infection | 8 (3.5) | 4 (1.7) |
| Respiratory complication | 3 (1.3) | 2 (0.8) |
| Cardiac complication | 1 (0.4) | 0 |
| Cerebrovascular complication | 1 (0.4) | 0 |
| Other | 12 (5.2) | 7 (3.0) |
The categories (intraoperative, within 30 days postoperatively, and within 6 months [after 30 days]) are not mutually exclusive.
Mortality Within 30 Days Postoperatively
Mortality was a rare event, with 4 deaths (0.9%) among 466 patients (2 in each group). All deaths were related to the surgical intervention and involved a septic complication.
Postoperative Bladder and Sexual Function
Patient self-reported assessment of bladder function between baseline and 6 months was complete in 351 of 466 cases (75.3%). Patient self-reported assessment of sexual function was complete in 181 of 320 men (56.6%) and 54 of 151 women (35.8%). Exploratory analyses comparing prognostic factors between patients with and without complete data and also between the 2 treatment groups showed that the conclusions of the complete case analyses are robust to any potential effect of the missing data.
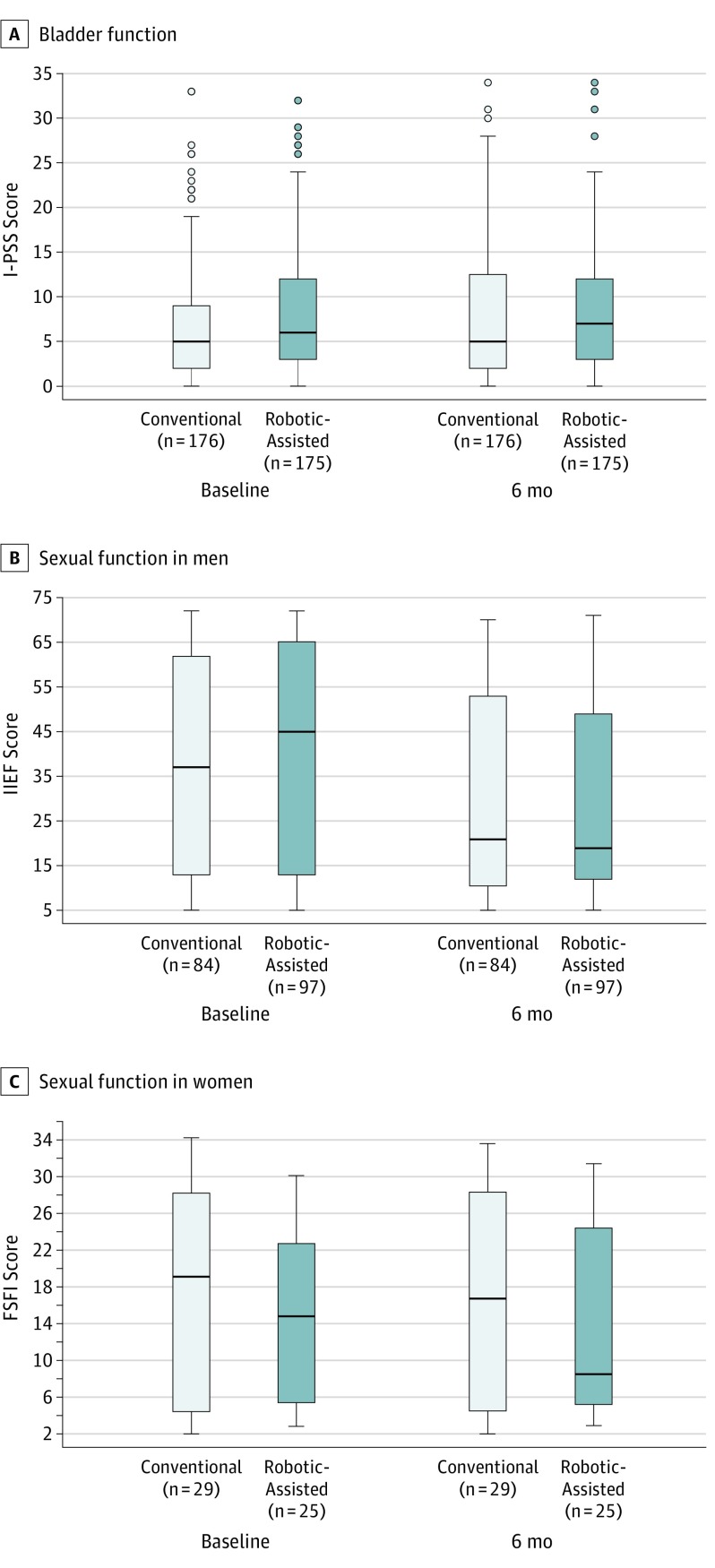
The I-PSS scores for bladder function (with higher scores indicating worse function on a scale of 0-35) for conventional and robotic-assisted laparoscopic surgery at baseline and 6 months are presented in Figure 2. The adjusted analysis comparing 6-month scores yielded an estimated difference (conventional laparoscopic group minus robotic-assisted laparoscopic group) of 0.743 (95% CI, −0.587 to 2.072; P = .27), indicating there was no statistically significant difference between the groups.
Figure 2. Bladder and Sexual Function at Baseline and 6 Months Postoperatively Following Conventional or Robotic-Assisted Laparoscopic Surgery.
Bladder function was assessed with the International Prostate Symptom Score (I-PSS; scores range from 0-35, with higher scores indicating worse function) (A), sexual function in men with the International Index of Erectile Function (IIEF; scores range from 5-75, with higher scores indicating worse function) (B), and sexual function in women with the Female Sexual Function Index (FSFI; scores range from 2-36, with higher scores indicating better function) (C). Horizontal line in the middle of each box indicates median; lower and upper borders of each box, 25th and 75th percentiles, respectively; whiskers, 1.5 times the interquartile range; and circles, extreme outliers.
