Key Points
Question
Does routine prophylactic low-dose oxygen supplementation after acute stroke improve functional outcome?
Findings
In this randomized clinical trial, 8003 patients with acute stroke were randomized within 24 hours of admission to 3 days of continuous oxygen, nocturnal oxygen, or control. After 3 months, there was no significant difference in death and disability for the combined oxygen groups compared with control (odds ratio, 0.97) or for the continuous oxygen group compared with the nocturnal oxygen group (odds ratio, 1.03).
Meaning
Routine low-dose oxygen did not improve outcomes in nonhypoxic patients after acute stroke.
Abstract
Importance
Hypoxia is common in the first few days after acute stroke, is frequently intermittent, and is often undetected. Oxygen supplementation could prevent hypoxia and secondary neurological deterioration and thus has the potential to improve recovery.
Objective
To assess whether routine prophylactic low-dose oxygen therapy was more effective than control oxygen administration in reducing death and disability at 90 days, and if so, whether oxygen given at night only, when hypoxia is most frequent, and oxygen administration is least likely to interfere with rehabilitation, was more effective than continuous supplementation.
Design, Setting, and Participants
In this single-blind randomized clinical trial, 8003 adults with acute stroke were enrolled from 136 participating centers in the United Kingdom within 24 hours of hospital admission if they had no clear indications for or contraindications to oxygen treatment (first patient enrolled April 24, 2008; last follow-up January 27, 2015).
Interventions
Participants were randomized 1:1:1 to continuous oxygen for 72 hours (n = 2668), nocturnal oxygen (21:00 to 07:00 hours) for 3 nights (n = 2667), or control (oxygen only if clinically indicated; n = 2668). Oxygen was given via nasal tubes at 3 L/min if baseline oxygen saturation was 93% or less and at 2 L/min if oxygen saturation was greater than 93%.
Main Outcomes and Measures
The primary outcome was reported using the modified Rankin Scale score (disability range, 0 [no symptoms] to 6 [death]; minimum clinically important difference, 1 point), assessed at 90 days by postal questionnaire (participant aware, assessor blinded). The modified Rankin Scale score was analyzed by ordinal logistic regression, which yields a common odds ratio (OR) for a change from one disability level to the next better (lower) level; OR greater than 1.00 indicates improvement.
Results
A total of 8003 patients (4398 (55%) men; mean [SD] age, 72 [13] years; median National Institutes of Health Stroke Scale score, 5; mean baseline oxygen saturation, 96.6%) were enrolled. The primary outcome was available for 7677 (96%) participants. The unadjusted OR for a better outcome (calculated via ordinal logistic regression) was 0.97 (95% CI, 0.89 to 1.05; P = .47) for oxygen vs control, and the OR was 1.03 (95% CI, 0.93 to 1.13; P = .61) for continuous vs nocturnal oxygen. No subgroup could be identified that benefited from oxygen. At least 1 serious adverse event occurred in 348 (13.0%) participants in the continuous oxygen group, 294 (11.0%) in the nocturnal group, and 322 (12.1%) in the control group. No significant harms were identified.
Conclusions and Relevance
Among nonhypoxic patients with acute stroke, the prophylactic use of low-dose oxygen supplementation did not reduce death or disability at 3 months. These findings do not support low-dose oxygen in this setting.
Trial Registration
ISRCTN Identifier: ISRCTN52416964
Three months after acute stroke, this randomized clinical trial compares rates of death and disability among adult patients receiving continuous or nocturnal low-dose oxygen supplementation or control intervention (oxygen only if clinically indicated.
Introduction
Hypoxia is common during the first days after an acute stroke1 and associated with higher rates of neurological deterioration,2 death and institutionalization,3 and greater mortality.4 While cells in the ischemic penumbra are only viable for a few hours, brain cells beyond the ischemic core and penumbra remain at risk of delayed cell death for several days owing to vasogenic edema, inflammation, and programmed cell death, particularly if metabolic disturbances are compounded by hypoxia.5,6,7 Continuous monitoring is associated with better outcomes,8 but even in intensively monitored patients, hypoxia is not always identified and treated. Adverse outcomes were observed to be increased when only some desaturations of less than 90% were treated with oxygen and reduced when all were treated.3
Supplemental oxygen could improve outcomes by preventing hypoxia and secondary brain damage but could also have adverse effects.9 These include vasoconstriction and pulmonary toxicity with high concentrations,9 respiratory tract infection due to contamination of the nasal tubes, the tubing acting as an impediment to mobilization, stress, and the direct effects of oxygen on vascular tone and blood pressure.10 Three small trials of short-term (≤12 hours) high-flow (10 to 45 L/min) therapeutic oxygen, aimed at generating supraphysiological blood oxygen levels, have not shown improved outcomes.11,12,13 A larger trial (n = 550) using low-dose supplemental oxygen (3 L/min for 24 hours) also showed no benefit,14 but early neurological recovery was improved in a study giving low-dose oxygen over 72 hours.15
The primary aim of the Stroke Oxygen Study (SO2S) was to determine whether low-dose oxygen therapy during the first 3 days after an acute stroke improves outcome compared with usual care (oxygen only when needed). Because oxygen may restrict mobility and interfere with daytime activities, the secondary hypothesis was that oxygen given at night only, when hypoxia is most likely, is more effective than continuous oxygen supplementation.
Methods
Study Design
This was a multicenter randomized clinical trial of oxygen supplementation with single-blind outcome assessment. The protocol and statistical analysis plan (Supplement 1 and Supplement 2),16,17 and data collection forms18 are published. Fully informed written or witnessed oral consent was given by the participants or, if they did not have capacity to consent, by a legal representative. The protocol was approved by the North Staffordshire Research Ethics Committee (06/Q2604/109).
Participants
Adults (aged ≥18 years) with a clinical diagnosis of acute stroke within 24 hours of hospital admission (136 participating centers in the United Kingdom), who had no clinical indications for or contraindications to oxygen treatment or any concomitant condition likely to limit life expectancy to less than 12 months were eligible (see eAppendix in Supplement 3 for definition of acute stroke).
