Abstract
Importance
Latent cytomegalovirus (CMV) infection is present in more than half the adult population, and a viral reactivation (ie, when the virus becomes measurable in body fluids such as blood) can occur in up to one-third of these individuals during episodes of critical illness.
Objective
To determine whether antiviral therapy is safe and effective for preventing CMV reactivation in a general population of critically ill patients.
Design, Setting, and Participants
A single-center, open-label, randomized, controlled clinical trial recruited 124 CMV-seropositive patients undergoing mechanical ventilation for at least 24 hours in the intensive care unit between January 1, 2012, and January 31, 2014. The mean baseline Acute Physiology and Chronic Health Evaluation II score of all patients was 17.6.
Interventions
Patients were randomized to receive anti-CMV prophylaxis with valacyclovir hydrochloride (n = 34) or low-dose valganciclovir hydrochloride (n = 46) for up to 28 days to suppress viral reactivation, or to a control group with no intervention (n = 44).
Main Outcomes and Measures
Time to first CMV reactivation in blood within the 28-day follow-up period following initiation of the study drug.
Results
Among the 124 patients in the study (46 women and 78 men; mean [SD] age, 56.9 [16.9] years), viral reactivation in the blood occurred in 12 patients in the control group, compared with 1 patient in the valganciclovir group and 2 patients in the valacyclovir group (combined treatment groups vs control: hazard ratio, 0.14; 95% CI 0.04-0.50). Although this trial was not powered to assess clinical end points, the valacyclovir arm was halted prematurely because of higher mortality; 14 of 34 patients (41.2%) had died by 28 days, compared with 5 of 37 (13.5%) patients in the control arm at the point of the decision to halt this arm. Other safety end points showed similar outcomes between groups.
Conclusions and Relevance
Antiviral prophylaxis with valacyclovir or low-dose valganciclovir suppresses CMV reactivation in patients with critical illness. However, given the higher mortality, a large-scale trial would be needed to determine the clinical efficacy and safety of CMV suppression.
Trial Registration
clinicaltrials.gov Identifier: NCT01503918
This randomized clinical trial assesses whether antiviral therapy is safe and effective for preventing cytomegalovirus reactivation in a general population of critically ill patients.
Key Points
Question
Is antiviral prophylaxis safe and effective for preventing cytomegalovirus (CMV) reactivation in critically ill patients?
Findings
In this randomized clinical trial, valganciclovir hydrochloride and valacyclovir hydrochloride both suppressed CMV reactivation compared with a control arm, although the valacyclovir arm was stopped early because of higher mortality.
Meaning
Prophylaxis prevents CMV reactivation in critically ill patients; further research is needed to determine clinical efficacy and safety.
Introduction
Herpesvirus infections are widely prevalent within the human population and establish a state of chronic infection. Primary infection with cytomegalovirus (CMV) is usually clinically silent; most individuals become chronically infected during their lifetime. The presence of measurable CMV in body fluids such as blood is prevented by a competent host immune system acting to suppress the virus. If this response is inadequate, reactivation occurs (ie, CMV is present in body fluids). Cytomegalovirus is thus often detectable in immunosuppressed patients. Viral reactivation is associated with a wide range of clinical problems, and antiviral prophylaxis has become well established as therapy in high-risk settings, such as transplantation and therapeutic immunosuppression.
Critical illness impairs host defense mechanisms, particularly in patients with a systemic inflammatory response; this impairment increases the risk of CMV reactivation, which has been reported as affecting up to 30% of critically ill patients. Clinical risk factors associated with reactivation of CMV include the duration of intensive care unit (ICU) stay, pneumonia, sepsis, and high disease severity. There are also many biological factors that act to increase the frequency of CMV reactivation in critical illness, including direct stimulation of viral replication resulting from release of endotoxins and inflammatory cytokines and increased levels of catecholamines. In addition to direct tissue damage, CMV viremia may itself suppress normal immune function and increase the risk of secondary infectious complications. Systematic reviews have demonstrated that mortality among patients with CMV reactivation was, on average, doubled compared with those without viremia. Limaye et al have further demonstrated a direct correlation between CMV viral load and mortality.
Several antiviral agents are available with activity against CMV. Both valganciclovir hydrochloride and valacyclovir hydrochloride (orally active forms of ganciclovir sodium and acyclovir sodium) are used for prophylaxis against CMV reactivation in organ transplant recipients. However, despite the potential for benefit, to our knowledge, there are currently no data evaluating the efficacy of antiviral agents as prophylaxis for viral reactivation in nonimmunosuppressed patients in the ICU.
We performed a proof-of-principle study, designed to assess the efficacy, safety, and feasibility of antiviral prophylaxis for suppressing CMV reactivation in critically ill patients receiving care in the ICU. Two active treatment arms were chosen: 1 using low-dose valganciclovir and the other using valacyclovir. Both regimens have been used widely outside critical care settings. Valganciclovir has been shown to have a less favorable adverse effect profile but demonstrates increased activity against CMV. In contrast, valacyclovir requires administration of high dosages because of its relatively limited activity against CMV, but it is generally well tolerated. Here we report the results of this study.
