Key Points
Question
Is self-monitoring blood glucose levels effective for people with non–insulin-treated type 2 diabetes in terms of improving either hemoglobin A1c levels or health-related quality of life (HRQOL) in primary care practice?
Findings
In this pragmatic randomized clinical trial that included 450 patients randomized to 1 of 3 groups: no self-monitoring of blood glucose (SMBG), once-daily SMBG, and once-daily SMBG with enhanced patient feedback. There were no significant differences in glycemic control across all groups, nor were there significant differences found in HRQOL.
Meaning
Routine self-monitoring of blood glucose levels does not significantly improve hemoglobin A1c levels or HRQOL for most patients with non–insulin-treated type 2 diabetes; patients and clinicians should consider the specifics of each clinical situation as they decide whether to test or not to test.
This randomized trial compares 3 approaches of self-monitoring of blood glucose levels for effects on hemoglobin A1c levels and health-related quality of life among patients with non–insulin-treated type 2 diabetes in primary care practice.
Abstract
Importance
The value of self-monitoring of blood glucose (SMBG) levels in patients with non–insulin-treated type 2 diabetes has been debated.
Objective
To compare 3 approaches of SMBG for effects on hemoglobin A1c levels and health-related quality of life (HRQOL) among people with non–insulin-treated type 2 diabetes in primary care practice.
Design, Setting, and Participants
The Monitor Trial study was a pragmatic, open-label randomized trial conducted in 15 primary care practices in central North Carolina. Participants were randomized between January 2014 and July 2015. Eligible patients with type 2 non–insulin-treated diabetes were: older than 30 years, established with a primary care physician at a participating practice, had glycemic control (hemoglobin A1c) levels higher than 6.5% but lower than 9.5% within the 6 months preceding screening, as obtained from the electronic medical record, and willing to comply with the results of random assignment into a study group. Of the 1032 assessed for eligibility, 450 were randomized.
Interventions
No SMBG, once-daily SMBG, and once-daily SMBG with enhanced patient feedback including automatic tailored messages delivered via the meter.
Main Outcomes and Measures
Coprimary outcomes included hemoglobin A1c levels and HRQOL at 52 weeks.
Results
A total of 450 patients were randomized and 418 (92.9%) completed the final visit. There were no significant differences in hemoglobin A1c levels across all 3 groups (P = .74; estimated adjusted mean hemoglobin A1c difference, SMBG with messaging vs no SMBG, −0.09%; 95% CI, −0.31% to 0.14%; SMBG vs no SMBG, −0.05%; 95% CI, −0.27% to 0.17%). There were also no significant differences found in HRQOL. There were no notable differences in key adverse events including hypoglycemia frequency, health care utilization, or insulin initiation.
Conclusions and Relevance
In patients with non–insulin-treated type 2 diabetes, we observed no clinically or statistically significant differences at 1 year in glycemic control or HRQOL between patients who performed SMBG compared with those who did not perform SMBG. The addition of this type of tailored feedback provided through messaging via a meter did not provide any advantage in glycemic control.
Trial Registration
clinicaltrials.gov Identifier: NCT02033499
Introduction
The value of self-monitoring of blood glucose (SMBG) for patients with non–insulin-treated type 2 diabetes mellitus (T2DM) has been debated, yet over 75% perform regular SMBG. Several trials showed significant benefit from SMBG on glycemic control, while others found no evidence of benefit. Proponents postulate that testing promotes better awareness of glucose levels, leading to improvements in diet and lifestyle. However, harms from routine SMBG in patients with non–insulin-treated T2DM are possible.
Studies of enhanced SMBG, where patients and/or clinicians were educated to better interpret SMBG values, found hemoglobin A1c reductions close to 0.5%, compared with simple SMBG, where levels were reduced by 0.2%, an amount that was statistically significant but of doubtful clinical significance. This pattern suggests that, for SMBG to be an effective self-management tool in non–insulin-treated T2DM, the patient and physician must actively engage in performing, interpreting, and acting on the SMBG values.
Our goal was to answer the following question: Is SMBG effective for people with non–insulin-treated T2DM in terms of improving either hemoglobin A1c levels or health-related quality of life (HRQOL)?
Methods
Trial Design
We performed this pragmatic trial across 15 primary care practices in central North Carolina. The trial was funded by the Patient-Centered Outcomes Research Institute Diabetes stakeholders provided input during grant design, implementation, and dissemination. The trial protocol (Supplement 1) was reviewed and approved by the University of North Carolina institutional review board. Written informed consent was obtained and participants were compensated with $50 for filling out baseline and follow-up surveys. Participants in the testing arms also received a meter and test strips. Patients with non–insulin-treated T2DM were randomly assigned to 1 of 3 arms: (1) no SMBG; (2) standard once-daily SMBG consisting of glucose values immediately reported to the patient through the meter; and (3) enhanced once-daily SMBG consisting of glucose values immediately reported to the patient plus automated, tailored messaging delivered to the patient through a Telcare meter. The messaging algorithm accounted for blood glucose value, time of day, and relationship to food intake. Messages were intended to educate and motivate patients (eTable 2 in Supplement 2). Time- and date-stamped data uploaded from the meters allowed the study team to monitor daily meter use in the SMBG arms. Following randomization, primary care clinicians guided participants’ routine diabetes management. Clinicians received summaries of SMBG data and potential treatment options based on American Diabetes Association Standards of Care through the electronic health record for patients in both testing arms. The recommendations were not prescriptive and clinicians were encouraged to use them based on clinical situation. Participants were reassessed at 52 weeks following randomization.