Randomization and Interventions
Participants were allocated 1:1:1 via central web-based minimized randomization19 to (1) continuous oxygen supplementation; (2) nocturnal oxygen supplementation only; or (3) no routine oxygen (control). The factors for which imbalances were minimized were the Six Simple Variables prognostic index for independent survival at 6 months20 (cutoffs: ≤0.1, >0.1 to ≤0.35, >0.35 to ≤0.70, >0.70), oxygen treatment before randomization (yes, no, unknown), baseline oxygen saturation on air (<95%, ≥95%), and time since stroke onset (cutoffs: ≤3, >3 to ≤6, >6 to ≤12, >12 to ≤24, >24 hours). Stroke onset was defined as the last time well for wake-up strokes. No blocking was used. Oxygen was administered per nasal tubes either continuously (day and night) during the first 72 hours after randomization or overnight (21:00 hours to 07:00 hours) for 3 nights. Oxygen was given at a flow rate of 3 L/min if baseline saturation was 93% or below or at a flow rate of 2 L/min if baseline saturation was greater than 93%. In the control group, no routine oxygen supplementation was given.
Vital signs were observed at least 4 times per day, with any abnormal findings treated independently of trial allocation. Patients requiring oxygen in the control group, patients in the nocturnal oxygen group during the day, or patients needing changes in oxygen dosage for clinical reasons were given the appropriate concentration of oxygen irrespective of treatment group. In addition, for 4144 patients recruited in the latter half of the study, spot checks of treatment adherence were undertaken at midnight and 6 am.
Outcomes and Blinding
Outcomes were assessed at 1 week by a member of the local research team and at 90 days via postal questionnaire. Telephone interviews were conducted with nonresponders or to clarify unclear or missing answers. The primary outcome was the modified Rankin Scale (mRS) 21 score (disability range, 0 [no symptoms] to 6 [death]; minimum clinically important difference 1 point) assessed at 90 days. Secondary outcomes were number of participants with neurological improvement (≥4-point decrease on the National Institutes of Health Stroke Scale [NIHSS])22,23 between randomization and day 7, the highest and lowest oxygen saturations within the first 72 hours, and mortality at 1 week. Further secondary outcomes at 90 days were mortality, number of participants alive and independent (mRS ≤2), number of participants living at home, Barthel Index activities of daily living (ADL) score,24 quality of life (EuroQol [EQ5D-3L]) score,25 and Nottingham Extended Activities of Daily Living score.26 For the NIHSS and Barthel Index, deaths were recorded as the worst outcome on the scale.27 Participants, their physicians, and local research staff who recorded the 1-week outcomes were not blind to the study interventions. Ninety-day assessments were undertaken by the SO2S study office, which was blind to treatment allocation.
Study Size
The initial recruitment target was 6000 participants, which was estimated to provide 90% power to detect small (0.2 mRS-point [eg, a 1-point improvement among 1 in 5 participants]) differences between oxygen (continuous and night-only groups combined) and no oxygen at a P value of less than or equal to .01 and 90% power at a P value of less than or equal to .05 to detect small differences between continuous oxygen and nocturnal-only oxygen. The study size was subsequently revised to 8000 participants, using ordinal methods,16,17 without knowledge of interim results, to increase the number of patients with severe stroke and thereby provide greater power to investigate any differential effectiveness of oxygen vs control within subgroups (defined by severity).
Statistical Analysis
The trial was designed to answer 2 key questions: whether oxygen supplementation improves outcome (mRS at 90 days) and whether giving oxygen at night is more effective than giving it continuously. The main comparisons, therefore, were of the 2 combined oxygen groups (continuous and nocturnal only) vs control, and of continuous oxygen vs nocturnal-only oxygen. The statistical analysis plan describes the analysis methods in detail (Supplement 1 and Supplement 2).17
The mRS was analyzed by ordinal logistic regression, which yields a common odds ratio (OR) for a move from one level to the next better (lower) level with an OR more than 1.00 indicating an improvement. For this and other outcome variables, a primary unadjusted analysis and a secondary covariate-adjusted analysis were performed. Adjusted analyses incorporated the following covariates: age, sex, baseline NIHSS score, baseline oxygen saturation, and the Six Simple Variables prognostic index for 6-month independence (or for analysis of mortality, the Six Simple Variables prognostic index for 30-day survival). Sensitivity analysis for the mRS used multiple imputation of missing values (using a chained equations method with 20 imputed data sets). Additional imputations were performed to allow for the possibility that data were missing not at random and were either better or worse than expected; missing values were thereby replaced by either very good (ie, lowest) or very poor (ie, highest) scores on the mRS as appropriate (eTable 3 in Supplement 3). Subgroups, for the mRS only, were analyzed by an interaction term and were predefined in the statistical analysis plan.17
For continuous outcomes, means and standard deviations or medians and interquartile ranges (IQRs) are reported, as appropriate. Unadjusted analyses used unrelated t tests, with the mean difference between treatments and corresponding CIs reported. The adjusted analysis used analysis of covariance, with the covariates specified earlier included in the analysis. For dichotomous outcomes, percentages were compared across the treatment comparisons using a χ2 test (unadjusted analyses). Adjusted analyses of dichotomous outcomes used binary logistic regression, with the covariates listed earlier; ORs and CIs are reported.
All analyses were by intention to treat, ie, according to the treatment group to which participants were allocated, irrespective of treatment actually received. Statistical significance was set at a P value of less than or equal to .05 with 95% CIs for the primary outcome and at a P value of less than or equal to .01 with 99% CIs for secondary outcomes. All reported P values are 2-sided. The main analysis was performed in SAS software for Windows, version 9.4 (SAS Institute Inc), and IBM SPSS for Windows, version 22 was used for sensitivity analyses.
Interim analyses of safety and effectiveness were reviewed annually by an independent data monitoring and safety committee. No α-spending adjustments were made.
