Key Points
Question
Does oral biotin supplementation interfere with hormone and nonhormone assays that use biotinylation in their design?
Findings
In this nonrandomized crossover study of 6 healthy adults (2 women, 4 men), 10 mg/d of biotin ingested for 1 week was associated with potentially clinically important assay interferences in some but not all biotinylated hormone and nonhormone assays studied.
Meaning
Oral biotin use may be associated with false hormone and nonhormone assay results.
Abstract
Importance
Biotinylated antibodies and analogues, with their strong binding to streptavidin, are used in many clinical laboratory tests. Excess biotin in blood due to supplemental biotin ingestion may affect biotin-streptavidin binding, leading to potential clinical misinterpretation. However, the degree of interference remains undefined in healthy adults.
Objective
To assess performance of specific biotinylated immunoassays after 7 days of ingesting 10 mg/d of biotin, a dose common in over-the-counter supplements for healthy adults.
Design, Setting, and Participants
Nonrandomized crossover trial involving 6 healthy adults who were treated at an academic medical center research laboratory
Exposure
Administration of 10 mg/d of biotin supplementation for 7 days.
Main Outcomes and Measures
Analyte concentrations were compared with baseline (day 0) measures on the seventh day of biotin treatment and 7 days after treatment had stopped (day 14). The 11 analytes included 9 hormones (ie, thyroid-stimulating hormone, total thyroxine, total triiodothyronine, free thyroxine, free triiodothyronine, parathyroid hormone, prolactin, N-terminal pro-brain natriuretic peptide, 25-hydroxyvitamin D) and 2 nonhormones (prostate-specific antigen and ferritin). A total of 37 immunoassays for the 11 analytes were evaluated on 4 diagnostic systems, including 23 assays that incorporated biotin and streptavidin components and 14 assays that did not include biotin and streptavidin components and served as negative controls.
Results
Among the 2 women and 4 men (mean age, 38 years [range, 31-45 years]) who took 10 mg/d of biotin for 7 days, biotin ingestion–associated interference was found in 9 of the 23 (39%) biotinylated assays compared with none of the 14 nonbiotinylated assays (P = .007). Results from 5 of 8 biotinylated (63%) competitive immunoassays tested falsely high and results from 4 out of 15 (27%) biotinylated sandwich immunoassays tested falsely low.
Conclusions and Relevance
In this preliminary study of 6 healthy adult participants and 11 hormone and nonhormone analytes measured by 37 immunoassays, ingesting 10 mg/d of biotin for 1 week was associated with potentially clinically important assay interference in some but not all biotinylated assays studied. These findings should be considered for patients taking biotin supplements before ordering blood tests or when interpreting results.
Trial Registration
clinicaltrials.gov Identifier: NCT03034707
This nonrandomized crossover trial evaluated associations between biotin supplement ingestion and the accuracy of common diagnostic assays that rely on biotinylation, such as tests of thyroid function, vitamin D, and prostate specific antigen.
Introduction
Inaccuracy of laboratory medicine diagnostic tests may be associated with ingestion of over-the-counter vitamin and herbal supplements. One such example is interference associated with biotin. Biotin (vitamin B7), a water-soluble vitamin found in meat, fish, eggs, and dairy, serves as a catalyst for carboxylase enzymes important in macronutrient metabolism. Biotin supplements, especially very large doses (eg, 10-15 mg/d, or 333-fold greater than the dietary recommendation of 30 µg/d) have become popular for presumptive health benefits such as stimulating hair growth and treating certain medical conditions including biotinidase deficiency, diabetes, lipid disorders, and diabetic peripheral neuropathy. Ingestion of up to 300 mg/d may be beneficial for multiple sclerosis. Taking high-doses of biotin may result in inaccurate laboratory results because biotin is commonly used, in the form of biotinylated antibodies or analogues, as a critical component in immunoassays. These assays exploit the strong, stable, and specific binding between biotin and streptavidin to amplify the assay sensitivity for detecting low analyte levels. Excessive biotin in a blood sample can compete with biotinylated components in the assay, potentially falsely decreasing results in sandwich immunoassays or falsely increasing results in competitive immunoassays (eFigures 1 and 2 in the Supplement).
Due to a lack of systematic studies, little is known about whether or how performance of specific biotinylated immunoassays may be associated with biotin ingestion at doses common in over-the-counter supplements (10 mg/d) in healthy adults. Therefore, this study was designed to assess the association of short-term biotin ingestion for 7 days with performance of assays that measure 11 hormone and nonhormone analytes: thyroid-stimulating hormone (TSH), total thyroxine (T4), total triiodothyronine (T3), free T4, free T3, intact parathyroid hormone (PTH), prolactin, N-terminal pro-brain natriuretic peptide (NT-proBNP), 25-hydroxyvitamin D (25-OHD) in 6 healthy adults, and ferritin and prostate-specific antigen (PSA) in the 4 men, using 4 assay systems.
Methods
Study Design
This study was approved by the institutional review board at the University of Minnesota. Six healthy adults responded to study announcement fliers, gave informed consent before participation, and were not compensated. Exclusion criteria including but not limited to conditions that potentially affect biotin or hormones were being pregnant or lactating, having known thyroid disease, undergoing thyroid hormone treatment, ingesting over-the-counter dietary or nutritional supplements (excluding standard multivitamin preparations containing no more than 100% of the daily value for biotin and calcium), working the night shift, smoking, being treated with anticonvulsant medicine, or lacking the capacity to consent. Participants were asked to stop taking multivitamins 2 weeks before study participation.
Intervention
Participants were instructed to take 10 mg/d of biotin (Nature Made) at the same time each morning for 7 days. Blood specimens were collected by venipuncture at baseline prior to starting biotin, after 1 week of biotin supplementation (day 7), and 1 week after participants stopped taking biotin (day 14). On the last day of treatment (day 7), the blood specimen was drawn approximately 2 hours after taking their last dose. Blood specimens were labeled with participant study identification number and date, promptly centrifuged and processed to produce serum sample aliquots that were stored up to a year at −70°C until further analysis.
Outcomes
Eighteen serum samples, collected from the 6 participants at the 3 time points, were each split into 4 aliquots and sent for testing at 4 clinical laboratories using different diagnostic assay systems: Johns Hopkins Medical Institutions used Roche cobas e602 for measurement of all 9 hormones and ferritin; Children’s Mercy Hospital used the OCD Vitros 5600 for TSH, PTH, total T4, total T3, free T4, NT-proBNP, and ferritin and Siemens Immulite 2000 for prolactin. The University of Minnesota Medical Center used Siemens Vista Dimension 1500 for TSH, PTH, total T4, free T4, free T3, NT-proBNP, ferritin, and PSA and Siemens Advia Centaur XP for total T3 and PTH; Boston Medical Center used Abbott Architect 2000 for TSH, PTH, total T4, total T3, free T4, prolactin, 25-OHD, ferritin, and PSA. eTable 1 in the Supplement summarizes information on the 37 assays for the 11 analytes evaluated on 4 systems: 23 incorporated biotin and streptavidin components and 14 did not include those components, serving as negative controls. Analytical imprecision data for these 37 assays are included in eTable 2 in the Supplement. Two immunoassay principals were used (eFigures 1 and 2 in the Supplement): the sandwich immunoassay for TSH, PTH, prolactin, NT-proBNP, PSA, and ferritin and the competitive immunoassay for total T4, total T3, free T4, free T3, and 25-OHD.