Patients
Eligibility criteria included: (1) T2DM, (2) 30 years or older, (3) established patient at a participating practice, (4) hemoglobin A1c levels between 6.5% and 9.5% within 6 months preceding screening, and (5) willing to be randomly assigned to a study group. Patients were excluded if they planned to see an endocrinologist in the upcoming year, currently or planned to use insulin during study period, planned to become pregnant or relocate in the next year, or had other conditions that would put them at risk in following study protocol. Patients were not excluded if they had prior SMBG experience.
Baseline Procedures
After study field staff obtained written informed consent, patients completed an interview that included demographic, health history, and patient-reported measures. Patients had a hemoglobin A1c blood test and height and weight were recorded. The field coordinator then opened a numbered, opaque randomization envelope containing group assignment. Randomization was stratified by practice and used randomly permuted blocks of sizes 15 and 18 generated by a biostatistics research assistant not otherwise involved in the study. The field coordinator taught patients randomized to the testing groups how to use the meter. All participants received educational brochures describing blood glucose goals and symptoms of hypoglycemia and hyperglycemia.
Outcomes
The 2 primary outcomes were change in hemoglobin A1c levels and in HRQOL. Hemoglobin A1c levels were measured at baseline and again at a mean (SD) 52 (6) weeks from baseline visit. For the first 40 patients enrolled, baseline hemoglobin A1c levels were measured by total glycated hemoglobin; glycosylated hemoglobin was calculated using a published formula by the processing laboratory. The remainder of patients had their hemoglobin A1c levels measured by glycosylated hemoglobin by a single laboratory at baseline and follow up visits. Intermediate hemoglobin A1c values were captured passively from the electronic health record. We assessed HRQOL using physical and mental component scores of the Short-Form 36 (SF-36). Secondary outcomes included Problem Areas In Diabetes, Diabetes Symptoms Checklist, and Diabetes Empowerment Scale to assess diabetes-specific HRQOL and self-efficacy. We examined diabetes self-care through the Summary of Diabetes Self-Care Activities measure. Treatment satisfaction and provider-patient communication were assessed through the Diabetes Treatment Satisfaction Questionnaire and the Communication Assessment Tool.
Preidentified potentially study-related adverse events (AEs) included finger stick infections and severe hypoglycemia. Emergency department and hospitalizations alerts from the electronic health record allowed review of intrastudy events, which were adjudicated by committee. At follow-up, participants were queried regarding any urgent care, emergency department visit, hospitalization, finger stick infection, and hypoglycemic episode over the past 52 weeks.
Statistical Analysis
We calculated power for the 2 df overall tests comparing our primary outcomes across all 3 groups. Assuming a common standard deviation for change in hemoglobin A1c levels of 0.8% and no more than 10% loss to follow-up, randomizing 150 patients per group would provide at least 90% power to detect a mean difference of −0.325% between the SMBG and no SMBG groups at the .05 significance level. Assuming a HRQOL standard deviation of 10 points, this sample size would provide at least 80% power to detect an overall difference between groups if the mean difference between the highest and lowest groups was at least 4 points on either component of the HRQOL scale at the .025 level (Bonferroni-corrected for 2 components).
For primary analyses, all randomized patients were analyzed according to their group regardless of the extent to which they performed SMBG (intention-to-treat, ITT). The statistician remained blinded to treatment groups until after finalization of programming for primary comparisons. Missing 52-week outcome data were ignored for primary analyses. We compared change in hemoglobin A1c levels from baseline through 52 weeks across the 3 randomization groups using an analysis of covariance (ANCOVA) conducted at the .05 significance level. This model controlled for site, baseline hemoglobin A1c levels, whether baseline hemoglobin A1c levels were directly measured or calculated, use of SMBG at baseline, duration of diabetes, baseline use of antihyperglycemic treatment (sulfonylurea or glinide), age, race/ethnicity, health literacy, and number of comorbidities. Had the overall test been rejected, we planned to compare each SMBG group to the no testing group separately using the Dunnett-Tamhane Step-Up procedure. We also conducted a contrast test comparing the average of the 2 SMBG groups to the no SMBG group at the .05 level. ANCOVA similar models were used to compare groups for change in HRQOL component scores as well as listed secondary outcomes; besides the covariates listed above, each of these models controlled for corresponding baseline scale score. Additionally, we explored potential for effect modification by each baseline variable included in the models by adding appropriate interaction terms to the ANCOVA model 1 at a time.