Results
Participants
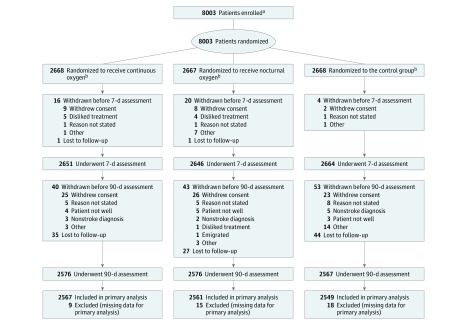
A total of 8003 participants from 136 collaborating centers in the United Kingdom were randomized and followed up between April 24, 2008, and January 27, 2015, (Figure 1). Baseline demographic and clinical characteristics, including stroke severity and oxygen saturation at randomization, were well-balanced in the 3 groups (Table 1). The mean (SD) age of participants was 72 (13) years, 4398 (55%) were men, and 7332 (92%) could undertake activities of daily living independently before the stroke. The mean (SD) NIHSS score was 7 (6) and the median score was 5 (IQR, 3 to 9). Prior to randomization, oxygen had been given to 1601 (20%) participants either in the ambulance or in the hospital. Patients were enrolled at a median of 20:43 hours (IQR, 11:59 to 25:32 hours) after symptom onset. The mean (SD) oxygen saturation at randomization was 96.6% (1.7%). All participants had a clinical diagnosis of stroke at the time of enrollment. The final diagnosis at 7 days was ischemic stroke in most cases (n = 6555; 82%), 588 (7%) had a primary intracerebral hemorrhage, and 294 (4%) were strokes without computed tomography diagnosis. There were 168 (2%) participants who were given a final diagnosis of transient ischemic attack, and 292 (4%) were found to have other nonstroke diagnoses with missing data in 106 (1%).
Figure 1. Flow of Participants Enrolled in the Continuous Oxygen, Nocturnal Oxygen, and Control Groups.
aThe number of patients screened for eligibility was not available.
bSee eTable 2 in Supplement 3 for adherence data.
Table 1. Baseline Characteristics.
| No. (%)a | |||
|---|---|---|---|
| Continuous Oxygen (n = 2668) |
Nocturnal Oxygen (n = 2667) |
Control (n = 2668) |
|
| Demographic characteristics | |||
| Age, mean (SD), yb | 72 (13) | 72 (13) | 72 (13) |
| Men | 1466 (55) | 1466 (55) | 1466 (55) |
| Prognostic factors | |||
| Living alone before the strokeb | 861 (32) | 857 (32) | 907 (34) |
| Independent in basic ADLs before the strokeb | 2451 (92) | 2431 (91) | 2450 (92) |
| Normal verbal responseb,c | 2190 (82) | 2207 (83) | 2196 (82) |
| Able to lift both armsb | 1998 (75) | 2022 (76) | 1996 (75) |
| Able to walkb | 660 (25) | 704 (26) | 677 (25) |
| Probability of 30-d survival, median (IQR)20 | 0.92 (0.86-0.95) |
0.92 (0.86-0.95) |
0.92 (0.86-0.95) |
| Alive and independent at 6 m, probability, median (IQR)d,e | 0.44 (0.12-0.71) |
0.42 (0.12-0.71) |
0.42 (0.12-0.71) |
| Blood glucose, mean (SD), mg/dL | 127 (46) | 126 (43) | 128 (45) |
| Concomitant medical problems | |||
| Atrial fibrillation | 638 (24) | 673 (25) | 684 (26) |
| Ischemic heart disease | 573 (21) | 515 (19) | 514 (19) |
| Heart failure | 224 (8) | 217 (8) | 216 (8) |
| Chronic obstructive pulmonary disease/asthma | 253 (9) | 242 (9) | 245 (9) |
| Other chronic lung problem | 29 (1) | 24 (1) | 19 (1) |
| Details of the Qualifying Event | |||
| Time since symptom onset, hh:mm median (IQR)d | 20:44 (11:53-25:33) |
20:32 (12:05-25:31) |
20:45 (11:57-25:31) |
| Diagnosisf,g | |||
| Transient ischemic attack | 52 (1.9) | 50 (1.9) | 66 (2.5) |
| Ischemic stroke | 2187 (82.0) | 2165 (81.1) | 2203 (82.6) |
| Intracerebral hemorrhage | 185 (6.9) | 207 (7.8) | 196 (7.3) |
| Stroke without imaging diagnosis | 104 (3.9) | 106 (4.0) | 84 (3.1) |
| Not stroke or transient ischemic attack | 101 (3.8) | 98 (3.7) | 93 (3.5) |
| Missing | 39 (1.5) | 41 (1.5) | 26 (1.0) |
| Glasgow Coma Scale score, median (IQR) [range]h | 15 (15-15) [4-15] | 15 (15-15) [5-15] | 15 (15-15) [3-15] |
| Thrombolyzedg | 447 (17) | 410 (15) | 447 (17) |
| NIHSS score, median (IQR)i | 5 (3-9) | 5 (3-9) | 5 (3-9) |
| Oxygenation | |||
| Oxygen given prior to randomizationd | 531 (20) | 531 (20) | 539 (20) |
| Oxygen saturation on room air, % mean (SD)d | 96.6 (1.7) | 96.6 (1.6) | 96.7 (1.7) |
Abbreviations: ADL, activities of daily living; IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale.
SI conversion factor: To convert glucose to mmol/L, multiply values by 0.0555.
Data are reported as No. (%) unless otherwise indicated. Data were collected before randomization unless otherwise indicated.
Characteristic is one of the Six Simple Variables.20
Normal verbal response was taken from the verbal subitem of the Glasgow Coma Scale.
Characteristic is a minimization variable.
The probability of being alive and independent was calculated using the Six Simple Variables prognostic index for independent survival at 6-month assessment.20
See eAppendix in Supplement 3 for definitions for diagnoses.
Indicates data that were recorded on day 7. All other data were collected before randomization.