Four analytes (NT-proBNP, ferritin, 25-OHD, and PSA) were performed as a second tier after the initial analysis of 7 analytes (TSH, intact PTH, total T4, total T3, free T4, free T3, prolactin). As a result, the sample size for some tests was less than 6 due to an insufficient volume in the blood sample or sex differences in the reference range. Prostate-specific antigen and ferritin were measured for only the 4 men.
Assays were performed for each analyte as a single batch, on automated systems, by clinical laboratory technologists blinded to the nature of the study. Serum biotin was measured by the Cambridge Biomedical Research Group (Boston, Massachusetts) using a microbial growth assay. Serum calcium was measured at Johns Hopkins Medical Institutions with the Roche cobas c701 chemistry analyzer.
Statistical Analysis
The study was powered to detect changes larger than the expected assay coefficient of variations (imprecisions) at normal reference levels for common hormone tests (eTable 2 in the Supplement). Because significant biotin ingestion–associated changes were observed in some assays studied, study recruitment was terminated after data analysis from the first 6 participants.
Analyte levels below or above their reportable ranges were assigned the following values: for Ortho Vitros NT-proBNP, 11 pg/mL was used for levels reported to be lower than 11.1 pg/mL; for both Siemens Vista Dimension and Roche cobas NT-proBNP, 4 pg/mL was used for levels reported that were less than 5 pg/mL. For biotin more than 3600 pg/mL, 3601 pg/mL was used. These value assignments would underestimate any biotin interference that was present. (To convert biotin from pg/mL to nmol/L, multiply by 0.00409; prolactin from ng/mL to pmol/L, multiply by 43.478; free T3 from pg/mL to pmol/L, multiply by 1.54; total T3 from ng/mL to nmol/L, multiply by 1.54; free T4 from ng/dL to pmol/L, multiply by 12.871; total T4 from µg/dL to nmol/L, multiply by 12.871.)
Each combination of an analyte and a system was analyzed separately with repeated measures of an analysis of variance (ANOVA) (mixed linear model), for which the random effect was a participant and the within-participant fixed effect was time (day of study). The primary analysis compared study day 7, the last day participants took biotin, with the mean of baseline and study day 14, before participants took biotin and after they stopped taking biotin, using a contrast in the ANOVA. To test this contrast for each analyte and system, P < .05 was the criterion for statistical significance. For each combination of an analyte and a system, we also present comparisons of pairs of times using the Tukey honest significant difference post hoc test. Data are reported as mean and 95% CIs. The Fisher exact test was used to compare biotin interference outcomes between the biotinylated assays and nonbiotinylated assays. All tests were 2-sided. All analyses used JMP Pro v13 (SAS Institute Inc).
Results
Characteristics of the Study Population
Six healthy adults enrolled in the study, 2 women and 4 men, with a mean age of 38 years (range, 31-45 years). Baseline analyte concentrations for the 6 participants were within the manufacturer’s reference ranges for 7 analytes measured by 29 assays, except for 4 analytes measured by 8 assays: (1) PTH by OCD Vitros 5600 and Siemens Advia Centaur XP; (2) total T4 by Abbott Architect; (3) prolactin by Roche cobas e602, Siemens Immulite 2000, and Abbott Architect; and (4) ferritin by the Roche cobas e602 and Abbott Architect. In each case, this involved no more than 1 or 2 participants. None of the participants had abnormal baseline results across all systems for a given analyte.
Biotin Ingestion and Assay Performance
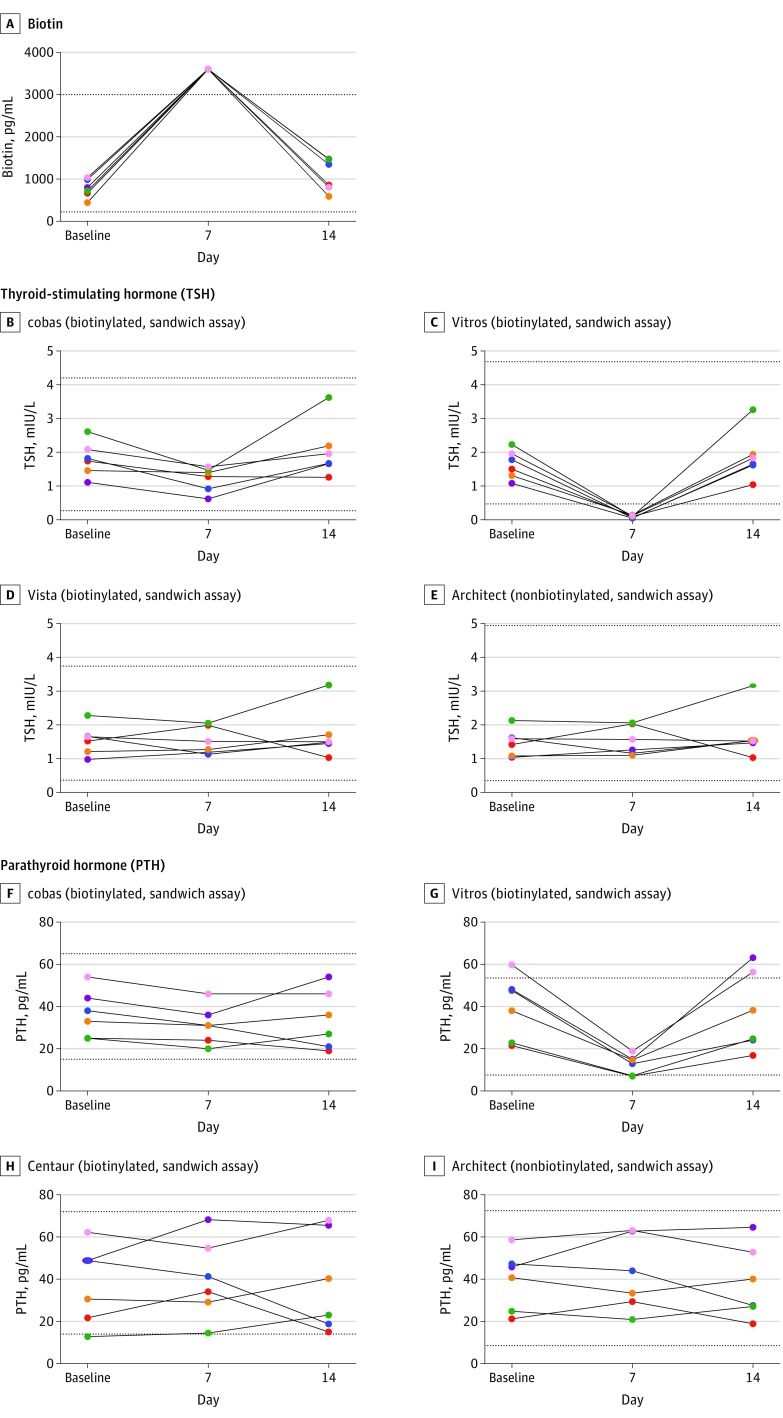
The mean baseline serum biotin concentration was 774 pg/mL (95% CI, 554-1004 pg/mL) compared with a mean study day-7 concentration of more than 3600 pg/mL (95% CI, 3601-3601, mg/mL; P value <.001) for all 6 participants (Figure 1A). On study day 14, the mean biotin concentration decreased to 1090 pg/mL (95% CI, 687-1493 pg/mL). Baseline and study day 14 biotin concentrations did not differ statistically (eTable 4 in the Supplement).