We conducted 3 prespecified sensitivity analyses for the hemoglobin A1c comparison. First, we repeated ITT analysis using a per-protocol population that excluded participants who initiated insulin use during the study or who were not sufficiently compliant with their assigned treatment. In the testing arms, we excluded participants who uploaded a meter reading fewer than 80% of their days in the study, and in the no testing arm we excluded participants who admitted to ever testing with any regularity during the study. Second, we repeated the ANCOVA model using linear mixed models that included all intermediate hemoglobin A1c values captured from the electronic health record, excluding any following initiation of insulin use. This model included fixed effects for linear and quadratic time trends and time-by-treatment group interactions, as well as random intercepts and slopes for each patient. As a final sensitivity analysis, we used last observation carried forward to impute the 52-week hemoglobin A1c value for any patient who was lost to follow-up or who initiated insulin during the study.
Results
Overview of Trial Conduct
A total of 450 patients underwent randomization from January 2014 to July 2015 (Figure 1). A total of 92.9% of patients completed the final visit and provided data on both outcomes (hemoglobin A1c levels and HRQOL). The demographic and clinical characteristics were similar among groups (Table 1). The mean age was 61 years old, patients had diabetes an average of 8 years, 75% were performing SMBG at baseline, and 38% had low health literacy (less than 4 on the Newest Vital Sign). Patient testing preference at baseline was similar among groups with 22% preferring no SMBG and 40% preferring to self-monitor. The majority were taking metformin (80%), followed by sulphonylurea (35%).
Figure 1. The Monitor Trial CONSORT Flow Diagram.
SMBG indicates self-monitoring of blood glucose.
Table 1. Baseline Characteristics.
| Characteristic | Randomization Group | |||
|---|---|---|---|---|
| No SMBG (n = 152) | SMBG, No Messaging (n = 150) | SMBG With Messaging (n = 148) | Total (n = 450) | |
| Age, median (range), y | 61 (31-89) | 63 (32-82) | 61 (35-92) | 61 (31-92) |
| Sex, No. (%) | ||||
| Male | 74 (48.7) | 67 (44.7) | 66 (44.6) | 207 (46.0) |
| Female | 78 (51.3) | 83 (55.3) | 82 (55.4) | 243 (54.0) |
| Race, No. (%) | ||||
| Black | 42 (27.6) | 55 (36.7) | 51 (34.5) | 148 (32.9) |
| White | 104 (68.4) | 89 (59.3) | 86 (58.1) | 279 (62.0) |
| Other | 6 (3.9) | 6 (4.0) | 11 (7.4) | 23 (5.1) |
| Ethnicity, non-Latino Hispanic, No. (%) | 148 (97.4) | 147 (98.7) | 146 (98.6) | 441 (98.2) |
| Education, No. (%) | ||||
| <High school | 6 (4.0) | 10 (6.7) | 9 (6.1) | 25 (5.6) |
| High school/some college | 95 (62.9) | 87 (58.0) | 89 (60.1) | 271 (60.4) |
| College or higher | 50 (33.1) | 53 (35.3) | 50 (33.8) | 153 (34.1) |
| BMI, median (range) | 33 (22-58) | 33 (21-62) | 34 (21-75) | 33 (21-75) |
| Low health literacy, No. (%)a | 62 (40.8) | 54 (36.5) | 55 (37.2) | 171 (38.2) |
| Years with diabetes, median (range) | 6 (0-45) | 6 (0-44) | 6 (0-50) | 6 (0-50) |
| Diabetes 1 y or less, No. (%) | 25 (16.4) | 27 (18.0) | 14 (9.5) | 66 (14.7) |
| No. of comorbidities, median (range) | 3 (0-9) | 3 (0-10) | 3 (0-8) | 3 (0-10) |
| Use of SMBG, No. (%) | ||||
| Current | 114 (75.0) | 108 (72.0) | 116 (78.4) | 338 (75.1) |
| Ever | 138 (90.8) | 135 (90.0) | 143 (96.6) | 416 (92.4) |
| Testing preference, No. (%) | ||||
| Any SMBG | 63 (41.4) | 56 (37.3) | 59 (39.9) | 178 (39.6) |
| No SMBG | 31 (20.4) | 34 (22.7) | 32 (21.6) | 97 (21.6) |
| Uncertain | 2 (1.3) | 1 (0.7) | 1 (0.7) | 4 (0.9) |
| No preference | 56 (36.8) | 59 (39.3) | 56 (37.8) | 171 (38.0) |
| Diabetes medications, No. (%)b | ||||
| Metformin | 123 (80.9) | 115 (76.7) | 120 (81.1) | 358 (79.6) |
| Sulfonylurea or glinide | 51 (33.6) | 50 (33.3) | 60 (40.5) | 161 (35.8) |
| Thiazolidinedione | 8 (5.3) | 3 (2.0) | 10 (6.8) | 21 (4.7) |
| GLP-1 agonist | 5 (3.3) | 2 (1.3) | 10 (6.8) | 17 (3.8) |
| DPP-4 inhibitor | 12 (7.9) | 11 (7.3) | 17 (11.5) | 40 (8.9) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); SMBG, self-monitoring of blood glucose.