Methods
Study Design
We conducted a single-center, proof-of-principle, open-label, randomized, controlled clinical, 3-armed trial of 2 anti-CMV prophylaxis treatments and standard care (no antiviral prophylaxis; control group) for patients who were seropositive for CMV receiving care in the ICU at Queen Elizabeth Hospital Birmingham, Birmingham, England, between January 1, 2012, and January 31, 2014. The study was approved by the National Research Ethics Service Committee, London, England. The study protocol is in Supplement 1. We adopted a 2-stage contingent consent process: first for a screening sample to determine CMV positivity, and second for recruitment to the interventional trial in the event of positive screening. Generally, sedated patients did not have the capacity to give informed consent, so consent was sought from a personal or professional legal representative prior to randomization. Patient consent to continue as a trial participant was sought once capacity had been regained.
Participants
Patients were eligible for the study if they were seropositive for CMV, already in the ICU for more than 24 hours, and mechanically ventilated, with the ICU stay and mechanical ventilation anticipated to continue for at least 48 hours. Because the study was designed to examine patients without preexisting immune suppression, the following exclusions were applied: known or suspected congenital or acquired immunodeficiency, receipt of immunosuppressive medication within 30 days, and receipt of chemotherapeutic agents within 6 months. Corticosteroids were not an exclusion criterion if the dosage was less than 10 mg/d of prednisolone sodium phosphate (or equivalent), short courses of up to 1 mg/kg of prednisolone (or equivalent) for exacerbations of chronic obstructive pulmonary disease for up to 14 days, or “stress dose” (given for relative adrenal insufficiency of critical illness) hydrocortisone sodium succinate up to 400 mg/d as part of intensive care support. Patients were also excluded from randomization if they tested as CMV IgG seronegative, if they were younger than 18 years of age, if onset of acute illness was more than 7 days at the point of randomization, if they were in receipt of systemic antiviral medication within 7 days (use of oseltamivir phosphate was allowed), if expected survival was less than 48 hours, if they had neutropenia (neutrophil count, <1000/µL [to convert to ×109 per liter, multiply by 0.001]), if they had experienced an isolated brain injury, if they had a known allergy to any of the study drugs, or if they were known to be pregnant or breastfeeding.
Intervention and Randomization
Eligible participants were randomized in a 1:1:1 ratio by telephone access to a computer-generated random treatment allocation sequence (Birmingham Clinical Trials Unit, Birmingham, England) to receive valganciclovir, valacyclovir, or control. The randomization was stratified by age (≤50 years or >50 years). Although patients and treating physicians were not masked to the assigned treatment group, CMV quantitative polymerase chain reaction (qPCR) results were not available during the study period and were processed in batches at a later date; laboratory staff were blinded to treatment allocation. Interim safety analyses were reviewed at 6 months, and at 2-month intervals thereafter by the independent Data Monitoring Committee.
Participants randomized to 1 of the 2 study drug arms received either valganciclovir or valacyclovir prophylaxis. Low-dose valganciclovir has been established as the mainstay of prophylaxis in other groups. High-dose valacyclovir has the benefit of activity against a wider group of viruses, as well as few toxic effects. Both low-dose valganciclovir and high-dose valacyclovir have been used successfully in trials of immunosuppressed patients and, subsequently, in the clinical setting. Patients randomized to the valganciclovir hydrochloride arm received 450 mg once a day by the enteral route. Patients in this group who were unable to receive enteral medication received intravenous ganciclovir sodium, 2.5 mg/kg ideal body weight, once a day until they were able to receive enteral medication. Patients randomized to the valacyclovir hydrochloride arm received 2 g 4 times a day by the enteral route. Patients unable to receive enteral medication received intravenous aciclovir sodium, 10 mg/kg of ideal body weight, 3 times a day until they were able to receive enteral medication.
In both arms, the study drug was initiated on the day of randomization and continued for 28 days in the ICU. The drug was discontinued after a minimum of 14 days if patients were discharged from the ICU to the ward. The drug was discontinued if patients were discharged from the hospital. Treatment dosing was modified in the presence of renal impairment (eTable 1 in Supplement 2). The study drug was withdrawn in the presence of severe neutropenia (neutrophil count, <1000/µL), as a requirement for granulocyte-colony stimulating factor therapy, or at the request of the clinical team overseeing patient care.
The patient group randomized to receive no antiviral prophylaxis received standard care. Antiviral medication could be initiated if the clinical team overseeing the care of the patient deemed it necessary for therapeutic reasons.