The IIEF scores for male sexual function (with higher scores indicating worse function on a scale of 5-75) and FSFI scores for female sexual function (with higher scores indicating better function on a scale of 2-36) for conventional and robotic-assisted laparoscopic surgery at baseline and 6 months are also presented in Figure 2. Adjusted analyses comparing the 6-month scores yielded an estimated IIEF total score difference (conventional laparoscopic group minus robotic-assisted laparoscopic group) of 0.802 (95% CI, −4.100 to 5.704; P = .75) and an estimated FSFI total score difference (conventional laparoscopic group minus robotic-assisted laparoscopic group) of 1.231 (95% CI, −3.541 to 6.003; P = .60), indicating there was no difference between the groups.
Health Economic Analysis
Multiple imputation was used to provide data for all 190 US and UK patients. The health care costs in the robotic-assisted laparoscopic group (mean of £11 853 or $13 668 [95% CI, $13 025-$14 350]) were higher than in the conventional laparoscopic group (mean of £10 874 or $12 556 [95% CI, $11 889-$13 223]), and this difference was statistically significant (mean difference = £980 or $1132 [95% CI, $191-$2072]; P = .02). The main drivers of higher operative costs were longer mean use of the operating theater (robotic-assisted laparoscopic group minus conventional laparoscopic group, difference = 50.88 minutes [95% CI, 20.26-81.56]; P = .001) and the mean cost of instruments (robotic-assisted laparoscopic group minus conventional laparoscopic group, difference = £513 or $593 [95% CI, $493-$693]; P < .001).
Health care resource allocation data were complete in 47 of 95 patients receiving conventional laparoscopic surgery and 52 of 95 patients receiving robotic-assisted laparoscopic surgery. Among the patients with complete data, the mean cost for patients receiving conventional laparoscopic surgery was slightly lower than the imputed analysis ($12 341 vs $12 556, respectively) and was almost identical to the mean cost for patients receiving robotic-assisted laparoscopic surgery ($13 691 vs $13 688, respectively).