Figure 1. Concentrations of Serum Biotin, Thyroid-Stimulating Hormone (TSH), and Parathyroid Hormone (PTH) From the 6 Study Participants .
Biotin ingestion of 10 mg/d for 7 days was associated with significant increased biotin concentrations (P < .001), as well as falsely decreased results in the Roche cobas e602 TSH (P = .006), OCD Vitros 5600 TSH (P < .001), and OCD Vitros 5600 PTH (P < .001) assays. A unique color is used for each participant across all panels. Dotted lines represent the lower and the upper reference range for the assay. Architect indicates Abbott Architect 2000; Centaur, Siemens Centaur XP; cobas, Roche cobas e602; Vista, Siemens Vista Dimension 1500; and Vitros, OCD Vitros 5600. For SI conversion factors, see the Methods section.
Biotin ingestion was associated with falsely decreased Roche cobas e602 TSH levels by a mean of 0.72 mIU/L (95% CI, −1.13 to −0.32 mIU/L; P = .006), a 37% reduction from baseline, although all results remained within the euthyroid reference range (Figure 1B). The interference was much greater when measured by the Vitros 5600 TSH assay (Figure 1C) for which TSH significantly decreased by a mean of 1.67 mIU/L (95% CI, −2.08 to −1.26 mIU/L; P < .001), a 94% reduction from baseline, with all results falsely decreased to below the reference range (ie, <0.15 mIU/L; reference range, 0.47-4.68 mIU/L).
Biotin ingestion was associated with significantly decreased OCD Vitros PTH results by a mean of 25.8 pg/mL (95% CI, −34.8 to −16.8 pg/mL; P < .001), a 61% reduction from baseline. In 2 participants with normal baseline PTH concentrations, biotin ingestion was associated with falsely decreased PTH results, slightly below the lower limit of the reference range at 7.0 pg/mL and 7.2 pg/mL (reference range, 7.5-53.5 pg/mL). Serum calcium concentrations in all participants remained stable within the reference range (Table 1).
Table 1. Concentrations of Biotin, Thyroid-Stimulating Hormone, and Parathyroid Hormone Across the Study Assay Systems .
| Biotin Use | Mean of Analyte Concentrations (95% CI) | Mean of Day 7 Difference From the Mean of Baseline and Day 14 (95% CI)a | Day 7 Difference P Value | Mean of Analyte Concentrations (95% CI) | Mean of Day 7 Difference From the Mean of Baseline and Day 14 (95% CI)a | Day 7 Difference P Value | ||
|---|---|---|---|---|---|---|---|---|
| Biotin, pg/mL | Calcium, mg/dLb | |||||||
| Baseline | Without | 774 (554 to 1004) | 2669 (2440 to 2898) | <.001 | 9.1 (9.0 to 9.3) | 0.03 (–0.13 to 0.19) | .69 | |
| Day 7 | With | 3601 (3601 to 3601) | 9.1 (8.8 to 9.5) | |||||
| Day 14 | Without | 1090 (687 to 1493) | 9.1 (8.8 to 9.3) | |||||
| Thyroid-Stimulating Hormone, mIU/L | Parathyroid Hormone, pg/mL | |||||||
| cobas | ||||||||
| Baseline | Without | 1.80 (1.26 to 2.34) | –0.72 (–1.13 to –0.32) | .006 | 36.5 (24.6 to 48.4) | –3.83 (–0.47 to 1.80) | .21 | |
| Day 7 | With | 1.21 (0.83 to 1.59) | 31.3 (21.7 to 40.9) | |||||
| Day 14 | Without | 2.06 (1.19 to 2.93) | 33.8 (19.1 to 48.6) | |||||
| Vitros | ||||||||
| Baseline | Without | 1.64 (1.20 to 2.09) | –1.67 (–2.08 to –1.26) | <.001 | 39.6 (23.7 to 55.6) | –25.8 (–34.8 to –16.8) | <.001 | |
| Day 7 | With | 0.10 (0.06 to 0.13) | 12.6 (7.68 to 17.5) | |||||
| Day 14 | Without | 1.89 (1.11 to 2.67) | 37.2 (17.4 to 57.0) | |||||
| Vista | ||||||||
| Baseline | Without | 1.55 (1.08 to 2.02) | –0.11 (–0.49 to 0.26) | .56 | ||||
| Day 7 | With | 1.52 (1.10 to 1.95) | ||||||
| Day 14 | Without | 1.73 (0.94 to 2.51) | ||||||
| Architect | ||||||||
| Baseline | Without | 1.48 (1.06 to 1.90) | –0.06 (–0.44 to 0.31) | .74 | 39.8 (24.8 to 54.7) | 3.13 (–4.21 to 10.5) | .42 | |
| Day 7 | With | 1.53 (1.08 to 1.98) | 42.3 (23.7 to 60.8) | |||||
| Day 14 | Without | 1.71 (0.93 to 2.48) | 38.5 (20.2 to 56.8) | |||||
| Centaur | ||||||||
| Baseline | Without | 37.5 (17.7 to 57.2) | 2.37 (–7.88 to 12.6) | .66 | ||||
| Day 7 | With | 40.3 (20.3 to 60.3) | ||||||
| Day 14 | Without | 38.4 (13.7 to 63.1) | ||||||
Abbreviations: See Figure legends for full names of the assay systems.
SI conversion factors: To convert biotin from pg/mL to nmol/L, multiply by 0.00409; calcium from mg/dL to mmol/L, multiply by 0.25.
Each analysis was a repeated measures analysis of variance (ANOVA; mixed linear model), for which the random effect was a participant and the within-participant fixed effect was time. The primary comparison was study day 7 vs the mean of the baseline and study day 14 using a contrast in the ANOVA, ie: study day 7 −(½ baseline + study day 14). Data are presented as the absolute mean difference of study day 7 from the mean of baseline and study day 14. The units for the study day-7 difference are the same as those of analyte concentrations.
Calcium values were included because they are necessary to interpret parathyroid hormone results.