Data Collection
We obtained patients’ Acute Physiology and Chronic Health Evaluation II (APACHE II) scores from our local case-mix program database of the Intensive Care National Audit and Research Centre. Physiological and routine blood test results, including data allowing calculation of the daily Sequential Organ Failure Assessment (SOFA) score, were collected for 28 days or until death or discharge from hospital, if this was sooner. Patients were followed up until death or hospital discharge.
Specimens of plasma, urine, throat swab, and nondirected bronchiolar lavage (NDBL) fluid were collected on day 1 and at intervals of 5 (±1) days during the 28-day study period (on days 1, 6, 11, 16, 21, and 26), or until patient death or discharge from hospital, if this was sooner. Anonymized specimens were sent to the microbiology department of the University Hospitals Birmingham to be analyzed for the presence of CMV DNA using a qPCR assay (limit of detection, 20 copies/mL; Abbott Diagnostics). Nondirected bronchiolar lavage samples were obtained from patients with no contraindication, whose tracheas remained intubated at the required sampling time point. Blood specimens underwent analysis for levels of tumor necrosis factor (TNF) and interleukin 6 (IL-6) using Proseek Multiplex (Olink Bioscience). Protein levels were evaluated using assay-specific units: normalized protein expression (NPX) units, on a log2 scale, normalized to minimize intra-assay and interassay variation, in which a high value corresponds to a high protein level.
Outcomes
The primary outcome measure was time to first reactivation of CMV in blood (defined as above the lower limit of the qPCR assay[20 copies/mL]) from initiation of the study drug until day 28, excluding patients if viral reactivation had already taken place before initiation of the study drug on the day of enrollment. Secondary outcomes were time to first reactivation of CMV by day 28 in urine, throat swab, and NDBL specimens. Time to more than 1000 copies/mL and more than 10 000 copies/mL, peak CMV viral load, and area under the curve were also planned analyses. Secondary clinical outcome measures included mortality by 28 days after randomization; organ failure–free days (SOFA score, <2) and moderate organ dysfunction–free days (SOFA score, <5) at 28 days; and time to discharge from the ICU and time to discharge from the hospital. Assessments of drug safety were time to neutropenia, time to thrombocytopenia (platelet count, <50 × 103/µL [to convert to ×109 per liter is a 1:1 conversion]), requirement for rescue granulocyte-colony stimulating factor therapy or premature cessation of study drug, number of platelet transfusions, and development of renal impairment (creatinine clearance, <60 mL/min [to convert to milliliters per minute per 1.73 m2, divide by body surface area and then multiply by 1.73]) or severe renal impairment (creatinine clearance, <30 mL/min or requirement for renal replacement therapy). Cytokine analysis was performed on blood specimens, with change in TNF and IL-6 from time of randomization to day 14 and day 28 selected a priori as exploratory outcome measures.
Statistical Analysis
The sample size was based on studies of CMV reactivation rates among patients in the ICU who were seropositive for CMV, where reactivation rates of up to 30% have been observed, and high drug efficacy has been seen in other patient populations. The target sample of 141 patients (47 patients in each group) was determined using 90% power at P = .05 to detect a difference in CMV reactivation from 30% in the control group to 5% in the treatment group in critically ill patients who were seropositive for CMV.
Primary analyses compared the combined treatment groups with the control group. As the valacyclovir arm was ended early owing to safety concerns, it was decided by the trial team and Birmingham Clinical Trials Unit statisticians (who were blinded to the data at this time) to also compare valganciclovir with the control group. Time-to-event analyses were performed using survival analysis methods to compare time to first CMV reactivation between groups. Kaplan-Meier plots were produced, and unadjusted Cox proportional hazards regression models were used to report hazard ratios (HRs) and 95% CIs. All analyses were performed on an intention-to-treat principle, whereby patients included in the analysis were analyzed according to the treatment group to which they were randomized regardless of whether they received this treatment. As the primary outcome of the study was to measure the efficacy of antiviral drugs to prevent CMV reactivation, patients were excluded from the analyses of CMV viral load if viral reactivation had already taken place before initiation of the study drug on the day of recruitment. In the event of patient discharge from hospital or death, the results were censored at the most proximate blood CMV qPCR sample point. Analysis of clinical and safety outcomes included all patients and therefore included those who had reactivated CMV in any body fluid at baseline. All analyses were undertaken using SAS, version 9.2 (SAS Institute, Inc).