Discussion
In this study, to our knowledge the largest randomized clinical trial of robotic-assisted laparoscopic surgery for patients with rectal adenocarcinoma suitable for curative resection, there were no statistically significant differences in the rates of conversion to open laparotomy for robotic-assisted laparoscopic surgery compared with conventional laparoscopic surgery (8.1% vs 12.2%, respectively), and there were no statistically significant differences in CRM+, complication rates, or quality of life at 6 months. There is insufficient evidence to conclude that robotic-assisted laparoscopic surgery, compared with conventional laparoscopic surgery, reduces the risk of conversion to open laparotomy when performed by surgeons of varying experience with robotic-assisted surgery.
The primary outcome measure was conversion to open surgery, based on the hypothesis that the technological advantages of the robot should facilitate rectal cancer resection and avoid the need to convert to an open operation. The sample size calculations were based on best available evidence in 2009, including the largest randomized clinical trial of conventional laparoscopic rectal cancer surgery, the MRC CLASICC trial, which reported a 34% conversion rate to open surgery. A 25% conversion rate from conventional laparoscopic to open surgery was assumed, giving a sample size of 400 patients to demonstrate a 50% relative reduction in the conversion rate with robotic-assisted surgery. The actual overall conversion rate was much lower, 10.1%. A similar reduction in conversion rates with time has been reported in other conventional laparoscopic rectal cancer trials: COLOR II, 16%; ACOSOG Z6051, 11%; and ALaCaRT, 9%. In our trial, the difference in conversion rates between conventional laparoscopic surgery (12.2%) and robotic-assisted laparoscopic surgery (8.1%) was not statistically significant. The statistically significant lower overall conversion in patients undergoing low anterior resection, as compared with abdominoperineal resection, probably reflects that the majority of the oncological component of the operation is performed from the perineum in the abdominoperineal approach and is less affected by the laparoscopic approach. Similarly, the higher overall conversion rates for men (as compared with women) and obese patients (as compared with underweight or normal-weight patients) probably reflect the increasing technical difficulty in these patients.
The sensitivity analysis exploring learning effects suggested a potential benefit of robotic surgery when performed by surgeons with substantial prior robotic-assisted laparoscopic experience, regardless of their level of conventional laparoscopic experience. This suggests that most participating surgeons were experts in conventional laparoscopic surgery but still in their learning phases for robotic-assisted laparoscopic surgery and that at the higher end of the spectrum of experience in robotic-assisted laparoscopic surgery there is evidence of a benefit (in terms of conversion rate) over conventional laparoscopic surgery.
In almost all of the subgroup analyses, there were insufficient numbers of patients to produce statistically meaningful comparisons between the groups regarding the need to convert to an open operation. However, differences were apparent in the conversion rates for the conventional and robotic-assisted laparoscopic groups in men, with robotic-assisted laparoscopic surgery appearing to offer a benefit. While results yielded by a subgroup analysis must be interpreted with caution, the moderate evidence of interaction between sex and treatment effect, evidence of a difference between treatments in men, and the clinical plausibility of the robot facilitating dissections in the narrower male pelvis with more operator-controlled retraction, better optics, and instrument precision all warrant further investigation into the potential benefit of robotic-assisted laparoscopic surgery in this subgroup of technically challenging patients.
The experience of the participating surgeons was also evident in the low CRM+ rate (overall, 5.7%), which was lower than previous trials studying conventional laparoscopy for rectal cancer: COLOR II, 10%; ACOSOG Z6051, 12.1%; and ALaCaRT, 7%. Pathological grading of the plane of surgery showed a good standard, with mesorectal plane surgery observed in 75.3% overall. This is lower than reported in COLOR II (88%) and ALaCaRT (87%), but similar to ACOSOG Z6051 (72.9%), and is probably due to the recognized variation in reporting between pathologists. In our trial, reporting of the pathological plane of surgery was standardized to the method described by Nagtegaal and Quirke.