Glasgow Coma Scale (score range, 3 [deep coma] to 15 [alert and oriented]).
NIHSS range, 0 (no deficit) to 42 (most-severe deficit).
Informed consent was provided by 6991 (87%) participants, and 1012 (13%) had consent given by a relative, caregiver, or an independent legal representative (eTable 1 in Supplement 3). Of the participants who were unable to personally provide consent and were included by a representative, 6 (0.1%) refused consent at the 1-week reassessment and 22 (2%) refused at the 90-day assessment and were withdrawn.
Treatment Adherence
Adherence was similar in the continuous oxygen group (2158 [81%]) and the nocturnal oxygen group (2225 [83%]), all of whom were prescribed the full course of treatment (eTable 2 in Supplement 3). Use of oxygen was discontinued prematurely among 433 (16%) participants in the continuous oxygen group and 361 (14%) in the nocturnal oxygen group. The most common reason for early discontinuation of oxygen was discharge from the hospital. In the control group, trial oxygen was recorded as being given to 33 (1.2%) participants, with no recording of whether oxygen was given among 406 (15%).
Effect on Oxygenation
Oxygen treatment resulted in a significant increase of 0.8% in the highest oxygen saturation and 0.9% in the lowest oxygen saturation during the 72 hours of the intervention period in the continuous oxygen group compared with controls, and of 0.5% in the highest oxygen saturation and 0.4% in the lowest oxygen saturation during the 72 hours of the intervention period in the nocturnal oxygen group compared with controls (P < .001 for all comparisons; Table 2). Significantly more participants in the combined oxygen groups (n = 463 [9%]) required oxygen for clinical reasons during the intervention period than in the control group (n = 176 [7%]) (P < .001). Similarly, more participants in the continuous oxygen group (n = 254 [10%]) required oxygen than in the nocturnal oxygen group (n = 209 [8%]); P = .03.
Table 2. Secondary, Exploratory, and Safety Outcomes.
| No. (N = 8003) |
Continuous Oxygen (n = 2668) |
Nocturnal Oxygen (n = 2667) |
Control (n = 2668) |
Comparison 1 | Comparison 2 | |||
|---|---|---|---|---|---|---|---|---|
| Combined Oxygen vs Control, Statistic (99% CI) |
P Value | Continuous vs Nocturnal, Statistic (99% CI) |
P Value | |||||
| Secondary Outcomes at 72 h | ||||||||
| Highest oxygen saturation, mean (99% CI), %a | 7860 | 99.1 (99.1 to 99.2) | 98.8 (98.7 to 98.9) | 98.3 (98.2 to 98.3) | MD, 0.69 (0.61 to 0.77) | <.001b | MD, 0.32 (0.22 to 0.41) | <.001b |
| Lowest oxygen saturation, mean (99% CI), %a | 7860 | 95.0 (94.9 to 95.1) | 94.5 (94.4 to 94.6) | 94.1 (94.0 to 94.2) | MD, 0.62 (0.48 to 0.76) | <.001b | MD, 0.48 (0.32 to 0.63) | <.001b |
| Oxygen saturation <90%, No. (%) | 7860 | 39 (1.5) | 30 (1.1) | 74 (2.8) | OR, 0.46 (0.30 to 0.71)c | <.001d | OR, 1.30 (0.69 to 2.44)c | .28d |
| Oxygen saturation <95%, No. (%) | 7860 | 861 (32.9) | 1119 (42.9) | 1354 (51.5) | OR, 0.57 (0.51 to 0.65)c | <.001d | OR, 0.65 (0.56 to 0.76)c | <.001d |
| Need for additional oxygen, No. (%) | 7809 | 254 (9.8) | 209 (8.1) | 176 (6.7) | OR, 1.36 (1.07 to 1.73)c | .001d | OR, 1.23 (0.96 to 1.59)c | .03d |
| Secondary Outcomes at 7 d | ||||||||
| NIHSS, median (99% CI)e | 7778 | 2 (2 to 3) | 2 (2 to 3) | 2 (2 to 3) | MdD, 0 (0 to 0) | .56f | MdD, 0 (0 to 0) | .95f |
| Neurological improvement, No. (%)g | 7778 | 1016 (39.2) | 1029 (39.7) | 1037 (39.9) | OR, 0.98 (0.86 to 1.11)c | .68d | OR, 0.98 (0.85 to 1.13)c | .71d |
| Death by 7 d, No. (%) | 7959 | 50 (1.9) | 35 (1.3) | 45 (1.7) | OR, 0.95 (0.59 to 1.53)c | .78d | OR, 1.43 (0.81 to 2.54)c | .11d |
| Secondary Outcomes at 90 d | ||||||||
| Death by 90 d, No. (%)h | 7677 | 257 (10.0) | 236 (9.2) | 246 (9.7) | OR, 1.00 (0.81 to 1.23)c | .96d | OR, 1.10 (0.86 to 1.40)c | .30d |
| Alive and independent, No. (%)i | 7677 | 1325 (51.6) | 1316 (51.4) | 1337 (52.5) | OR, 0.96 (0.85 to 1.09)c | .43d | OR, 1.01 (0.87 to 1.17)c | .87d |
| Living at home, No. (%) | 6859 | 1961 (85.8) | 1947 (84.8) | 1947 (85.4) | OR, 0.99 (0.82 to 1.20)c | .91d | OR, 1.08 (0.87 to 1.34)c | .35d |