Results
Patients
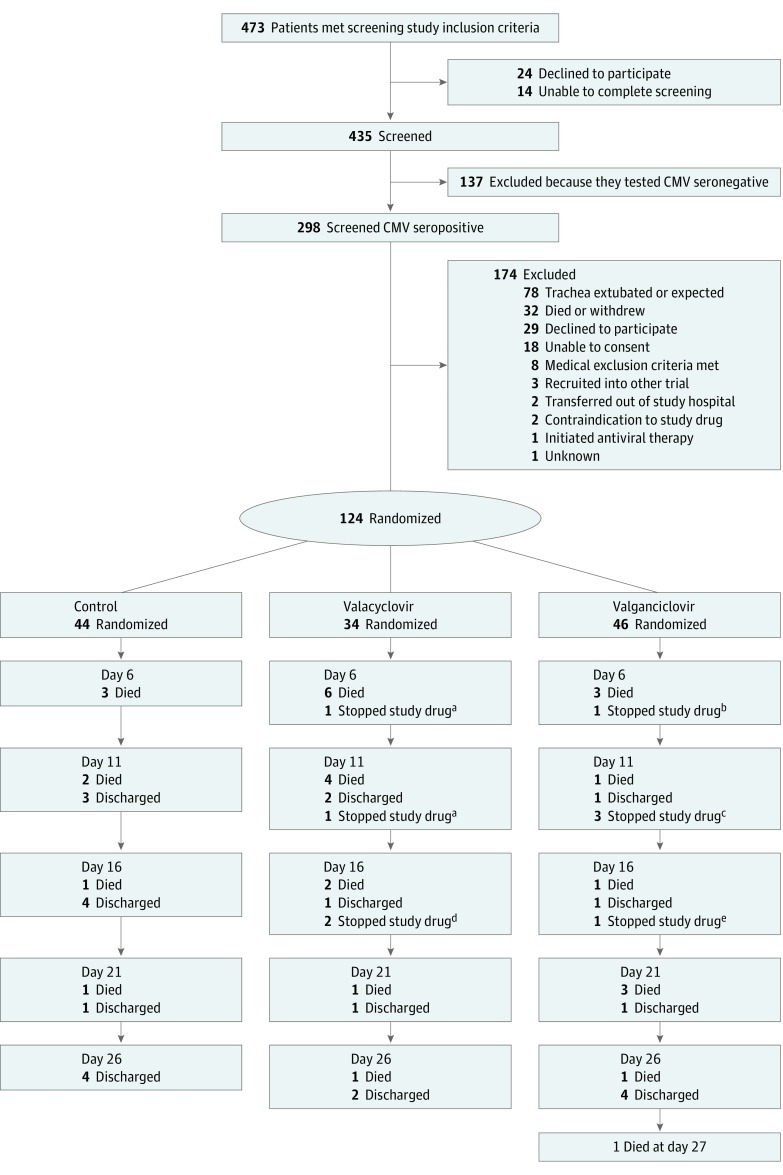
Between January 1, 2012, and January 31, 2014, a total of 124 patients were randomized into the trial: 44 in the control group, 34 in the valacyclovir group (recruitment to this arm stopped prematurely), and 46 in the valganciclovir group (Figure 1). Baseline characteristics were similar across the 3 groups (eTable 2 in Supplement 2). Disease severity scoring at admission to the ICU was similar between the groups, with a mean APACHE II score of 17.5 in the control group, 17.9 in the valacyclovir group, and 17.4 in the valganciclovir group. The main category of diagnosis at enrollment is shown in eTable 2 in Supplement 2.
Figure 1. Trial Flowchart.
Following randomization, the number of deaths and numbers having study drug stopped for each group are shown between planned sampling time points.
aPalliative care initiated (died on the same day).
bLow platelet count (died on the same day).
cLow platelet count (died 10 days later), rash (discharged 20 days later), or drug stopped at request of the family.
dRash (died 4 days later) or rash (discharged).
eAllergic reaction to study drug (discharged 12 days later).
Cessation of Valacyclovir Arm
Recruitment into the valacyclovir arm stopped prematurely in September 2013, following an interim analysis presented to the independent Data Monitoring Committee, which advised that this arm be closed because of significantly higher mortality in this group. At this point, 34 participants had been recruited into the valacyclovir arm, 14 (41.2%; 95% CI, 24.6%-57.7%) of whom had died by 28 days, compared with 5 of 37 participants (13.5%; 95% CI, 2.5%-24.5%) in the control arm and 7 of 34 participants (20.6%; 95% CI, 7.0%-34.2%) in the valganciclovir arm. To investigate potential associations between valacyclovir and cause of death, an independent case record review was performed. Reviewers were intensive care physicians independent of the study team; each set of case notes was examined by 2 reviewers blinded to group allocation. The reviewers identified all deaths as expected and attributable to the underlying disease. By the end of the study, mortality in the control group increased from 13.5% to 15.9% (7 of 44 participants) for 28-day mortality and to 20.5% (9 of 44 participants) for in-hospital mortality.