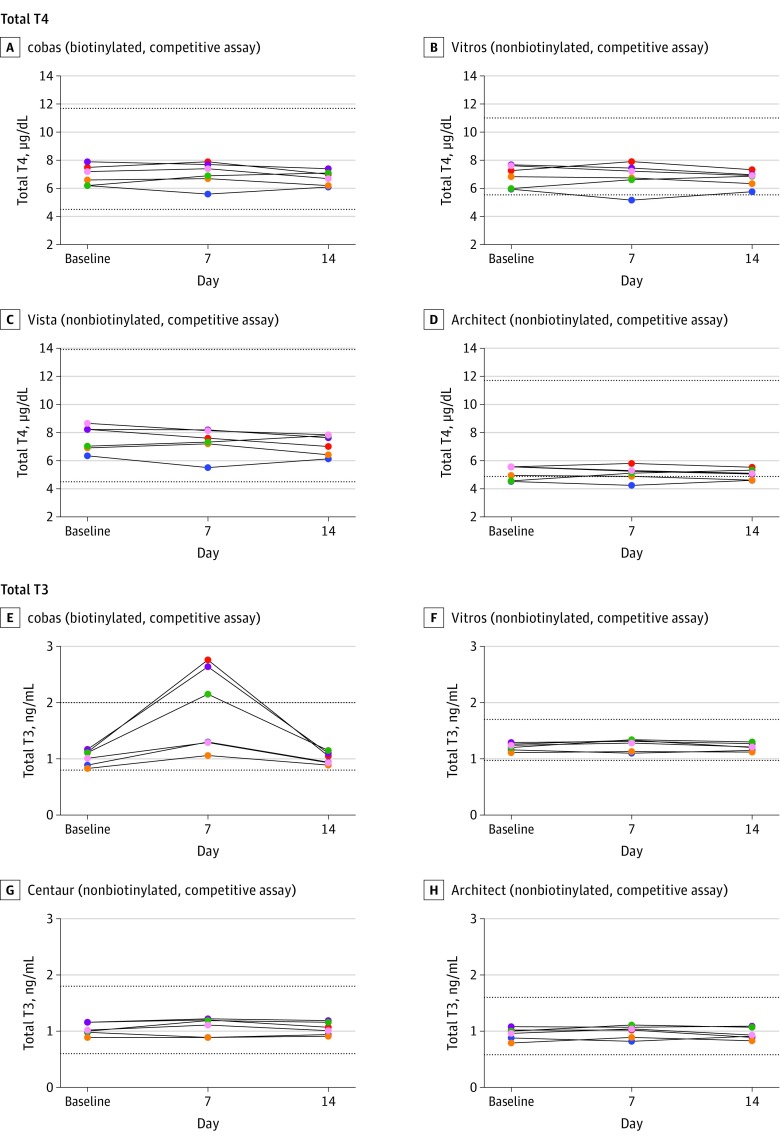
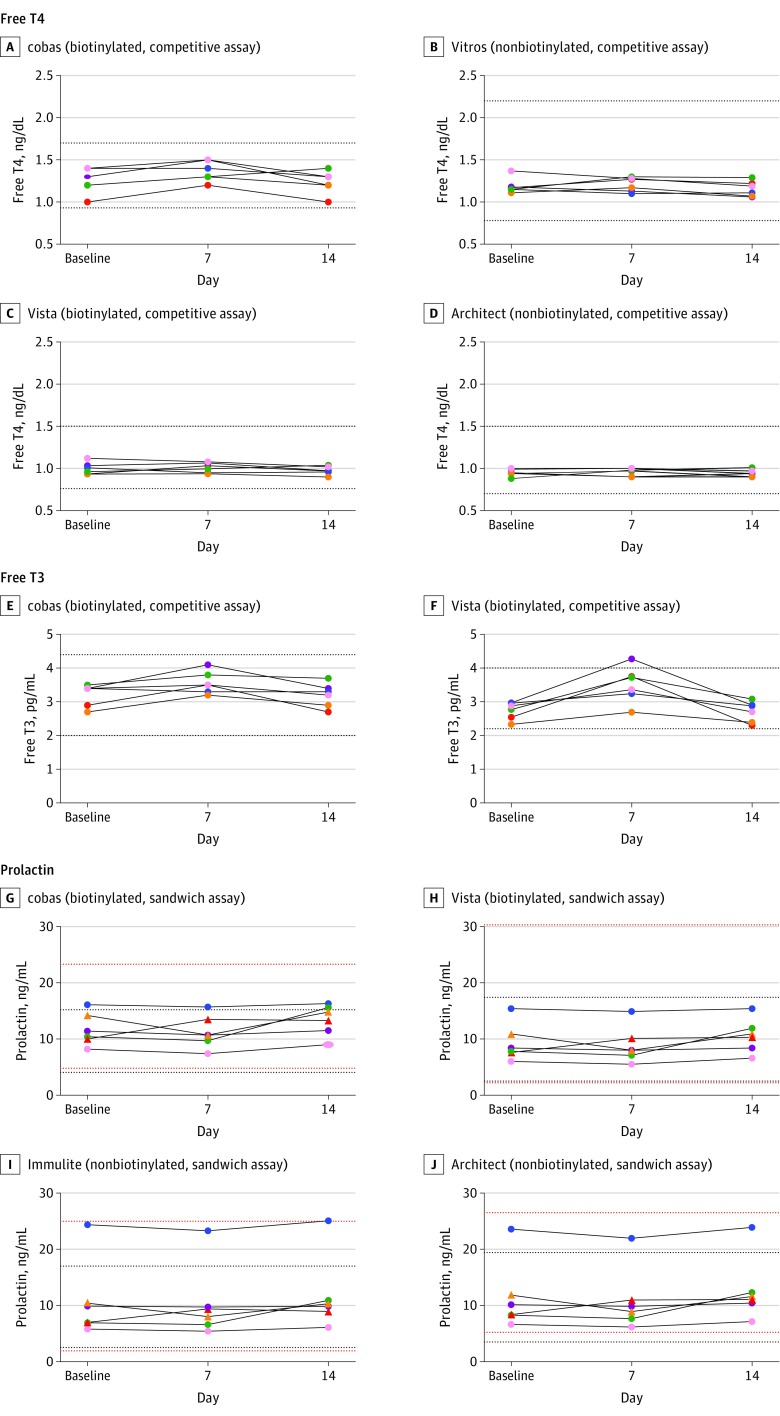
Biotin ingestion was associated with statistically significant false increases in 4 assays: 3 Roche cobas e602 assays measuring total T3, free T3, and free T4; and the Siemens Vista Dimension 1500 measuring free T3 (Figure 2 and Figure 3). Roche cobas e602 total T3 concentrations falsely increased by a mean of 0.85 ng/mL (95% CI, 0.49-1.22 ng/mL; P = .001), whereas Siemens Vista free T3 concentration falsely increased by a mean of 0.78 pg/mL (95% CI, 0.50-1.06 pg/mL; P < .001; Table 2). In 3 participants, the Roche cobas e602 total T3 results and in 1 participant Siemens Vista free T3 result were higher than their respective reference ranges. Table 3 shows the results for prolactin, which did not have biotin-associated changes.
Figure 2. Concentrations of Total Thyroxine (T4) and Total Triiodothyronine (T3) From the 6 Study Participants.
Taking 10 mg/d of biotin for 7 days was associated with falsely increased results from the Roche cobas e602 Total T3 (P =.001). A unique color is used for each participant across all panels. The dotted lines represent the lower and the upper reference range for each assay. Architect indicates Abbott Architect 2000; Centaur, Siemens Centaur XP; cobas, Roche cobas e602; Vista, Siemens Vista Dimension 1500; and Vitros, OCD Vitros 5600. For SI conversion factors, see the Methods section.