| Barthel ADL index, mean (99% CI)j | 6549 | 70.2 (68.2 to 72.2) | 71.1 (69.1 to 73.1) | 70.9 (68.9 to 72.8) | MD, −0.18 (−2.60 to 2.24) | .85b | MD, −0.86 (−3.65 to 1.93) | .43b |
| Nottingham Extended ADL, mean (99% CI)k | 7528 | 9.66 (9.29 to 10.02) | 9.54 (9.17 to 9.90) | 9.77 (9.40 to 10.14) | MD, −0.17 (−0.62 to 0.28) | .32b | MD, 0.12 (−0.40 to 0.64) | .55b |
| EQ5D-3L for quality of life, mean (99% CI)l | 7248 | 0.50 (0.48 to 0.51) | 0.50 (0.48 to 0.51) | 0.49 (0.48 to 0.51) | MD, 0.004 (−0.02 to 0.03) | .71b | MD, 0.003 (−0.03 to 0.03) | .78b |
| VAS for quality of life, mean (99% CI)m | 6675 | 55.4 (53.8 to 57.1) | 55.7 (54.1 to 57.3) | 55.5 (53.8 to 57.1) | MD, 0.10 (−1.93 to 2.12) | .90b | MD, −0.24 (−2.57 to 2.09) | .79b |
| Exploratory Outcomesn | ||||||||
| Highest heart rate within 72 h, mean (99% CI), beats/minb | 7859 | 87.2 (86.3 to 88.0) | 88.0 (87.2 to 88.8) | 87.7 (86.9 to 88.4) | MD, −0.07 (−1.06 to 0.92) | MD, −0.83 (−2.01 to 0.35) | ||
| Highest systolic BP within 72 h, mean (99% CI), mm Hg | 7864 | 162.4 (161.2 to 163.7) | 162.8 (161.5 to 164.0) | 164.6 (163.3 to 165.8) | MD, −1.96 (−3.48 to −0.44) | MD, −0.35 (−2.11 to 1.41) | ||
| Highest diastolic BP within 72 h, mean (99% CI), mm Hg | 7861 | 89.5 (88.7 to 90.2) | 90.2 (89.4 to 91.0) | 90.9 (90.1 to 91.7) | MD, −1.10 (−2.06 to −0.15) | MD, −0.72 (−1.82 to 0.37) | ||
| Highest temperature within 7 d, mean (99% CI), °C | 7877 | 37.1 (37.1 to 37.2) | 37.2 (37.1 to 37.2) | 37.1 (37.1 to 37.2) | MD, 0.01 (−0.03 to 0.04) | MD, −0.01 (−0.05 to 0.03) | ||
| Antibiotics given within 7 d, No. (%) | 7916 | 400 (15.2) | 393 (14.9) | 403 (15.2) | OR, 0.99 (0.83 to 1.17)c | OR, 1.02 (0.84 to 1.24)c | ||
| Sedatives given within 7 d, No. (%) | 7916 | 140 (5.3) | 161 (6.1) | 154 (5.8) | OR, 0.98 (0.76 to 1.28)c | OR, 0.86 (0.63 to 1.17)c | ||
| Sleep as good as before the stroke, No. (%) | 6584 | 1407 (64) | 1436 (65) | 1419 (65) | OR, 0.98 (0.85 to 1.13)c | OR, 0.96 (0.82 to 1.13)c | ||
| No significant speech problems, No. (%) | 6716 | 1957 (88) | 1957 (87) | 1939 (87) | OR, 1.09 (0.89 to 1.32)c | OR, 1.06 (0.84 to 1.34)c | ||
| Memory as good as before the stroke, No. (%) | 6646 | 981 (44) | 1000 (45) | 971 (44) | OR, 1.02 (0.89 to 1.16)c | OR, 0.97 (0.83 to 1.13)c | ||
| Safety Outcomes | ||||||||
| Serious adverse events, mean (99% CI) | 8003 | 0.16 (0.14 to 0.18) | 0.13 (0.11 to 0.16) | 0.16 (0.13 to 0.18) | RR, 0.94 (0.78 to 1.13) | .37o | RR, 1.19 (0.96 to 1.47) | .03o |
| Participants with ≥1 serious adverse event, No. (%) | 8003 | 348 (13.0) | 294 (11.0) | 322 (12.1) | OR, 1.00 (0.83 to 1.20)c | .96d | OR, 1.21 (0.97 to 1.51)c | .02d |
Abbreviations: ADL, activities of daily living; EQ5E-3L, EuroQol quality of life measure of health outcome; MD, mean difference; MdD, median difference; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; RR, rate ratio; VAS, Visual Analog Scale.
The highest and lowest oxygen saturations were the highest and lowest records of oxygen saturation on the participant’s observation chart during the 72 hours after randomization.
Significance testing was by unrelated t test.
ORs of less than 1 indicate the outcome is less likely with oxygen than with control (reference category) in comparison 1 and less likely with continuous oxygen than with nocturnal oxygen (reference category) in comparison 2.
Significance testing was by χ2 test.
NIHSS range, 0 (no deficit) to 42 (most-severe deficit).
Significance was by Wilcoxon rank-sum test.
Neurological improvement was indicated by a decrease of 4 or more or to zero on the NIHSS.
Evaluated by an mRS score of 6 (mRS disability range, 0 [no symptoms] to 6 [death]; minimum clinically important difference, 1 point).
Evaluated by an mRS score of 0, 1, or 2.
Barthel ADL index range (0 [worst] to 100 [best]).
Nottingham Extended ADL range (0 [worst] to 21 [best]).
EQ5D-3L range (−0.59 [worst] to 1 [best]).
VAS range (0 [worst] to 100 [best]).
As outlined in the statistical analysis (Supplement 1 and Supplement 2), tests were not conducted on the exploratory data and the outcomes suggested by patients and caregivers.
Significance testing was by negative binomial regression.