Scoring less than 4 on Newest Vital Sign.
Other diabetes medications were less than 5%.
Primary Outcomes
At 1 year, we found no evidence that SMBG led to improved glycemic control (estimated adjusted mean hemoglobin A1c difference: SMBG with messaging vs no SMBG, −0.09%; 95% CI, −0.31% to 0.14%; SMBG vs no SMBG, −0.05%; 95% CI, −0.27% to 0.17%; average over SMBG arms vs no SMBG, −0.07%; 95% CI, −0.26% to 0.12%) (Table 2). There were also no significant differences found in HRQOL (estimated adjusted mean difference for SF-36 Physical score: SMBG with messaging vs no SMBG, −0.83 points; 95% CI, −2.33 to 0.67; SMBG vs no SMBG, −0.05 points; 95% CI, −1.54 to 1.44; average over SMBG arms vs no SMBG, −0.44 points; 95% CI, −1.73 to 0.85; estimated adjusted mean difference for SF-36 Mental score: SMBG with messaging vs no SMBG, −0.19 points; 95% CI, −1.82 to 1.44; SMBG vs no SMBG, 0.19 points; 95% CI, −1.43 to 1.81; average over SMBG arms vs no SMBG, 0 points; 95% CI, −1.40 to 1.40).
Table 2. Summary of Primary Outcomes by Randomization Group.
| Variable | Randomization Group | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| No SMBG | SMBG, No Messaging | SMBG With Messaging | ||||||
| No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | Overalla | Contrastb | |
| Hemoglobin A1c, %c | ||||||||
| Baseline | 152 | 7.52 (1.12) (58.70 [12.24] mmol/mol) |
150 | 7.55 (1.10) (59.06 [12.07] mmol/mol) |
148 | 7.61 (0.97) (59.65 [10.64] mmol/mol) |
.74 | .48 |
| Follow-up | 147 | 7.55 (1.24) (59.01 [13.56] mmol/mol) |
141 | 7.49 (1.12) (58.41 [12.23] mmol/mol) |
139 | 7.51 (1.13) (58.55 [12.34] mmol/mol) |
||
| Change | 147 | 0.04 (1.12) (0.41 [12.27] mmol/mol) |
141 | −0.05 (1.00) (−0.57 [10.89] mmol/mol) |
139 | −0.10 (1.14) (−1.04 [12.42] mmol/mol) |
||
| Health-Related Quality of Life, SF-36 | ||||||||
| Physical score | ||||||||
| Baseline | 152 | 48.72 (8.00) | 150 | 47.27 (8.40) | 148 | 46.22 (10.13) | .48 | .50 |
| Follow-up | 143 | 48.47 (7.21) | 142 | 47.42 (9.03) | 135 | 46.44 (9.68) | ||
| Change | 143 | −0.43 (6.86) | 142 | 0.07 (6.77) | 135 | −0.35 (6.95) | ||
| Mental score | ||||||||
| Baseline | 152 | 53.52 (9.29) | 150 | 52.94 (8.77) | 148 | 53.43 (9.58) | .90 | >.99 |
| Follow-up | 143 | 53.39 (10.55) | 142 | 52.04 (9.57) | 135 | 52.57 (10.39) | ||
| Change | 143 | −0.94 (7.46) | 142 | −0.71 (7.72) | 135 | −1.39 (6.85) | ||
Abbreviation: SMBG, self-monitoring of blood glucose.
SI conversion factor: To convert percent of total hemoglobin to proportion of total hemoglobin, multiply by .01.
Test comparing all 3 groups from ANCOVA model controlling for site, baseline hemoglobin A1c, prior use of SMBG, duration of T2DM, baseline anti-hyperglycemic treatment, age, race/ethnicity, health literacy, and number of baseline comorbidities; for health-related quality of life scores, we also controlled for baseline score, and for hemoglobin A1c we also controlled for how hemoglobin A1c was measured at baseline.
Contrast test from same ANCOVA model comparing average of SMBG groups with no SMBG group.
Dual reported as percentage and as mmol/mol.
Secondary Outcomes
We did not find significant differences in patient-reported outcomes by the Problem Areas in Diabetes, Diabetes Symptom Checklist, Diabetes Empowerment Scale, Diabetes Treatment Satisfaction, or the Communication Assessment Tool (Table 3). There were significant differences in the Summary of Diabetes Self-Care Activities (mean change 0.01 points, 0.51 points, and 0.45 points, in the no SMBG, SMBG, and SMBG with messaging, respectively; overall, P < .001). However, this was owing to the influence of the SMBG intervention (blood glucose testing subscale mean change, −1.46 points, 2.94 points, 2.81 points in the no SMBG, SMBG, and SMBG with messaging, respectively; overall, P < .001). Among the arms, there were no significant differences in insulin initiation (8.6%, 4.0%, 5.4% in the no SMBG, SMBG, SMBG with messaging, respectively; overall, P = .23). Patients in the SMBG groups taking a GLP-1 agonist at baseline were significantly more likely to increase their dose compared with patients in the no SMBG group (P = .02), but the numbers were small (eTable 1 in Supplement 2). In addition, patients in the SMBG with messaging group were significantly more likely to start using thiazolidinedione (P = .01), but again the numbers were small. No other comparisons of medication use differed significantly between groups.