Figure 3. Concentrations of Free Thyroxine (T4), Free Triiodothyronine (T3), and Prolactin From the 6 Study Participants.
Taking 10 mg/d of biotin for 7 days was associated with falsely increased results from the Roche cobas e602 assay for free T4 (P= .01) and free T3 (P= .005) and from the Siemens Vista Dimension 1500 assay for free T3 (P< .001). A unique color is used for each participant across all panels. The dotted lines represent the lower and the upper reference range for each assay. For prolactin, 2 women were represented by triangles and 4 men by dots. Red dotted lines represent normal range of prolactin for women and black for men. Architect indicates Abbott Architect 2000; Centaur, Siemens Centaur XP; cobas, Roche cobas e602; Vista, Siemens Vista Dimension 1500; and Vitros, OCD Vitros 5600. For SI conversion factors, see the Methods section.
Table 2. Concentrations of Triiodothyronine (T3), Thyroxine (T4), Free T3, and Free T4 Across the Study Assay Systems.
| Biotin Use | Mean of Analyte Concentrations (95% CI) | Mean of Day 7 Difference From the Mean of Baseline and Day 14 (95% CI)a | Day 7 Difference P Value | Mean of Analyte Concentrations (95% CI) | Mean of Day 7 Difference From the Mean of Baseline and Day 14 (95% CI)a | Day 7 Difference P Value | |
|---|---|---|---|---|---|---|---|
| Total T4, µg/dL | Free T4, ng/dL | ||||||
| cobas | |||||||
| Baseline | Without | 6.93 (6.19 to 7.68) | 0.19 (−0.17 to 0.55) | .32 | 1.25 (1.09 to 1.41) | 0.13 (0.05 to 0.20) | .01 |
| Day 7 | With | 7.03 (6.15 to 7.91) | 1.37 (1.24 to 1.49) | ||||
| Day 14 | Without | 6.75 (6.21 to 7.29) | 1.23 (1.09 to 1.38) | ||||
| Vitros | |||||||
| Baseline | Without | 6.88 (6.07 to 7.69) | 0.06 (−0.32 to 0.44) | .77 | 1.19 (1.09 to 1.29) | 0.04 (−0.03 to 0.10) | .29 |
| Day 7 | With | 6.85 (5.85 to 7.84) | 1.21 (1.12 to 1.30) | ||||
| Day 14 | Without | 6.69 (6.11 to 7.28) | 1.16 (1.06 to 1.25) | ||||
| Vista | |||||||
| Baseline | Without | 7.57 (6.60 to 8.54) | –0.03 (–0.44 to 0.38) | .90 | 1.00 (0.92 to 1.07) | 0.02 (–0.02 to 0.06) | .31 |
| Day 7 | With | 7.33 (6.30 to 8.35) | 1.01 (0.95 to 1.07) | ||||
| Day 14 | Without | 7.14 (6.36 to 7.91) | 0.98 (0.92 to 1.03) | ||||
| Architect | |||||||
| Baseline | Without | 5.12 (4.59 to 4.65) | 0.02 (–0.25 to 0.28) | .90 | 0.95 (0.90 to 1.0) | 0.02 (–0.02 to 0.06) | .37 |
| Day 7 | With | 5.10 (4.55 to 5.64) | 0.97 (0.92 to 1.01) | ||||
| Day 14 | Without | 5.05 (4.64 to 5.43) | 0.94 (0.90 to 0.99) | ||||
| Total T3, ng/mL | Free T3, pg/mL | ||||||
| cobas | |||||||
| Baseline | Without | 1.02 (0.88 to 1.16) | 0.85 (0.49 to 1.22) | .001 | 3.22 (2.87 to 3.56) | 0.36 (0.17 to 0.55) | .005 |
| Day 7 | With | 1.87 (1.08 to 2.65) | 3.57 (3.22 to 3.92) | ||||
| Day 14 | Without | 1.01 (0.90 to 1.12) | 3.20 (2.82 to 3.58) | ||||
| Vitros | |||||||
| Baseline | Without | 1.21 (1.14 to 1.28) | 0.04 (–0.002 to 0.08) | .10 | |||
| Day 7 | With | 1.25 (1.14 to 1.36) | |||||
| Day 14 | Without | 1.21 (1.14 to 1.28) | |||||
| Centaur | |||||||
| Baseline | Without | 1.04 (0.92 to 1.15) | 0.04 (–0.01 to 0.09) | .18 | |||
| Day 7 | With | 1.08 (0.92 to 1.25) | |||||
| Day 14 | Without | 1.05 (0.93 to 1.17) | |||||
| Architect | |||||||
| Baseline | Without | 0.96 (0.85 to 1.06) | 0.04 (–0.01 to 0.09) | .18 | |||
| Day 7 | With | 0.99 (0.87 to 1.11) | |||||
| Day 14 | Without | 0.95 (0.84 to 1.06) | |||||
| Vista | |||||||
| Baseline | Without | 2.74 (2.47 to 3.01) | 0.78 (0.50 to 1.06) | <.001 | |||
| Day 7 | With | 3.51 (2.94 to 4.07) | |||||
| Day 14 | Without | 2.71 (2.39 to 3.03) | |||||
Abbreviations: See Figure legends for full names of the assay systems.
SI conversion factors: To convert total T3 from ng/mL to nmol/L, multiply by 1.54; total T4 from µg/dL to nmol/L, multiply by 12.871; free T3 from pg/mL to pmol/L, multiply by 1.54; free T4 from ng/dL to pmol/L, multiply by 12.871.
Each analysis was a repeated measures of analysis of variance (ANOVA; mixed linear model), for which the random effect was a participant and the within-participant fixed effect was the study day. The primary comparison was study 7 vs the mean of the baseline and study day 14 measures using a contrast in the ANOVA, ie: study day 7 −(½ baseline + day 14). Data are presented as the absolute mean difference of day 7 from the mean of baseline and day 14 (95% CI). The units for the day 7 difference are the same as those of analyte concentrations.
Table 3. Concentrations of Prolactin Across the Study Assay Systems .
| Biotin Use | Prolactin, ng/mL | Day 7 Difference P Value | ||
|---|---|---|---|---|
| Mean of Analyte Concentrations (95% CI) | Mean of Day 7 Difference From the Mean of Baseline and Day 14 (95% CI)a | |||
| cobas | ||||
| Baseline | Without | 11.7 (8.66 to 14.8) | –1.28 (–2.83 to 0.26) | .13 |
| Day 7 | With | 11.3 (8.22 to 14.3) | ||
| Day 14 | Without | 13.4 (10.5 to 16.3) | ||
| Vista | ||||
| Baseline | Without | 9.35 (5.82 to 12.9) | –1.03 (–2.26 to 0.19) | .13 |
| Day 7 | With | 8.93 (5.49 to 12.4) | ||
| Day 14 | Without | 10.6 (7.41 to 13.8) | ||
| Immulite | ||||
| Baseline | Without | 10.7 (3.45 to 18.0) | –0.89 (–2.02 to 0.24) | .15 |
| Day 7 | With | 10.40 (3.55 to 17.3) | ||
| Day 14 | Without | 11.9 (4.83 to 18.9) | ||
| Architect | ||||
| Baseline | Without | 11.4 (4.94 to 18.0) | –1.19 (–2.39 to 0.02) | .08 |
| Day 7 | With | 10.9 (4.94 to 16.8) | ||
| Day 14 | Without | 12.7 (6.68 to 18.8) | ||
Abbreviations: See Figure legends for full names of the assay systems.