In accordance with other studies, robotic-assisted laparoscopic surgery was associated with longer operating times and no benefit over conventional laparoscopic surgery in length of hospital stay. A full health care economics analysis will be reported separately.
The complication rates following conventional and robotic-assisted laparoscopic surgery were similar, and there were no safety issues attributable to the robotic system. Overall 30-day mortality was low at 0.9%, in keeping with the results of meta-analyses. The leading causes of intraoperative morbidity were iatrogenic damage to an organ or structure and significant hemorrhage. In contrast to other studies, hemorrhage was not more frequently associated with robotic-assisted laparoscopic surgery. Rectal cancer surgery is a high-risk intervention, with 32.4% of patients experiencing a complication within 30 days and 15.5% of patients having complications between 30 days and 6 months.
Previous studies have shown that both conventional and robotic-assisted laparoscopic surgery for rectal cancer can result in bladder and sexual dysfunction, but the studies suggest that recovery is earlier for robotic-assisted laparoscopic surgery. This analysis of bladder and sexual function, at the same time points and using the same research questionnaires, does not support previous findings. There was little change in the I-PSS, IIEF, and FSFI scores between baseline and 6 months postoperatively, suggesting that the surgeons were accomplished in autonomic nerve preservation and that clinically relevant bladder and sexual dysfunction were an infrequent event.
Results from the health economics analysis suggest that robotic-assisted laparoscopic surgery for rectal cancer is unlikely to be cost-saving. The mean difference per operation, excluding the acquisition and maintenance costs, was £980 ($1132) and driven by longer operating theater time and increased costs for robotic instruments. When considering robotic-assisted laparoscopic surgery as a whole, rather than just rectal cancer surgery, one has to consider the cost of purchase and maintenance of the system, the operational life, and the total utilization of the robot per year for all robotic procedures. Estimates of acquisition costs in 2017 vary between $0.6 million and $2.5 million, with maintenance costs between $0.08 million and $0.17 million per year. Assuming a midpoint acquisition cost of $1.55 million and midpoint maintenance cost of $0.125 million per year, with an operational life and amortization period of 7 years, the total cost of a robot would be approximately $2.425 million. Estimates for total utilization of the robot per year in 2017 vary between 819 000 and 843 000 procedures across 3919 installed systems, or 1505 procedures per robot over 7 years. This gives the total fixed cost of around $1611 per procedure, in addition to the variable costs.
Alternatively stated, the net benefits (excluding fixed costs) of any robotic procedure included in a set of cost-effective procedures need to be positive, and the entire set of cost-effective procedures needs to have an average net benefit of at least $1611. On average, all robotic procedures combined must exceed this figure, with all procedures making at least some positive contribution. On the basis of the evidence presented here, robotic-assisted laparoscopic surgery for rectal cancer does not appear to provide a positive contribution and does not appear to be justified given the extra costs and equivalency of clinical outcomes.
Limitations
This study has several limitations. The much lower than anticipated rate of conversion to open laparotomy limits the ability to provide conclusive evidence about our primary question of how robotic-assisted laparoscopic surgery compares with conventional laparoscopic surgery in odds of conversion. However, the fact that no statistically significant differences between the treatment groups were seen in any of the end points does suggest that robotic-assisted laparoscopic surgery, when performed by surgeons with varying robotic experience, does not confer a clinically important benefit over conventional laparoscopic surgery in the short term.
No blinding to treatment allocation was incorporated into this trial. The primary end point and the measure of mortality were certainly unaffected by this as objective end points. However, there is the potential for end points that are not completely objective to have been affected. In our pathology end points, including CRM+, we have guarded against this by carrying out a blinded central review of pathology assessments.