Table 3. Secondary Outcomes by Randomization Group Diabetes Mellitus Patient-Reported Outcomes.
| Variable | Randomization Group | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| No SMBG | SMBG, No Messaging | SMBG With Messaging | ||||||
| No. | Mean (SD) | No. | Mean (SD) | No. | Mean (SD) | Overalla | Contrastb | |
| Problem areas in diabetes (PAID) | ||||||||
| Baseline | 152 | 13.12 (15.53) | 150 | 12.54 (14.89) | 148 | 13.67 (18.16) | .21 | .08 |
| Follow-up | 143 | 11.06 (15.45) | 142 | 8.96 (13.90) | 135 | 9.04 (14.54) | ||
| Change | 143 | −1.97 (15.44) | 142 | −4.01 (12.16) | 135 | −3.84 (13.53) | ||
| Diabetes symptoms checklist (DSC) | ||||||||
| Baseline | 152 | 19.04 (19.56) | 150 | 21.55 (21.88) | 148 | 20.73 (22.62) | .06 | .06 |
| Follow-up | 143 | 21.43 (23.73) | 142 | 19.46 (20.10) | 135 | 19.80 (21.42) | ||
| Change | 143 | 2.15 (14.37) | 142 | −2.36 (15.37) | 135 | 0.53 (14.78) | ||
| Diabetes empowerment scale (DES-SF) | ||||||||
| Baseline | 152 | 4.35 (0.48) | 149 | 4.33 (0.50) | 148 | 4.27 (0.58) | .28 | .28 |
| Follow-up | 143 | 4.43 (0.49) | 142 | 4.42 (0.47) | 135 | 4.46 (0.49) | ||
| Change | 143 | 0.08 (0.53) | 141 | 0.11 (0.50) | 135 | 0.20 (0.49) | ||
| Summary of diabetes self-care activities (total score) | ||||||||
| Baseline | 152 | 3.42 (1.32) | 150 | 3.64 (1.42) | 148 | 3.46 (1.34) | <.001 | <.001 |
| Follow-up | 143 | 3.39 (1.23) | 142 | 4.12 (1.30) | 135 | 3.87 (1.32) | ||
| Change | 143 | 0.01 (1.00) | 142 | 0.51 (1.14) | 135 | 0.45 (1.67) | ||
| Summary of diabetes self-care activities (blood sugar subscale) | ||||||||
| Baseline | 152 | 2.54 (2.62) | 149 | 2.65 (2.77) | 148 | 2.64 (2.87) | <.001 | <.001 |
| Follow-up | 143 | 0.95 (2.00) | 142 | 5.60 (2.29) | 135 | 5.39 (2.30) | ||
| Change | 143 | −1.46 (2.83) | 141 | 2.94 (3.23) | 135 | 2.81 (3.30) | ||
| Diabetes Treatment Satisfaction | ||||||||
| Baseline | 149 | 31.74 (5.52) | 147 | 31.71 (4.92) | 148 | 31.89 (4.96) | .48 | .48 |
| Follow-up | 135 | 31.66 (6.27) | 141 | 32.21 (4.89) | 135 | 31.74 (5.90) | ||
| Change | 133 | −0.16 (6.26) | 138 | 0.67 (4.95) | 135 | −0.28 (5.89) | ||
| Communication assessment tool | ||||||||
| Baseline | 152 | 4.53 (0.69) | 150 | 4.35 (0.70) | 148 | 4.49 (0.76) | .68 | .45 |
| Follow-up | 141 | 4.57 (0.68) | 142 | 4.52 (0.74) | 134 | 4.53 (0.71) | ||
| Change | 141 | 0.03 (0.68) | 142 | −0.02 (0.65) | 134 | 0.01 (0.75) | ||
Abbreviation: SMBG, self-monitoring of blood glucose.
Test comparing all 3 groups from ANCOVA model controlling for site, baseline scale score, baseline hemoglobin A1c, prior use of SMBG, duration of T2DM, baseline antihyperglycemic treatment, age, race/ethnicity, health literacy, and number of baseline comorbidities.
Contrast test from same ANCOVA model comparing average of testing groups with no testing group.