SI conversion factors: To convert prolactin from ng/mL to pmol/L, multiply by 43.478.
Each analysis was a repeated measures of analysis of variance (ANOVA; mixed linear model), for which the random effect was a participant and the within-participant fixed effect was the study day. The primary comparison was study 7 vs the mean of the baseline and study day 14 measures using a contrast in the ANOVA, ie: study day 7 −(½ baseline + day 14). Data are presented as the absolute mean difference of day 7 from the mean of baseline and day 14 (95% CI). The units for the day 7 difference are the same as those of analyte concentrations.
eFigure 3 in the Supplement shows that biotin ingestion was associated with falsely reduced OCD Vitros 5600 NT-proBNP results by an average of more than 13.9 pg/mL (95% CI, −24.7 to −3.12 pg/mL; P = .03) to less than 11.1 pg/mL in all participants. The actual reduction was underestimated because results while participants were taking biotin were below the assay’s reportable range. Biotin ingestion was associated with falsely increased Roche cobas 25-OHD results by a mean of 9.25 ng/mL (95% CI, 5.72-12.8 ng/mL; P < .001) higher than the baseline (eTable 3 in the Supplement). None of the 11 analytes measured by 37 assays differed between baseline and day 14, except ferritin measured by the Siemens Vista Dimension (eTable 4 in the Supplement). Ferritin at day 7 of biotin treatment did not differ significantly from the mean of baseline and day 14 or from baseline alone in all 4 systems, supporting that biotin ingestion was not associated with the difference between baseline and day 14.
Biotin interference was not observed in any of the 14 nonbiotinylated assays (Table 4). Biotin interference was not observed in 14 of the 23 (61%) biotinylated assays; however, it was observed in 9 of the 23 (39%) biotinylated assays: falsely decreasing results in 4 sandwich immunoassays (4 of 15 [27%]); falsely increasing results in 5 competitive immunoassays (5 of 8 [63%]). Biotin interference outcomes were significantly different between biotinylated assays (9 of 23 [39%]) and nonbiotinylated assays (none) (Fisher exact test, P = .007).
Table 4. Summary of Predicted vs Observed Results of Biotin Interference Effectsa.
| Immunoassay Format | Predicted Direction of Biotin Interference | Analyzer | Analyte | Observed Direction of Biotin Interference |
|---|---|---|---|---|
| No Biotin Used in the Assay | ||||
| Competitive | Not affected | Architect | Free T4 Total T3 Total T4 |
Not affected |
| Vitros | Free T4 Total T3 Total T4 |
|||
| Centaur | Total T3 | |||
| Vista | Total T4 | |||
| Sandwich | Not affected | Architect | PSA PTH TSH Ferritin Prolactin |
Not affected |
| Immulite | Prolactin | |||
| Biotin Used in the Assay | ||||
| Competitive | Falsely high | Architect | 25-OHD | Not affected |
| cobas e602 | 25-OHD Free T3 Free T4 Total T3 |
Falsely high | ||
| Total T4 | Not affected | |||
| Vista | Free T3 | Falsely high | ||
| Free T4 | Not affected | |||
| Sandwich | Falsely low | Vitros | Ferritin | Not affected |
| NT-proBNP PTH TSH |
Falsely low | |||
| cobas e602 | Ferritin NT-proBNP PTH Prolactin |
Not affected | ||
| TSH | Falsely low | |||
| Centaur | PTH | Not affected | ||
| Vista | PSA Ferritin NT-proBNP TSH Prolactin |
Not affected | ||
Abbreviations: Free T3, free triiodothyronine; free T4, free thyroxine; NT-proBNP, N-terminal pro-brain natriuretic peptide; PSA, prostate-specific antigen; PTH, parathyroid hormone; TSH, thyroid-stimulating hormone; total T3,triiodothyronine; total T4, total thyroxine; 25-OHD, 25-hydroxyvitamin D. See Figure legends for full names of the assay systems.
Prediction of whether biotin ingestion–associated interference is absent or present is based on whether an immunoassay uses biotinylated components in the reagents and biotin-streptavidin binding in the assay design. Interference is predicted to be absent in nonbiotinylated assays that do not use biotinylated components in the assay reagents but present in biotinylated assays. Direction of the predicted interference is based on the immunoassay principle: sandwich or competitive. Biotin ingestion is predicted to be associated with falsely decreased results in sandwich biotinylated immunoassays and with falsely increased results in competitive biotinylated immunoassays. A 2-tailed Fisher exact test was used to compare interference outcomes, which were present in 9 of the 23 biotinylated assays and none of the 14 nonbiotinylated assays (P = .007).
Discussion
This study involving 6 healthy adults demonstrated that oral biotin was associated with potentially clinically important assay interference in some but not all biotinylated assays. Among the 23 biotinylated assays studied, biotin interference was of greatest clinical significance in the OCD Vitros TSH assay, where falsely decreased TSH concentrations (to <0.15 mU/L) could have resulted in misdiagnosis of thyrotoxicosis in otherwise euthyroid individuals. Likewise, falsely decreased OCD Vitros NT-proBNP, to lower than assay detection limits, could possibly result in failure to identify congestive heart failure. Because healthy study participants had normal baseline NT-proBNP, further study of patients with high baseline NT-proBNP concentration would be required to establish the effect of biotin interference on the diagnosis of heart failure. The smaller changes observed in other assays, namely OCD Vitros PTH; Roche cobas e602 TSH, total and free T3, free T4, and 25-OHD; and Siemens Vista free T3, although primarily producing false results within the reference range among participants while taking biotin, could lead to falsely normal or abnormal interpretation of the results for individuals starting from baseline levels closer to the reference range limits.
Many of the studied biotinylated assays were not affected by 7 days of biotin, despite what would have been predicted (Table 4) based on the assay biotin and streptavidin components. For some assays (eg, Roche cobas e602 PTH, total T4, free T4, and prolactin and Siemens Vista 1500 TSH, PTH, free T4, and prolactin), there are reports of biotin interference. For example, a falsely low Roche Elecsys PTH level of 48 ng/L was reported in a patient with hyperparathyroidism in chronic kidney disease (true PTH level 576 ng/L).
Potential reasons for discrepancies between predicted and actual biotin interference in an individual blood sample include inherent differential biotin interference tolerance among biotinylated assays owing to assay design, endogenous levels of free biotin and biotin metabolites present in the sample, the type of biotin supplement, the dose and duration of biotin ingestion, time of blood draws after the last dose, and the analyte concentration. Supraphysiologic doses of biotin may increase blood concentrations by 1.5 to 163 times higher than normal, depending on dose and measurement time after administration. Differential biotin interference tolerance among assays is likely due to the amounts of biotinylated antibodies or analogues used, the availability of streptavidin binding sites and areas in the assay reagents (eg, streptavidin-coated magnetic particles) (eFigures 1 and 2 in the Supplement), and possible effects from biotin metabolites.