Despite enforcing a mandatory minimum experience level for surgeon participation, operations in this trial were performed, on average, by a surgeon considered to be an expert in conventional laparoscopic surgery and who may still have been in his or her learning phase for robotic-assisted laparoscopic surgery. The prespecified sensitivity analysis of learning effects addresses this by extending the primary analysis model to analyze the interaction between operating surgeon experience and the treatment effect (eAppendix 2, eTables 2 and 3, and eFigure in Supplement 2).
The primary analysis adjusted only for stratification factors (including operating surgeon) and thus did not include an adjustment for treating site. A (prespecified) adjustment for treating site was considered in a sensitivity analysis, but model estimation issues were caused by the small sizes of the resulting strata, resulting in no meaningful output.
Conclusions
Among patients with rectal adenocarcinoma suitable for curative resection, robotic-assisted laparoscopic surgery, as compared with conventional laparoscopic surgery, did not significantly reduce the risk of conversion to open laparotomy. These findings suggest that robotic-assisted laparoscopic surgery, when performed by surgeons with varying experience with robotic surgery, does not confer an advantage in rectal cancer resection.
Trial Protocol
eAppendix 1. Supplementary Summary of Cost Analysis
eAppendix 2. Sensitivity Analysis of Potential Learning Effects
eAppendix 3. Subgroup Analyses for Conversion to Open
eTable 1. Cost Breakdown and Distributions
eTable 2. Number of Laparoscopic and Robotic Procedures Performed Before the Current Operation Summarized Across Patients
eTable 3. Estimated Adjusted Odds Ratios (Robotic vs Laparoscopic) for Conversion to Open Surgery vs Operating Surgeon’s Level of Previous Robotic Experience
eTable 4. Treatment x Sex Interaction Effects
eTable 5. Treatment x BMI Interaction Effects
eTable 6. Treatment x Procedure Performed Interaction Effects
eFigure. Estimated Adjusted Odds Ratios (Robotic vs Laparoscopic) for Conversion to Open Surgery vs Operating Surgeon’s Level of Previous Robotic Experience
References
- 1.Bonjer HJ, Deijen CL, Abis GA, et al. ; COLOR II Study Group . A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372(14):1324-1332. [DOI] [PubMed] [Google Scholar]
- 2.Jeong SY, Park JW, Nam BH, et al. . Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15(7):767-774. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson AR, Solomon MJ, Lumley JW, et al. ; ALaCaRT Investigators . Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356-1363. [DOI] [PubMed] [Google Scholar]
- 4.Fleshman J, Branda M, Sargent DJ, et al. . Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes: the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pigazzi A, Luca F, Patriti A, et al. . Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17(6):1614-1620. [DOI] [PubMed] [Google Scholar]
- 6.Baik SH, Kwon HY, Kim JS, et al. . Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16(6):1480-1487. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ. Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot. 2012;8(3):360-370. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Wang F, Zhang P, et al. . Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19(12):3727-3736. [DOI] [PubMed] [Google Scholar]
- 9.Luca F, Valvo M, Ghezzi TL, et al. . Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg. 2013;257(4):672-678. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Kim N-K, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19(8):2485-2493. [DOI] [PubMed] [Google Scholar]
- 11.Kim CW, Baik SH, Roh YH, et al. . Cost-effectiveness of robotic surgery for rectal cancer focusing on short-term outcomes: a propensity score-matching analysis. Medicine (Baltimore). 2015;94(22):e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai A, Marecik SJ, Park JJ, Melich G, Sulo S, Prasad LM. Oncologic and clinicopathologic outcomes of robot-assisted total mesorectal excision for rectal cancer. Dis Colon Rectum. 2015;58(7):659-667. [DOI] [PubMed] [Google Scholar]
- 13.Collinson FJ, Jayne DG, Pigazzi A, et al. . An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27(2):233-241. [DOI] [PubMed] [Google Scholar]
- 14.Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH. Attaining surgical competency and its implications in surgical clinical trial design: a systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol. 2014;21(3):829-840. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization BMI classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed September 29, 2017.