Sensitivity Analyses
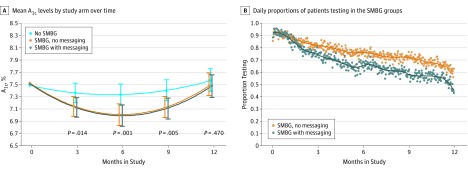
In per-protocol and last observation carried forward analyses, results were not notably different from those in the primary analyses. We did find evidence that mean hemoglobin A1c values differed across groups over time. At 6 months, the estimated mean hemoglobin A1c difference between the testing arms and the no testing arm was −0.33% (95% CI, −0.54% to -0.12%; P = .002). By 12 months, the mean differences between groups are similar to the primary analysis and do not show a significant difference. (Figure 2A).
Figure 2. Mean Hemoglobin A1c by Study Arm Over Time and Daily Proportions of Patients Testing in the SMBG Groups.
A, Model-estimated mean hemoglobin A1c values obtained by fitting a quadratic polynomial regression with linear mixed models using all observed hemoglobin A1c values, including those at interim visits, but excluding any following insulin use. The model included 1875 total hemoglobin A1c measurements from 450 patients; only 10 patients contributed no interim hemoglobin A1c measurements and the median number was 4. The intervals represent pointwise 95% CIs for each group, and the P values compare the average of the SMBG groups with the no SMBG group. B, Daily proportions of patients in the SMBG groups uploading a result with the meter on each study day. Lines represent locally weighted smoothing using local quadratic polynomials across the observed proportions. SMBG Indicates self-monitoring of blood glucose.
Effect Modification
In analyses exploring potential for effect modification of prespecified subgroups (prior experience using SMBG, T2DM duration, baseline glycemic control, baseline insulin secretagogue use, age, race/ethnicity, health literacy, and number of baseline comorbidities), there were no significant interactions for glycemic control. For the HRQOL physical component score, we did identify a significant interaction by race (P = .02); African Americans in the SMBG with messaging group reported significantly lower HRQOL than the no testing group, but the same was not true for the SMBG without messaging group (estimated adjusted mean differences of SF-36 physical component score for African Americans: SMBG with messaging vs no SMBG, −2.91 points; 95% CI, −5.69 to −0.13; SMBG vs no SMBG, 0.78 points; 95% CI, −1.91 to 3.47) (eFigures 1-3 in Supplement 2).
Testing Compliance
Compliance dropped consistently in both SMBG groups, with a larger initial decrease after 1 month in the SMBG with messaging arm (Figure 2B). In the no SMBG arm, 36 (23.7% ) patients reported that they tested a few times per month or more, 2 (1%) tested once per month, and 13 (8.5%) tested less than once per month during the study.
Safety and Adverse Events
The following adverse events occurred during the study: 0 fingerstick infections, 1 severe hypoglycemia (secondary to urosepsis, recurrent bladder neoplasm, and acute kidney injury), 62 hospitalizations (no difference by arm), and 2 deaths (1 during cardiac surgery and 1 owing to amyotrophic laterals sclerosis). None of the adverse events were adjudicated to be study-related.
Discussion
After 1 year, we identified no clinically or statistically significant differences in glycemic control or HRQOL between patients who performed once-daily SMBG compared with those who did not perform SMBG. The addition of instant tailored feedback messages via a meter did not improve glycemic control. This null result occurred despite training participants and primary care clinicians on the use and interpretation of the meter results. These findings align with earlier studies and a group that reinforce the limited utility of SMBG in patients with non–insulin-treated T2DM. Surprisingly, SMBG has remained a cornerstone in the clinical management of non–insulin-treated T2DM, in part fueled by other studies and groups supporting glycemic control with SMBG. As the first large pragmatic US trial of SMBG, our findings provide evidence to guide patients and clinicians making important clinical decisions about routine blood glucose monitoring. Health care clinicians are typically divided on this issue; most universally either do or do not recommend SMBG monitoring. In addition, patient testing preferences are variable; in our study, patient testing preference at baseline was split. Based on these findings, patients and clinicians should engage in dialogue regarding SMBG with the current evidence suggesting that SMBG should not be routine for most patients with non–insulin-treated T2DM. Our study was not powered to determine effectiveness in certain clinical situations, such as initiation of new medication or medication dose changes. Patients and clinicians should consider each situation as they determine whether to test or not to test.
Patients were drawn from primary care practices; where most patients with T2DM receive their care. Most were on uncomplicated medical therapies including metformin (80%) and sulphonylureas (36%) and carried the diagnosis of T2DM for a median of 6 years. Given that only 66 (15%) patients had T2DM for a year or less, it is not surprising that most were experienced with SMBG (338, 75%) at baseline. In addition, compliance with testing showed progressive attrition in both SMBG monitoring groups. Although not a primary outcome, this may explain the statistically significant improvements in hemoglobin A1c levelsinitially seen between the testing and nontesting arms in the early months, but no significance at the primary outcome of 12 months. It is possible that the intervention was off putting in some way causing user fatigue or provided false reassurance.
Proponents of routine SMBG have cited evidence that this testing approach is useful for patients with newly diagnosed diabetes or patients with poorer glycemic control. Although disease duration, experience using SMBG, baseline glycemic control, antihyperglycemic treatment, age, race, health literacy, and number of comorbidities made no discernable difference in glycemic control at 52 weeks, absence of evidence is not evidence of absence. This trial was not powered for secondary analyses. Only race was significant by interaction testing for HRQOL physical component score; African Americans in the SMBG with messaging group had significantly lower scores. Given multiple comparisons across groups, the finding may be spurious.