Adherence to the Study Drug
Nine participants had receipt of the study drug stopped prematurely during the 28-day trial period (4 in the valacyclovir group and 5 in the valganciclovir group). Two patients in the valacyclovir group had receipt of the study drug stopped after 5 and 7 days, respectively, by the supervising clinician because of a change to palliative care. One patient in the valganciclovir group had receipt of the study drug stopped after 6 days at the request of his or her personal legal representative (with permission to continue sampling and data collection following withdrawal of study drug). The other 6 patients had receipt of the study drug stopped prematurely because of possible adverse events (n = 2) or serious adverse events (n = 4). In the valacyclovir group, 2 patients had receipt of the study drug stopped owing to rashes; 4 patients stopped receipt of the drug early in the valganciclovir group, 1 owing to allergic reaction, 1 owing to a rash, and 2 owing to clinical concerns associated with low platelet counts (Figure 1). No patients had receipt of the study drug stopped because of the predefined stopping points of neutropenia or use of granulocyte-colony stimulating factor therapy.
Primary Outcome
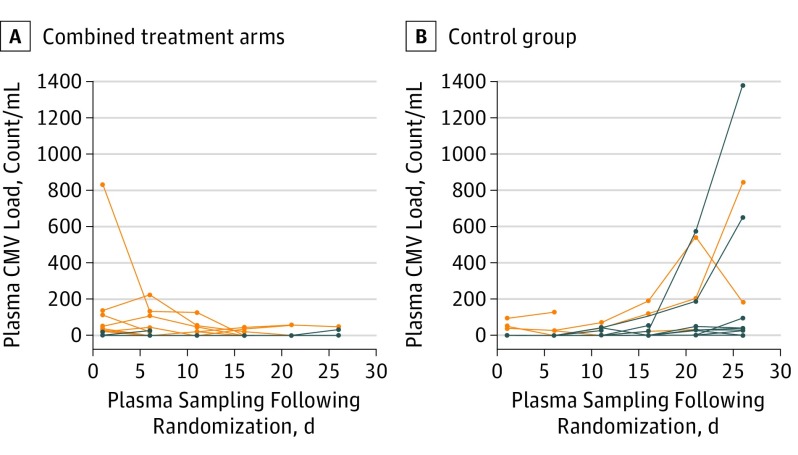
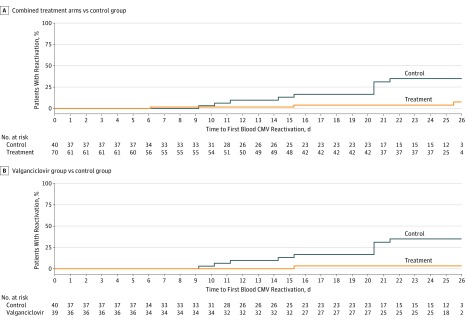
Figure 2 shows CMV blood viral load over time and includes all enrolled patients split into 2 groups: those receiving antiviral prophylaxis of any form and those in the control arm receiving no antivirals. Fourteen patients had CMV viremia on the day of enrollment (Figure 2) and were therefore excluded from the primary analysis of drug efficacy (time to reactivation). Viral reactivation in blood occurred in 12 patients randomized to the control group compared with 3 patients in the combined active treatment group (Kaplan-Meier, 35% vs 8%; HR, 0.14; 95% CI, 0.04-0.50; P = .002 for combined treatment group vs control) (Table 1 and Figure 3A). There was 1 reactivation in the low-dose valganciclovir group (HR, 0.08; 95% CI, 0.01-0.58; P = .01 for valganciclovir vs control; Figure 3B).
Figure 2. Cytomegalovirus (CMV) Viral Load in Blood .
A, Combined valacyclovir and valganciclovir arms. B, Control group. Each line represents a single patient. Orange lines represent patients who had CMV viremia on the day of enrollment and thus were excluded from the primary analysis of time to CMV reactivation. All enrolled patients are included in graphs to show differences in viral load over time with or without antiviral prophylaxis.
Table 1. Reactivation and Peak Viral Load Data for CMV Viral Load by PCRa.
| Data | Control (n = 44) |
Valacyclovir (n = 34) |
Valganciclovir (n = 46) |
Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|
| Combined vs Control | Valganciclovir vs Control | ||||
| Blood | |||||
| Reactivations, No. | 12 | 2 | 1 | 0.1 (0.04-0.5) |
0.08 (0.01-0.6) |
| Viral load for first positive PCR, median (range), copies/mL | 33.0 (22-95) |
29.5 (27-32) |
37 | NA | NA |
| Peak viral load in patients who had reactivated CMV, median (range), copies/mL | 37.5 (25-1382) |
29.5 (27-32) |
60 | NA | NA |
| Urine | |||||
| Reactivations, No. | 4 | 0 | 0 | NE | NE |
| Peak viral load in patients who had reactivated CMV, median (range), copies/mL | 48.5 (28-278) |
NA | NA | ||
| NDBL | |||||
| Reactivations, No. | 2 | 0 | 2 | 0.2 (0.02-2.6) |
0.3 (0.03-3.5) |
| P value | 0.2 | 0.3 | |||
| Peak viral load in patients who had reactivated CMV, median (range), copies/mL | 843 (22-1664) |
NA | 423.5 (25-822) |
NA | NA |
| Throatb | |||||
| Reactivations, No. | 4 | 2 | 1 | 0.5 (0.1-2.3) |
0.3 (0.03-2.4) |
| P value | 0.4 | 0.2 | |||
| Any body fluidc | |||||
| Reactivations, No. | 14 | 2 | 2 | 0.2 (0.05-0.5) |
0.1 (0.03-0.5) |
Abbreviations: CMV, cytomegalovirus; NA, not applicable; NDBL, nondirected bronchiolar lavage; NE, not estimable; PCR, polymerase chain reaction.