The time required for patients to stop taking supplements with biotin to avoid assay interference appears to be variable and may depend on the patient population, time of blood collection relative to the last biotin dose, biotin dose, chronicity of biotin exposure, and half-life of free biotin and biotin metabolites in plasma. Maximal assay interference was demonstrated 2 hours after a single 30-mg biotin dose. Peak biotin blood concentration occurred 1.25 hours and 1.5 hours after a 100-mg and a 300-mg single dose, respectively, with half-life up to 18.8 hours following a single 300-mg dose. In contrast, a 1.8-hour half-life was reported from a 600-µg biotin dose. Biotin metabolite concentrations (ie, bisnorbiotin) were significantly higher following months of taking 100 mg of biotin 3 times a day than they were following a single 300-mg dose, suggesting that biotin metabolites are affected by chronicity of biotin exposure. Spiking studies showed interference at higher in vitro biotin concentrations than have been measured in vivo, indicating the importance of biotin metabolites. Biotin assay interference following discontinuation of biotin has been demonstrated at 24 hours and at 16 hours following a single 30-mg dose and 3 daily 100-mg doses, respecively. Falsely abnormal hormone levels returned to normal 3 days after ceasing to take 300 mg of biotin in 3 daily doses and 2 days after ceasing to take up to 300 mg of biotin. In pediatric populations, biotin interference was found 2 days after the last dose of biotin (10 mg/d for 4 days), and disappeared at a week in infants and young children receiving between 2 and 15 mg/kg/d. In the current study, mean biotin concentration returned to baseline and the biotin ingestion–associated interferences resolved 1 week after a 10-mg/d 7-day course.
Limitations
To our knowledge, the current study is the first to systematically assess the association of biotin ingestion (10 mg/d for 7 days) in healthy adults with performance of 37 assays that measure 11 analytes over 4 major diagnostic systems (Table 4). The study has several limitations. First, only healthy adults with mostly normal analyte concentrations were evaluated. Second, the study was neither randomized nor blinded to the investigators or participants, although it was blinded to the clinical laboratories. The study did not have a placebo group, but the crossover design and repeated measures analysis allowed each participant’s baseline values to serve as his/her own controls, making it more efficient (smaller sample size is needed) than a randomized design, because between-person variability in overall level of an analyte is eliminated. Third, because no formal dose-response pharmacokinetic study of biotin at various doses was performed, the minimal dose and duration required to alter assay results remains undetermined. Fourth, the sample size was small; power may have been insufficient to detect smaller effects of biotin ingestion. Definitive studies of the effect of biotin on specific assays and analytes will require further investigation.
Despite these limitations, this study reinforces cautionary advice regarding potential limitations of assays that use biotin streptavidin binding for clinical evaluation of individuals who ingest large doses of biotin. A few assay manufacturers package inserts acknowledge biotin interference, recommending delayed sample collection after biotin intake. Based on these findings, manufacturers may need to consider modifying biotinylated assays to minimize the effects of biotin ingestion. Laboratories could identify assays that contain biotinylated components. Clinicians may want to ask about biotin ingestion even if assay results are not suspect because biotin interferences can cause either falsely normal or abnormal results. It may be advisable for patients to stop taking biotin, preferably for a week as studied herein, before undergoing laboratory testing. Alternatively, in the presence of biotin ingestion, nonbiotinylated assays would be preferred. Future studies, including patients with normal and abnormal analyte concentrations, are recommended to further clarify the extent and pharmacokinetics of ingested biotin interference on various assay platforms.
Conclusions
In this preliminary study of 6 healthy adult participants and 11 hormone and nonhormone analytes measured by 37 immunoassays, ingesting 10 mg/d of oral biotin for 1 week was associated with potentially clinically important assay interference in some but not all biotinylated assays studied. These findings should be considered for patients taking biotin supplements before ordering blood tests or when interpreting results.
eFigure 1. Principle of the Roche cobas e602 sandwich immunoassay for measurement of TSH in a blood sample (A and B) and effect of excess biotin in a blood sample (C)
eFigure 2. Principle of the Roche cobas e602 competitive immunoassay for measurement of total T4 in a blood sample (A and B) and effect of excess biotin in a blood sample (C).
eFigure. 3. Concentrations of ferritin (A-D), NT-proBNP (E-H), 25-OHD (I-L), PSA (M-N) at baseline, day 7 on biotin and day 14 off biotin
eTable 1. Summary of the 37 assays evaluated in the study
eTable 2. Assay imprecisions
eTable 3. Analyte mean individual comparisons over time between baseline off-biotin, day 7 on-biotin and day 14 off-biotin
eTable 4. Concentrations of NT-proBNP, 25-OHD, ferritin and PSA across the study systems at baseline off-biotin, day 7 on-biotin and day 14 off-biotin
References
- 1.Catlin DH, Leder BZ, Ahrens B, et al. . Trace contamination of over-the-counter androstenedione and positive urine test results for a nandrolone metabolite. JAMA. 2000;284(20):2618-2621. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe RM, Kasten B, Young DS, MacLowry JD. False-negative stool occult blood tests caused by ingestion of ascorbic acid (vitamin C). Ann Intern Med. 1975;83(6):824-826. [DOI] [PubMed] [Google Scholar]
- 3.Staggs CG, Sealey WM, McCabe BJ, Teague AM, Mock DM. Determination of the biotin content of select foods using accurate and sensitive HPLC/avidin binding. J Food Compost Anal. 2004;17(6):767-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr. 2002;22:221-239. [DOI] [PubMed] [Google Scholar]
- 5.Institute of Medicine In: Otten JJ, Pitzi Hellwig J, Meyers LD, eds. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006, https://www.nal.usda.gov/sites/default/files/fnic_uploads/DRIEssentialGuideNutReq.pdf. [Google Scholar]
- 6.Koutsikos D, Agroyannis B, Tzanatos-Exarchou H. Biotin for diabetic peripheral neuropathy. Biomed Pharmacother. 1990;44(10):511-514. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Mejia C. Pharmacological effects of biotin. J Nutr Biochem. 2005;16(7):424-427. [DOI] [PubMed] [Google Scholar]
- 8.Zempleni J, Hassan YI, Wijeratne SS. Biotin and biotinidase deficiency. Expert Rev Endocrinol Metab. 2008;3(6):715-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shelley WB, Shelley ED. Uncombable hair syndrome: observations on response to biotin and occurrence in siblings with ectodermal dysplasia. J Am Acad Dermatol. 1985;13(1):97-102. [DOI] [PubMed] [Google Scholar]
- 10.Sedel F, Papeix C, Bellanger A, et al. . High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4(2):159-169. [DOI] [PubMed] [Google Scholar]
- 11.Tourbah A, Lebrun-Frenay C, Edan G, et al. ; MS-SPI study group . MD1003 (high-dose biotin) for the treatment of progressive multiple sclerosis: a randomised, double-blind, placebo-controlled study. Mult Scler. 2016;22(13):1719-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamandis EP, Christopoulos TK. The biotin-(strept)avidin system: principles and applications in biotechnology. Clin Chem. 1991;37(5):625-636. [PubMed] [Google Scholar]
- 13.Wilchek M, Bayer EA. The avidin-biotin complex in bioanalytical applications. Anal Biochem. 1988;171(1):1-32. [DOI] [PubMed] [Google Scholar]
- 14.Piketty ML, Polak M, Flechtner I, Gonzales-Briceño L, Souberbielle J-C. False biochemical diagnosis of hyperthyroidism in streptavidin-biotin-based immunoassays: the problem of biotin intake and related interferences. Clin Chem Lab Med. 2016;55(6):780-788. [DOI] [PubMed] [Google Scholar]
- 15.Sealey WM, Teague AM, Stratton SL, Mock DM. Smoking accelerates biotin catabolism in women. Am J Clin Nutr. 2004;80(4):932-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mock DM, Dyken ME. Biotin catabolism is accelerated in adults receiving long-term therapy with anticonvulsants. Neurology. 1997;49(5):1444-1447. [DOI] [PubMed] [Google Scholar]
- 17.Cambridge Biomedical. Cambridge Biomedical Vitamin B7 H (Biotin) in serum package insert. http://www.cambridgebiomedical.com/DesktopModules/Bring2mind/DMX/Download.aspx?Command=Core_Download&EntryId=1990&PortalId=0&TabId=155. Accessed May 3, 2017.