Main Outcome
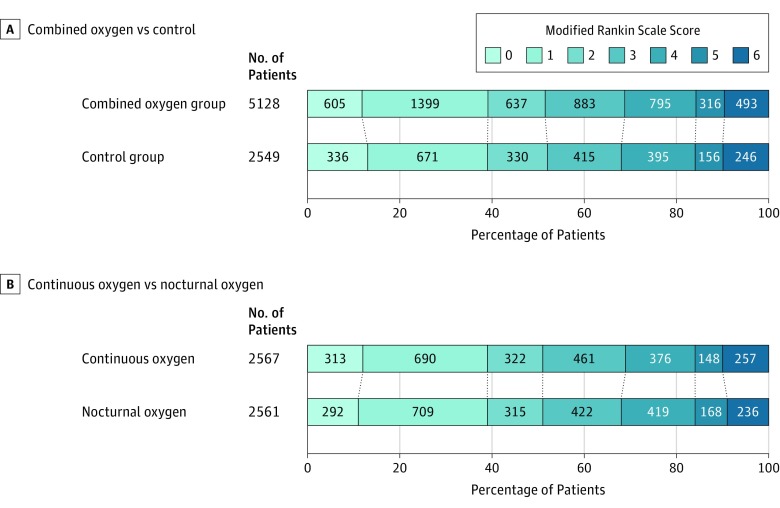
The primary analysis demonstrated that oxygen supplementation did not significantly improve functional outcome at 90 days (Figure 2). The unadjusted OR for a better outcome (lower mRS) was 0.97 (95% CI, 0.89 to 1.05; P = .47) for combined oxygen vs control, and 1.03 (95% CI, 0.93 to 1.13; P = .61) for continuous oxygen vs nocturnal oxygen. Secondary analyses adjusted for age, sex, baseline NIHSS score, baseline oxygen saturation, and the Six Simple Variables prognostic index yielded very similar results for the combined oxygen group vs control (OR, 0.97 [95% CI, 0.89 to 1.06]; P = .54) and for continuous oxygen vs nocturnal oxygen (OR, 1.01 [95% CI, 0.92 to 1.12]; P = .81). With similar numbers of missing responses in the 3 groups (continuous oxygen, n = 101; nocturnal oxygen, n = 106; and control, n = 119), findings were much the same in sensitivity analyses using multiple imputation or analyzing only participants who adherered to protocol (eTable 3 in Supplement 3).
Figure 2. Main Outcome Assessed by Modified Rankin Scale Score at 90-Day Follow-up.
From the ordinal regression analysis, the unadjusted odds ratio for a better outcome (lower modified Rankin Scale [mRS] score) was 0.97 (95% CI, 0.89 to 1.05; P = .47) for combined oxygen vs control, and 1.03 (95% CI, 0.93 to 1.13; P = .61) for continuous oxygen vs nightly oxygen (mRS score range, 0 to 6 [0, no symptoms; 1, few symptoms but able to carry out all previous activities and duties; 2, unable to carry out all previous activities but able to look after own affairs without assistance; 3, needs some help with looking after own affairs but able to walk without assistance; 4, unable to walk without assistance and unable to attend to own bodily needs without assistance but does not need constant care and attention; 5, major symptoms such as bedridden and incontinent and needs constant attention day and night; 6, death]).
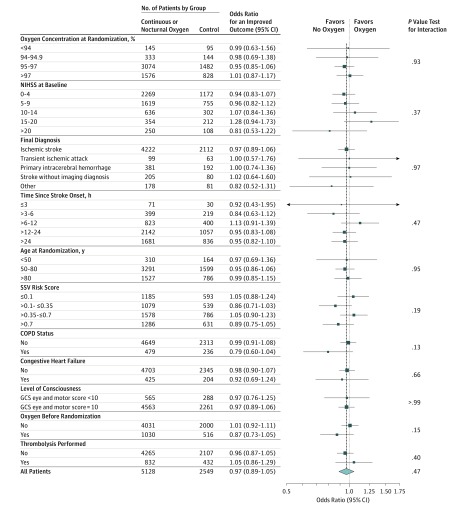
Subgroup analysis (Figure 3) found no indication that treatment effectiveness differed in any of the predefined subgroups, even those in whom most benefit might be expected such as patients with more severe stroke or those for whom oxygen supplementation was started early after stroke onset.
Figure 3. Subgroup Analyses for an Improved Outcome Assessed by Modified Rankin Scale Score Comparing Oxygen vs Control at 90 Days.
The x-axis depicts the common odds ratio (OR) for a better outcome over all 7 levels of the modified Rankin Scale score (mRS), derived from ordinal logistic regression. ORs greater than 1 indicate that a good outcome (low mRS) is more likely with oxygen than with control (reference category). The size of the markers reflects the total sample size in each subgroup, with larger markers indicating more precise estimates. The subgroup thresholds for oxygen concentration at randomization were revised from the prespecified thresholds because the analysis did not converge using the prespecified values. SSV indicates Six Simple Variables risk score; COPD, chronic obstructive pulmonary disease; GCS, Glasgow Coma Scale.
Secondary Outcomes
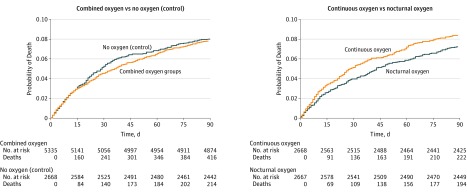
Analyses of secondary outcomes also showed no benefit from oxygen (Table 2). Neurological impairment at 1 week improved from baseline to the same degree in all 3 groups with median NIHSS scores of 2 (IQR, 1 to 6) by 1 week. Oxygen treatment did not increase the number of participants who were alive and independent or back in their home, the ability to perform basic (Barthel Index) or extended (Nottingham Extended Activities of Daily Living) activities of daily living, or quality of life (EuroQol-5D-3L) at 90 days. The results remained unchanged after adjustment for baseline prognostic factors (eTable 4 in Supplement 3). Mortality (Figure 4) was similar in the oxygen (both groups combined) and control groups (hazard ratio [HR], 0.97 [99% CI, 0.78 to 1.21]; P = .75), and for continuous oxygen vs nocturnal oxygen (HR, 1.15 [99% CI, 0.90 to 1.48]; P = .15).
Figure 4. Patient Mortality From 0 Through 90 Days.
Cutoff for mortality differs from the 90-day mortality reported in Table 2 and Figure 2, in which responses were accepted up to 6 months if 3-month outcomes were not returned. Median duration of follow-up was 90 days (range, 0 to 90) in each treatment group.