Patients who reactivated at baseline (on day 1) are excluded from this analysis (14 for blood, 2 for urine, 6 for NDBL, and 6 for throat swab), patients with only data at baseline are censored at day 1 (12 for blood, 11 for urine, 47 for NDBL, and 11 for throat swab), and patients are censored at discharge from hospital or death if this occurred before the end of scheduled sampling. Patients with no samples (0 for blood, 7 for urine, 38 for NDBL, and 0 for throat swab) are censored at day 1.
Throat swabs are either positive or negative, so viral loads are not presented.
Reactivation in blood, urine, NDBL, or throat swab at any point excluding baseline.
Figure 3. Time to Cytomegalovirus (CMV) Viral Reactivation in Blood.
A, Time to CMV viral reactivation in blood in combined treatment groups (valacyclovir and valganciclovir arms) vs control group (hazard ratio, 0.14; 95% CI, 0.04-0.50). B, Time to CMV viral reactivation in blood in valganciclovir group vs control group (hazard ratio, 0.08; 95% CI, 0.01-0.58).
Secondary Outcomes
Data on the reactivation of CMV in blood, urine, NDBL, and throat swab are presented in Table 1. Blood was the most sensitive body fluid for the demonstration of CMV reactivation, with 15 of 18 patients (83.3%) who experienced reactivation in any body fluid doing so in their blood, although this finding may reflect the more complete data set for blood. Nondirected bronchiolar lavage data were available beyond baseline for only 28.0% of patients (33 of 118) owing to difficulties in obtaining subsequent samples (compared with 89.1% for blood [98 of 110 patients], 85.2% for urine [104 of 122 patients], and 90.7% for throat swab [107 of 122 patients]), which means that these data should be interpreted with caution.
Clinical end points are shown in Table 2. In total, 9 of 44 patients died in the hospital in the control group compared with 15 of 34 patients in the valacyclovir group and 12 of 46 patients in the valganciclovir group. The relative risk for hospital mortality was 1.3 (95% CI, 0.6-2.7) in the valganciclovir group vs control and 2.2 (95% CI, 1.1-4.3) for the valacyclovir group vs control. Seven patients (15.9%) in the control group reported serious adverse events compared with 10 patients (29.4%) in the valacyclovir group and 16 patients (34.8%) in the valganciclovir group. The relative risk for a patient experiencing a serious adverse event was 1.8 (95% CI, 0.8-4.4) when comparing the valacyclovir group with the control group, 2.2 (95% CI, 1.0-4.8) when comparing the valganciclovir group with the control group, and 2.0 (95% CI, 1.0-4.3) when comparing the combined treatment groups with the control group. The time to renal impairment was similar between the combined treatment groups and the control group (HR, 1.2; 95% CI, 0.7-2.0), as was time to severe renal impairment (HR, 1.0; 95% CI, 0.6-1.8). Comparing valacyclovir with control gave similar results for renal impairment (HR, 1.5; 95% CI, 0.9-2.8) and severe renal impairment (HR, 1.2; 95% CI, 0.6-2.4). There was no evidence of any difference in levels of bone marrow suppression between groups. There were no reports of neutropenia, and the risk of thrombocytopenia was similar between the combined treatment groups and the control group (HR, 1.0; 95% CI, 0.5-2.2).
Table 2. Clinical, Safety, and Exploratory Cytokine Outcomes.