Incorporating technology into self-management activities has been touted as potentially transformative for patients, and to date some smaller studies support this notion. However, our findings do not. It is possible that the enhancement of SMBG with one-way messaging back to the patient does not adequately engage patients. This notion is supported by the sensitivity analyses that showed that over the first 6 months glycemic control improved for all patients engaging in SMBG regardless of messaging type. However, during months 6 through 12, improvements in glycemic control regressed back to baseline. A more interactive approach or the use of 2-way messaging between the patient and physician may improve the durability of this approach.
Although designed with an eye toward the real-world clinical setting, our study team did not engage with the patients beyond the baseline visit. Clinicians likewise had minimal interaction with the study team. Thus, we do not have data on what the clinicians did with the summary of blood glucose results. More active engagement of both patients and clinicians may have improved patient outcomes, although this would have diminished the pragmatic nature of this study. Most (338, 75%) patients had some experience with SMBG at baseline and 161 (36%) were taking oral hypoglycemics. Prior trials of SMBG were heterogeneous; many did not describe SMBG use at baseline.
Limitations
Although our resultant population is more of a test of continuing monitoring, rather than initiating monitoring, the question remains equally relevant. The population included were willing to be randomized; this may not reflect the typical population of patients with T2DM. In addition, not all patients adhered to the group to which they were assigned; however, per-protocol analyses were not notably different from the intent-to-treat analyses. There is also a possibility that those participating might be generally good at self-care, so an automated system may add less than in other populations. Because our population included patients with T2DM not using insulin, these results cannot be generalized to insulin users. Furthermore, participating primary care practices were affiliated with a single health care system, though patients were typical of those found in primary care nationally.
Conclusions
In patients with non–insulin-treated T2DM, there were no clinically or statistically significant differences at 1 year in glycemic control or HRQOL between patients who performed SMBG compared with those who did not perform SMBG. These findings suggest that glucose monitoring in patients with non–insulin-treated T2DM should not be routine.
Trial Protocol
eMonitor. Trial Group Members List
eFigure 1. Forest Plot of Prespecified Groups and A1c
eFigure 2. Forest Plot of Prespecified Groups and HRQOL SF-36 Physical Component Score
eFigure 3. Forest Plot of Prespecified Groups and HRQOL SF-36 Mental Component Score
eTable1. Summary of Glycemia Medication Usage
eTable2. Sample Tailored Meter Messages
References
- 1.American Diabetes Association 5. Glycemic targets. Diabetes Care. 2016;39(suppl 1):S39-S46. [DOI] [PubMed] [Google Scholar]
- 2.Allemann S, Houriet C, Diem P, Stettler C. Self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: a systematic review and meta-analysis. Curr Med Res Opin. 2009;25(12):2903-2913. [DOI] [PubMed] [Google Scholar]
- 3.Clar C, Barnard K, Cummins E, Royle P, Waugh N. Aberdeen Health Technology Assessment Group . Self-monitoring of blood glucose in type 2 diabetes: systematic review. Health Technol Assess. 2010;14(12):1-140. [DOI] [PubMed] [Google Scholar]
- 4.Farmer AJ, Perera R, Ward A, et al. Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ. 2012;344:e486-e486. [DOI] [PubMed] [Google Scholar]
- 5.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database of Systematic Reviews. http://onlinelibrary.wiley.com.libproxy.lib.unc.edu/doi/10.1002/14651858.CD005060.pub3/full. Login required. Accessed April 9, 2017. [DOI] [PMC free article] [PubMed]
- 6.Poolsup N, Suksomboon N, Rattanasookchit S. Meta-analysis of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients: an update. Diabetes Technol Ther. 2009;11(12):775-784. [DOI] [PubMed] [Google Scholar]
- 7.Towfigh A, Romanova M, Weinreb JE, et al. Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manag Care. 2008;14(7):468-475. [PubMed] [Google Scholar]
- 8.Wang J, Zgibor J, Matthews JT, Charron-Prochownik D, Sereika SM, Siminerio L. Self-monitoring of blood glucose is associated with problem-solving skills in hyperglycemia and hypoglycemia. Diabetes Educ. 2012;38(2):207-218. [DOI] [PubMed] [Google Scholar]
- 9.Barnett AH, Krentz AJ, Strojek K, et al. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen: a multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab. 2008;10(12):1239-1247. [DOI] [PubMed] [Google Scholar]
- 10.Durán A, Martín P, Runkle I, et al. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2(3):203-211. [DOI] [PubMed] [Google Scholar]
- 11.Guerci B, Drouin P, Grangé V, et al. ; ASIA Group . Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29(6):587-594. [DOI] [PubMed] [Google Scholar]