Exploratory Analyses
There was no evidence of increased stress levels (higher heart rates, higher blood pressure, and need for sedation) in the oxygen-treated group than in the control group or evidence that oxygen treatment was associated with more infections, with little difference in the highest temperature or the need for antibiotics (Table 2).
Safety Outcomes
The number of serious adverse events by 90 days was similar in the combined oxygen and control groups, but lower in the nocturnal oxygen group when compared with the continuous oxygen group (Table 2; eTable 5 in Supplement 3). No oxygen-related adverse events (respiratory depression, drying of mucous membranes) were reported.
Discussion
In this clinical trial of patients with acute stroke, routine prophylactic low-dose oxygen supplementation did not improve outcome among patients who were not hypoxic at baseline, whether oxygen was given continuously for 72 hours or at night only. This applied to the primary 90-day functional outcome and to all other tested outcomes, including early neurological recovery, mortality, disability, independence in basic and extended activities of daily living, and quality of life. The results remained unchanged in analyses adjusted for baseline prognostic factors and in sensitivity analyses using multiple imputation or analyzing adherers only. Subgroup analyses did not identify any characteristics that would make a patient more likely to benefit from oxygen treatment (includes enrollment between 3 to 6 hours after stroke onset, patients with a lower baseline oxygen saturation, severe strokes, a reduced level of consciousness, and a history of heart failure or lung disease [ie, characteristics for which benefit from oxygen was most anticipated]). Because of the large overall size of this trial, these patient subgroups were each sufficiently large for the lack of observed benefit to be likely real and not a false negative.
In contrast to the much smaller SOS Pilot study,15 this trial showed no evidence of better early neurological recovery with oxygen. Subgroup analysis of an earlier study of low-dose oxygen supplementation in acute stroke14 suggested that oxygen might adversely affect outcome in patients with mild strokes, possibly through formation of toxic free radicals. A more recent study of short-burst high-flow oxygen (45 L/min) was terminated early (after enrollment of 85 patients) because of excess mortality in the actively treated group.13 Hyperoxia was independently associated with mortality in a large retrospective cohort study of ventilated patients with stroke.28 Although suggestive of potential harm, these findings could be due to confounding factors.
As a large pragmatic trial, this study included unselected patients with a clinical diagnosis of acute stroke without radiological confirmation. The sample therefore included ischemic and hemorrhagic strokes and participants who were later found to have mimics or transient ischemic attacks.
More than half of all acute stroke services in the United Kingdom participated, and wide inclusion criteria allowed enrollment of a representative sample of patients with ischemic and hemorrhagic stroke across the whole range of severity. Stroke severity was similar to that of the UK stroke population as a whole, with a median NIHSS of 5 in this trial and 4 in the UK Sentinel Stroke National Audit Programme, which includes every stroke patient admitted to UK hospitals.29 The median NIHSS of 127 950 patients with acute ischemic stroke in the US Get with the Guidelines Register30 was 5, as in this trial. A median NIHSS of 5 at baseline was also recorded in a large Dutch study of antibiotic prophylaxis after stroke, with similarly wide inclusion criteria.31
This study has several limitations. Minor benefits from oxygen treatment might have been masked by poor adherence. However, this seems unlikely given the high statistical power to detect even small improvements. Moreover, sensitivity analyses did not show better outcomes in the adherers-only group (eTable 3 in Supplement 3). Furthermore, this trial found significant increases in the oxygen saturations in the treated groups compared with the control group. Patients with acute stroke are often restless and confused. Ensuring full adherence would ideally require a 1 to 1 nurse-to-patient ratio. However, this is not possible outside an intensive care setting. The main outcome was assessed by postal questionnaire and supported by telephone interviews with nonresponders. This method has been used successfully in large pragmatic trials32,33 but has been replaced by remote multiple-rater video-recorded interviews or in-person interview and examination by an allocation-blinded rater using formal structured assessments in several more recent studies.34 Low-dose oxygen supplementation may not be sufficient to prevent severe desaturations; both the SOS Pilot15 and this trial found no significant difference in severe desaturations between the treatment and control groups. A small (N = 46) nonrandomized study comparing high-flow oxygen treatment via mask with low-flow supplementation via nasal cannula showed a trend toward lower mortality with high flow that was not statistically significant. However, evidence from randomized trials of high-flow oxygen treatment in acute stroke11,12,13 does not show that higher doses of oxygen are associated with better outcomes. Early administration of high-dose oxygen might help maintain the viability of the ischemic penumbra and allow a broader time window for neuroprotection or thrombolysis. This question was not addressed in this trial of prophylactic oxygen, but will be tested in the PROOF trial.35
The median time from stroke onset to randomization in this trial was 20 hours, 43 minutes. However, 101 participants were enrolled early (within 3 hours of symptom onset). Subgroup analysis (Figure 3) showed a similar lack of effect for oxygen in the small subset of patients enrolled early as in those enrolled later but was underpowered. Larger trials in the early time window would be needed to definitely exclude a benefit.
Conclusions
Among nonhypoxic patients with acute stroke, the prophylactic use of low-dose oxygen supplementation did not reduce death or disability at 3 months. These findings do not support low-dose oxygen in this setting.