- 16.Royal College of Pathologists Dataset for colorectal cancer histopathology reports (3rd edition). 2007. https://www.rcpath.org/resourceLibrary/dataset-for-colorectal-cancer-histopathology-reports--3rd-edition-.html. Accessed September 29, 2017.
- 17.Barry MJ, Fowler FJ Jr, O’Leary MP, et al. ; Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5):1549-1557. [DOI] [PubMed] [Google Scholar]
- 18.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830. [DOI] [PubMed] [Google Scholar]
- 19.Rosen R, Brown C, Heiman J, et al. . The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. [DOI] [PubMed] [Google Scholar]
- 20.Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3(2):127-131. [DOI] [PubMed] [Google Scholar]
- 21.Guillou PJ, Quirke P, Thorpe H, et al. ; MRC CLASICC Trial Group . Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718-1726. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Crespi CM, Wong WK. Comparison of methods for estimating the intraclass correlation coefficient for binary responses in cancer prevention cluster randomized trials. Contemp Clin Trials. 2012;33(5):869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook JA, Bruckner T, MacLennan GS, Seiler CM. Clustering in surgical trials—database of intracluster correlations. Trials. 2012;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Pas MH, Haglind E, Cuesta MA, et al. ; Colorectal Cancer Laparoscopic or Open Resection II (COLOR II) Study Group . Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(3):210-218. [DOI] [PubMed] [Google Scholar]
- 25.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303-312. [DOI] [PubMed] [Google Scholar]
- 26.Memon S, Heriot AG, Murphy DG, Bressel M, Lynch AC. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19(7):2095-2101. [DOI] [PubMed] [Google Scholar]
- 27.Keller DS, Hashemi L, Lu M, Delaney CP. Short-term outcomes for robotic colorectal surgery by provider volume. J Am Coll Surg. 2013;217(6):1063-1069, e1. [DOI] [PubMed] [Google Scholar]
- 28.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP, Yun SH. Urinary and erectile function in men after total mesorectal excision by laparoscopic or robot-assisted methods for the treatment of rectal cancer: a case-matched comparison. World J Surg. 2014;38(7):1834-1842. [DOI] [PubMed] [Google Scholar]
- 29.Intuitive Surgical Intuitive Surgical investor presentation, Q1 2017. http://phx.corporate-ir.net/External.File?item=UGFyZW50SUQ9MzY3MzYwfENoaWxkSUQ9LTF8VHlwZT0z&t=1&cb=636229691488309586. Accessed September 29, 2017.
- 30.Iavazzo C, Papadopoulou EK, Gkegkes ID. Cost assessment of robotics in gynecologic surgery: a systematic review. J Obstet Gynaecol Res. 2014;40(11):2125-2134. [DOI] [PubMed] [Google Scholar]
- 31.Coronado PJ, Fasero M, Magrina JF, Herraiz MA, Vidart JA. Comparison of perioperative outcomes and cost between robotic-assisted and conventional laparoscopy for transperitoneal infrarenal para-aortic lymphadenectomy (TIPAL). J Minim Invasive Gynecol. 2014;21(4):674-681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. Supplementary Summary of Cost Analysis
eAppendix 2. Sensitivity Analysis of Potential Learning Effects
eAppendix 3. Subgroup Analyses for Conversion to Open
eTable 1. Cost Breakdown and Distributions
eTable 2. Number of Laparoscopic and Robotic Procedures Performed Before the Current Operation Summarized Across Patients
eTable 3. Estimated Adjusted Odds Ratios (Robotic vs Laparoscopic) for Conversion to Open Surgery vs Operating Surgeon’s Level of Previous Robotic Experience
eTable 4. Treatment x Sex Interaction Effects
eTable 5. Treatment x BMI Interaction Effects
eTable 6. Treatment x Procedure Performed Interaction Effects
eFigure. Estimated Adjusted Odds Ratios (Robotic vs Laparoscopic) for Conversion to Open Surgery vs Operating Surgeon’s Level of Previous Robotic Experience