| Outcome | Control (n = 44) |
Valacyclovir (n = 34) |
Valganciclovir (n = 46) |
|---|---|---|---|
| Secondary Clinical Measures | |||
| Organ failure–free days (SOFA score <2), median (IQR) [range] | 3.5 (0-18) [0-31] |
1.5 (0-13) [0-24] |
2.0 (0-11) [0-36] |
| Moderate organ failure–free days (SOFA score <5), median (IQR) [range] | 18.0 (2-24) [0-41] |
11.0 (0-22) [0-28] |
16.5 (4-21) [0-44] |
| Discharged from ICU by 3 mo, No. (%)a | 36 (81.8) | 21 (61.8) | 34 (73.9) |
| Discharged from hospital by 3 mo, No. (%)a | 30 (68.2) | 17 (50.0) | 28 (60.9) |
| ICU duration of stay, median (IQR), d | 11.5 (7-16) | 12.0 (7-31) | 16.0 (11-27) |
| SAEs forms returned, No. | 7 | 12 | 18 |
| Patients reporting SAEs, No. (%) | 7 (15.9) | 10 (29.4) | 16 (34.8) |
| Mortality at 28 d, No. (%) | 7 (15.9) | 14 (41.2) | 10 (21.7) |
| Mortality in the hospital, No. (%) | 9 (20.5) | 15 (44.1) | 12 (26.1) |
| Safety Measures | |||
| Requirement for G-CSF therapy, No. (%) | 0 | 0 | 0 |
| Neutropenia (<1000/µL), No. (%) | 0 | 0 | 0 |
| Platelet count (<50 × 103/µL), No. (%) | 10 (22.7) | 9 (26.5) | 10 (21.7) |
| Platelet transfusions, No. | 44 | 32 | 42 |
| Median (IQR) | 0 (0-0) | 0 (0-0.5) | 0.2 (0-1) |
| Renal insufficiency, No. (%) | |||
| CrCl <60 mL/min | 23 (52.3) | 22 (64.7) | 24 (52.2) |
| CrCl <30 mL/min or required dialysis | 19 (43.2) | 16 (47.1) | 18 (39.1) |
| Exploratory Cytokine Analyses | Control |
Mean Difference (95% CI)
(Valganciclovir vs Control) |
Valganciclovir |
| TNF, NPX unitsb | |||
| Mean change (SD) between day 0 and day 14 [No. of patients] | 0.07 (0.18) [n = 23] |
0.05 (0.33) [n = 26] |
|
| Mean difference (95% CI) | −0.01 (−0.16 to 0.14) | ||
| Mean change (SD) between day 0 and day 28 [No. of patients] | 0.04 (0.15) [n = 23] |
−0.01 (0.10) [n = 22] |
|
| Mean difference (95% CI) | −0.05 (−0.13 to 0.02) | ||
| IL-6, NPX unitsb | |||
| Mean change (SD) between day 0 and day 14 [No. of patients] | −1.09 (2.46) [n = 23] |
−1.34 (1.82) [n = 26] |
|
| Mean difference (95% CI) | −0.25 (−1.48 to 0.98) | ||
| Mean change (SD) between day 0 and day 28 [No. of patients] | −1.91 (2.14) [n = 23] |
−2.01 (2.18) [n = 22] |
|
| Mean difference (95% CI) | −0.11 (−1.41 to 1.20) | ||
Abbreviations: CrCl, creatinine clearance; G-CSF, granulocyte-colony stimulating factor; ICU, intensive care unit; IL-6, interleukin 6; IQR, interquartile range; NPX, normalized protein expression; SAEs, serious adverse events; SOFA, Sequential Organ Failure Assessment; TNF, tumor necrosis factor.
SI conversion factors: to convert neutrophils to ×109 per liter, multiply by 0.001; to convert platelets to ×109 per liter is a 1:1 conversion; to convert CrCl to milliliters per minute per 1.73 m2, divide by body surface area and then multiply by 1.73.
Based on patients who remained alive and could be discharged from hospital. By 3 months, all patients (except 1) who could have been discharged (ie, alive patients) had been discharged from the hospital.
For day 14, samples taken between days 13 and 15 were used. For day 28, samples taken between days 21 and 28 were used.
Exploratory Outcomes
A preplanned exploratory analysis assessing changes in TNF and IL-6 from time of randomization to day 14 and day 28 was undertaken. To accommodate variations in sampling times, we used time windows rather than a fixed time point; if samples for the prespecified days were not available, samples from days 13 to 15 were included in the day 14 analysis, and samples from days 21 to 28 were included in the day 28 analysis. These data were available only for patients who were in the hospital at the time the sample was taken, and, as such, the analysis is based on a limited group of patients. Results from patients randomly assigned to valganciclovir were compared with results from controls. The mean difference in change in TNF was –0.01 (95% CI, −0.16 to 0.14) between day 0 and day 14 and –0.05 (95% CI, −0.13 to 0.02) between day 0 and day 28 (Table 2). The mean difference in change in IL-6 was –0.25 (95% CI, −1.48 to 0.98) between day 0 and day 14 and –0.11 (95% CI, −1.41 to 1.20) between day 0 and day 28.
Time to more than 1000 copies/mL and more than 10 000 copies/mL and the area under the curve were also planned analyses for the data on blood, urine, NBDL, and throat swab samples. There were 3 samples with more than 1000 copies/mL (1 in blood and 2 in NDBL) and no samples with more than 10 000 copies/mL, and incomplete sample profiles caused by death or discharge from the hospital or nonavailability of sampling access (patients whose tracheas were extubated, preventing NDBL) limited the utility of area under the curve analyses.