Final Approved Trial Protocol and Statistical Analysis Plan
Changes to the Protocol and Statistical Analysis Plan
eAppendix. Definitions of Ischemic Stroke, Hemorrhagic Stroke, TIA, and Stroke Mimics
eTable 1. Informed Consent
eTable 2. Adherence to the Trial Intervention
eTable 3. Sensitivity Analyses
eTable 4. Secondary, Exploratory, and Safety Outcomes—Adjusted Analyses
eTable 5. Total Number of Serious Adverse Events by Event Categories
References
- 1.Roffe C, Sills S, Halim M, et al. . Unexpected nocturnal hypoxia in patients with acute stroke. Stroke. 2003;34(11):2641-2645. [DOI] [PubMed] [Google Scholar]
- 2.Rocco A, Pasquini M, Cecconi E, et al. . Monitoring after the acute stage of stroke. Stroke. 2007;38(4):1225-1228. [DOI] [PubMed] [Google Scholar]
- 3.Bravata DM, Wells CK, Lo AC, et al. . Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810. [DOI] [PubMed] [Google Scholar]
- 4.Rowat AM, Dennis MS, Wardlaw JM. Hypoxaemia in acute stroke is frequent and worsens outcome. Cerebrovasc Dis. 2006;21(3):166-172. [DOI] [PubMed] [Google Scholar]
- 5.Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci. 2012;1268:26-34. [DOI] [PubMed] [Google Scholar]
- 6.Alawneh JA, Jones PS, Mikkelsen IK, et al. . Infarction of ‘non-core-non-penumbral’ tissue after stroke. Brain. 2011;134(6):1765-1776. [DOI] [PubMed] [Google Scholar]
- 7.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439-447. [DOI] [PubMed] [Google Scholar]
- 8.Ciccone A, Celani MG, Chiaramonte R, Rossi C, Righetti E. Continuous versus intermittent physiological monitoring for acute stroke. Cochrane Database Syst Rev. 2013;5(5):CD008444. [DOI] [PubMed] [Google Scholar]
- 9.O’Driscoll BR, Howard LS, Earis J, Mak V; et al. . BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(suppl 1):ii1-ii90. [DOI] [PubMed] [Google Scholar]
- 10.Floyd TF, Clark JM, Gelfand R, et al. . Independent cerebral vasoconstrictive effects of hyperoxia and accompanying arterial hypocapnia at 1 ATA. J Appl Physiol (1985). 2003;95(6):2453-2461. [DOI] [PubMed] [Google Scholar]
- 11.Padma MV, Bhasin A, Bhatia R, et al. . Normobaric oxygen therapy in acute ischemic stroke. Ann Indian Acad Neurol. 2010;13(4):284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhal AB, Benner T, Roccatagliata L, et al. . A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36(4):797-802. [DOI] [PubMed] [Google Scholar]
- 13.Singhal AB. Normobaric oxygen therapy in acute ischemic stroke trial. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT000414726. Accessed June 30, 2017.
- 14.Rønning OM, Guldvog B. Should stroke victims routinely receive supplemental oxygen? Stroke. 1999;30(10):2033-2037. [DOI] [PubMed] [Google Scholar]
- 15.Roffe C, Ali K, Warusevitane A, et al. . The SOS pilot study. PLoS One. 2011;6(5):e19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roffe C, Nevatte T, Crome P, et al. . The Stroke Oxygen Study (SO2S). Trials. 2014;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim J, Gray R, Nevatte T, et al. . Statistical analysis plan for the Stroke Oxygen Study (SO2S). Trials. 2014;15:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroke Oxygen Study. http://www.so2s.co.uk/. Accessed July 14, 2016.
- 19.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. [PubMed] [Google Scholar]
- 20.Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke. 2002;33(4):1041-1047. [DOI] [PubMed] [Google Scholar]
- 21.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. [DOI] [PubMed] [Google Scholar]
- 22.Brott T, Adams HP Jr, Olinger CP, et al. . Measurements of acute cerebral infarction. Stroke. 1989;20(7):864-870. [DOI] [PubMed] [Google Scholar]
- 23.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25(2):362-365. [DOI] [PubMed] [Google Scholar]
- 24.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index. Int Disabil Stud. 1988;10(2):61-63. [DOI] [PubMed] [Google Scholar]
- 25.EuroQol Group EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 26.Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clin Rehabil. 1987;1(4):301-305. doi: 10.1177/026921558700100409 [DOI] [Google Scholar]
- 27.Ali M, Jüttler E, Lees KR, Hacke W, Diedler J; et al. . Patient outcomes in historical comparators compared with randomised-controlled trials. Int J Stroke. 2010;5(1):10-15. [DOI] [PubMed] [Google Scholar]
- 28.Rincon F, Kang J, Maltenfort M, et al. . Association between hyperoxia and mortality after stroke. Crit Care Med. 2014;42(2):387-396. [DOI] [PubMed] [Google Scholar]
- 29.Smith CJ, Bray BD, Hoffman A, et al. . Can a novel clinical risk score improve pneumonia prediction in acute stroke care? J Am Heart Assoc. 2015;4(1):e001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonarow GC, Pan W, Saver JL, et al. . Comparison of 30-day mortality models for profiling hospital performance in acute ischemic stroke with vs without adjustment for stroke severity. JAMA. 2012;308(3):257-264. [DOI] [PubMed] [Google Scholar]
- 31.Westendorp WF, Vermeij JD, Zock E, et al. . The Preventive Antibiotics in Stroke Study (PASS). Lancet. 2015;385(9977):1519-1526. [DOI] [PubMed] [Google Scholar]
- 32.Dennis MS, Lewis SC, Warlow C; et al. . Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD). Lancet. 2005;365(9461):764-772. [DOI] [PubMed] [Google Scholar]
- 33.IST-3 Collaborative Group The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]). Lancet. 2012;379(9834):2352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Cancio E, Salvat M, Cerdà N, et al. . Phone and video-based modalities of central blinded adjudication of modified Rankin Scores in an endovascular stroke trial. Stroke. 2015;46(12):3405-3410. [DOI] [PubMed] [Google Scholar]
- 35.Stroke Alliance for Europe PROOF trial. http://www.safestroke.eu/proof-trial/. Accessed September 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Final Approved Trial Protocol and Statistical Analysis Plan
Changes to the Protocol and Statistical Analysis Plan
eAppendix. Definitions of Ischemic Stroke, Hemorrhagic Stroke, TIA, and Stroke Mimics
eTable 1. Informed Consent
eTable 2. Adherence to the Trial Intervention
eTable 3. Sensitivity Analyses
eTable 4. Secondary, Exploratory, and Safety Outcomes—Adjusted Analyses
eTable 5. Total Number of Serious Adverse Events by Event Categories