- 12.Schwedes U, Siebolds M, Mertes G. SMBG Study Group . Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25(11):1928-1932. [DOI] [PubMed] [Google Scholar]
- 13.Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med. 2005;118(4):422-425. [DOI] [PubMed] [Google Scholar]
- 14.Farmer AJ, Wade AN, French DP, et al. Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial. Health Technol Assess. 2009;13(15):iii-iv, ix-xi, 1-50. [DOI] [PubMed] [Google Scholar]
- 15.Kleefstra N, Hortensius J, Logtenberg SJJ, et al. Self-monitoring of blood glucose in tablet-treated type 2 diabetic patients (ZODIAC). Neth J Med. 2010;68(1):311-316. [PubMed] [Google Scholar]
- 16.Muchmore DB, Springer J, Miller M. Self-monitoring of blood glucose in overweight type 2 diabetic patients. Acta Diabetol. 1994;31(4):215-219. [DOI] [PubMed] [Google Scholar]
- 17.O’Kane MJ, Bunting B, Copeland M, Coates VE. ESMON study group . Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336(7654):1174-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A; Diabetes Glycaemic Education and Monitoring Trial Group . Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008;336(7654):1177-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonomo K, De Salve A, Fiora E, et al. Evaluation of a simple policy for pre- and post-prandial blood glucose self-monitoring in people with type 2 diabetes not on insulin. Diabetes Res Clin Pract. 2010;87(2):246-251. [DOI] [PubMed] [Google Scholar]
- 20.Polonsky WH, Fisher L, Schikman CH, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34(2):262-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher L, Polonsky WH, Parkin CG, Jelsovsky Z, Petersen B, Wagner RS. The impact of structured blood glucose testing on attitudes toward self-management among poorly controlled, insulin-naïve patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;96(2):149-155. [DOI] [PubMed] [Google Scholar]
- 22.Selby JV, Forsythe L, Sox HC. Stakeholder-Driven Comparative Effectiveness Research: An Update From PCORI. JAMA. 2015;314(21):2235-2236. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. [PubMed] [Google Scholar]
- 24.Welch GW, Jacobson AM, Polonsky WH. The Problem Areas in Diabetes Scale: an evaluation of its clinical utility. Diabetes Care. 1997;20(5):760-766. [DOI] [PubMed] [Google Scholar]
- 25.Herschbach P, Duran G, Waadt S, et al. Psychometric properties of the Questionnaire on Stress in Patients with Diabetes–Revised (QSD-R). Health Psychol. 1997;16(2):171-174. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RM, Fitzgerald JT, Gruppen LD, Funnell MM, Oh MS. The Diabetes Empowerment Scale-Short Form (DES-SF). Diabetes Care. 2003;26(5):1641-1642. [DOI] [PubMed] [Google Scholar]
- 27.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943-950. [DOI] [PubMed] [Google Scholar]
- 28.Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7(5):445-451. [DOI] [PubMed] [Google Scholar]
- 29.Makoul G, Krupat E, Chang CH. Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67(3):333-342. [DOI] [PubMed] [Google Scholar]
- 30.Dunnett CW, Tamhane AC. Step-up multiple testing of parameters with unequally correlated estimates. Biometrics. 1995;51(1):217-227. [PubMed] [Google Scholar]
- 31.Weiss BD, Mays MZ, Martz W, et al. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3(6):514-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choosing Wisely: Society of General Internal Medicine. http://www.choosingwisely.org/clinician-lists/society-general-internal-medicine-daily-home-finger-glucose-testing-type-2-diabetes-mellitus/. Accessed February 10, 2017.
- 33.American Diabetes Association 6. Glycemic Targets. Diabetes Care. 2017;40(suppl 1):S48-S56. [DOI] [PubMed] [Google Scholar]
- 34.Czupryniak L, Barkai L, Bolgarska S, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe—recommendations from the international Central-Eastern European expert group. Diabetes Technol Ther. 2014;16(7):460-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kesavadev J, Sadikot S, Wangnoo S, et al. Consensus guidelines for glycemic monitoring in type 1/type 2 & GDM. Diabetes Metab Syndr. 2014;8(3):187-195. [DOI] [PubMed] [Google Scholar]
- 36.Chomutare T, Fernandez-Luque L, Arsand E, Hartvigsen G. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res. 2011;13(3):e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman ND, Woodley PD. Self-management support interventions that are clinically linked and technology enabled: can they successfully prevent and treat diabetes? J Diabetes Sci Technol. 2011;5(3):798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Census Bureau 2009-2013 5-Year American Community Survey. http://www.census.gov/data/developers/updates/acs-5-yr-summary-available-2009-2013.html. 2014. Accessed November, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMonitor. Trial Group Members List
eFigure 1. Forest Plot of Prespecified Groups and A1c
eFigure 2. Forest Plot of Prespecified Groups and HRQOL SF-36 Physical Component Score
eFigure 3. Forest Plot of Prespecified Groups and HRQOL SF-36 Mental Component Score
eTable1. Summary of Glycemia Medication Usage
eTable2. Sample Tailored Meter Messages