- 18.Januzzi JL, van Kimmenade R, Lainchbury J, et al. . NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27(3):330-337. [DOI] [PubMed] [Google Scholar]
- 19.Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13(11):1069-1078. [DOI] [PubMed] [Google Scholar]
- 20.Piketty M-L, Prie D, Sedel F, et al. . High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference. Clin Chem Lab Med. 2017;55(6):817-825. [DOI] [PubMed] [Google Scholar]
- 21.Meany DL, Jan de Beur SM, Bill MJ, Sokoll LJ. A case of renal osteodystrophy with unexpected serum intact parathyroid hormone concentrations. Clin Chem. 2009;55(9):1737-1739. [DOI] [PubMed] [Google Scholar]
- 22.Elston MS, Sehgal S, Du Toit S, Yarndley T, Conaglen JV. Factitious Graves’ disease due to biotin immunoassay interference—a case and review of the literature. J Clin Endocrinol Metab. 2016;101(9):3251-3255. [DOI] [PubMed] [Google Scholar]
- 23.Barbesino G. Misdiagnosis of Graves’ disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid. 2016;26(6):860-863. [DOI] [PubMed] [Google Scholar]
- 24.Willeman T, Casez O, Faure P, Gauchez AS. Evaluation of biotin interference on immunoassays: new data for troponin I, digoxin, NT-Pro-BNP, and progesterone. Clin Chem Lab Med. 2017;55(10):e226-e229. [DOI] [PubMed] [Google Scholar]
- 25.Mock DM, Lankford GL, Mock NI. Biotin accounts for only half of the total avidin-binding substances in human serum. J Nutr. 1995;125(4):941-946. [DOI] [PubMed] [Google Scholar]
- 26.Mock DM, Heird GM. Urinary biotin analogs increase in humans during chronic supplementation: the analogs are biotin metabolites. Am J Physiol. 1997;272(1 pt 1):E83-E85. [DOI] [PubMed] [Google Scholar]
- 27.Clevidence BA, Marshall MW, Canary JJ. Biotin levels in plasma and urine of healthy adults consuming physiological doses of biotin. Nutr Res. 1988;8(10):1109-1118. [Google Scholar]
- 28.Peyro Saint Paul L, Debruyne D, Bernard D, Mock DM, Defer GL. Pharmacokinetics and pharmacodynamics of MD1003 (high-dose biotin) in the treatment of progressive multiple sclerosis. Expert Opin Drug Metab Toxicol. 2016;12(3):327-344. [DOI] [PubMed] [Google Scholar]
- 29.Singh A, Moses FM, Deuster PA. Vitamin and mineral status in physically active men: effects of a high-potency supplement. Am J Clin Nutr. 1992;55(1):1-7. [DOI] [PubMed] [Google Scholar]
- 30.Wijeratne NG, Doery JCG, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology. 2012;44(7):674-675. [DOI] [PubMed] [Google Scholar]
- 31.Bitsch R, Salz I, Hötzel D. Studies on bioavailability of oral biotin doses for humans. Int J Vitam Nutr Res. 1989;59(1):65-71. [PubMed] [Google Scholar]
- 32.Henry JG, Sobki S, Arafat N. Interference by biotin therapy on measurement of TSH and FT4 by enzyme immunoassay on Boehringer Mannheim ES700 analyser. Ann Clin Biochem. 1996;33(pt 2):162-163. [DOI] [PubMed] [Google Scholar]
- 33.Kwok JS-S, Chan IH-S, Chan MH-M. Biotin interference on TSH and free thyroid hormone measurement. Pathology. 2012;44(3):278-280. [DOI] [PubMed] [Google Scholar]
- 34.Minkovsky A, Lee MN, Dowlatshahi M, et al. . High-dose biotin treatment for secondary progressive multiple sclerosis may interfere with thyroid assays. AACE Clin Case Rep. 2016;2(4):e370-e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batista MC, Ferreira CES, Faulhaber ACL, Hidal JT, Lottenberg SA, Mangueira CLP. Biotin interference in immunoassays mimicking subclinical Graves’ disease and hyperestrogenism: a case series. Clin Chem Lab Med. 2017;55(6):e99-e103. [DOI] [PubMed] [Google Scholar]
- 36.Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med. 2016;375(7):704-706. [DOI] [PubMed] [Google Scholar]
- 37.Trambas C, Lu Z, Yen T, Sikaris K. Depletion of biotin using streptavidin-coated microparticles: a validated solution to the problem of biotin interference in streptavidin-biotin immunoassays [published online January 1, 2017]. Ann Clin Biochem. doi: 10.1177/0004563217707783 [DOI] [PubMed] [Google Scholar]
- 38.Samarasinghe S, Meah F, Singh V, et al. . Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks. Endocr Pract. 2017;23(8):989-998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Principle of the Roche cobas e602 sandwich immunoassay for measurement of TSH in a blood sample (A and B) and effect of excess biotin in a blood sample (C)
eFigure 2. Principle of the Roche cobas e602 competitive immunoassay for measurement of total T4 in a blood sample (A and B) and effect of excess biotin in a blood sample (C).
eFigure. 3. Concentrations of ferritin (A-D), NT-proBNP (E-H), 25-OHD (I-L), PSA (M-N) at baseline, day 7 on biotin and day 14 off biotin
eTable 1. Summary of the 37 assays evaluated in the study
eTable 2. Assay imprecisions
eTable 3. Analyte mean individual comparisons over time between baseline off-biotin, day 7 on-biotin and day 14 off-biotin
eTable 4. Concentrations of NT-proBNP, 25-OHD, ferritin and PSA across the study systems at baseline off-biotin, day 7 on-biotin and day 14 off-biotin