Discussion
The results of this study, designed to assess the efficacy, safety, and feasibility of antiviral prophylaxis for critically ill patients who are seropositive for CMV and are not immunosuppressed, demonstrate that CMV reactivates in 35% of critically ill patients and that reactivation can be reduced to 3% in such patients through the use of antiviral prophylaxis with low-dose valganciclovir. Blood was the most sensitive body fluid for the detection of CMV reactivation in our study, with 83.3% of patients who experienced reactivation doing so in at least their blood. This finding may reflect the more complete data set for blood since it was not possible to collect other specimens as consistently because of patient constraints, including anuria (urine) or tracheal extubation (NDBL). Viral loads for patients with CMV viremia were generally low (range, 25-1382 copies/mL).
Although valacyclovir was effective at suppressing CMV, it was difficult to administer (high frequency of administration of both routes, inability to crush and administer valacyclovir enterally via nasogastric tube, and potential risk of thrombophlebitis from intravenous administration). Valacyclovir was associated with increased mortality, although it is unclear why. All study deaths were classed as expected and attributable to the underlying disease by independent blinded reviewers. The incidence of renal impairment and bone marrow suppression, both potential adverse effects of the treatment drugs, were similar between groups, although this study was not powered to identify differences. It is possible that critically ill patients, who commonly develop significant organ dysfunction, are more susceptible to the potential adverse effects of antiviral drugs, although these drugs have long been used safely for suppression of CMV reactivation in patients undergoing a transplant.
Herpesviruses have the capacity to modify host immune defenses, including TNF-regulated signaling pathways. Elevated levels of TNF and IL-6 may mediate the higher mortality rates associated with CMV antibody response in an elderly Latino population. Survivors of critical illness with CMV reactivation are reported to have a more marked proinflammatory biomarker profile (including elevated IL-6 levels) 3 months after ICU discharge than are survivors who are seronegative for CMV. However, in our exploratory analysis, we found no consistent pattern in trends for IL-6 or TNF between the study groups (Table 2). We suspect that if CMV-associated cytokine changes occur, they are likely to be masked by the proinflammatory and anti-inflammatory components of acute critical illness.
Limitations
There are several limitations to our study. Although this was a single-center study, and the representativeness of patients and generalizability of the results would have been improved by recruiting from multiple sites, this study was conducted in the largest ICU in Europe, with 100 beds, including both general and specialty intensive care beds. For practical reasons, the study was necessarily open label. The primary outcome measure of time to CMV reactivation in blood was felt to be robust because laboratory staff were blinded to treatment allocation. The lack of blinding could, however, have influenced other data, such as the recording of adverse events. Although one-third of patients in the control arm demonstrated CMV viremia, the viral loads were generally low when compared with those found in immunosuppressed patients, which may be relevant when assessing the importance of viral suppression with drugs. The valacyclovir arm was terminated early because of safety concerns, which led to a smaller sample size than planned in this group. Valacyclovir has been used for many years and has been examined in other studies outside of the critical care setting, with few adverse effects, with the additional benefit over valganciclovir of activity against other herpesviruses. However, the challenges of administering the drug to critically ill patients make it an unsuitable option for any subsequent trial powered to assess clinical outcomes. It could be questioned whether prophylactic therapy, as chosen in this study, is preferable to early treatment of active CMV reactivation. Prophylaxis is conceptually more attractive because it prevents viral reactivation before direct systemic and tissue injury takes place, and it is simpler to use when many ICUs do not have access to rapid CMV assay. Prophylaxis has been chosen as the standard in many other populations, with more effective CMV suppression, although at the expense of a higher incidence of adverse effects. However, treatment following reactivation minimizes the population’s exposure to the drug, which may be particularly important in the setting of polypharmacy associated with critical illness.
Conclusions
Our study demonstrates that antiviral prophylaxis effectively suppresses CMV reactivation in critically ill patients and is best achieved through the use of low-dose valganciclovir administered enterally or administered intravenously as ganciclovir. Valacyclovir was associated with increased mortality, although it was not possible to identify a causal link between drug administration and death. The safety and efficacy of antiviral prophylaxis to prevent CMV reactivation in this setting can be determined only by conducting a large-scale trial with close monitoring of clinical and patient-centered outcomes.
Trial Protocol
eTable 1. Study Drug Adjustment for Renal Impairment
eTable 2. Patient Baseline Characteristics
References
- 1.Tookey PA, Ades AE, Peckham CS. Cytomegalovirus prevalence in pregnant women: the influence of parity. Arch Dis Child. 1992;67(7 Spec No):779-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50(11):1439-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43(9):1143-1151. [DOI] [PubMed] [Google Scholar]
- 4.Yahav D, Gafter-Gvili A, Muchtar E, et al. . Antiviral prophylaxis in haematological patients: systematic review and meta-analysis. Eur J Cancer. 2009;45(18):3131-3148. [DOI] [PubMed] [Google Scholar]
- 5.Hodson EM, Craig JC, Strippoli GF, Webster AC. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2008;(2):CD003774. [DOI] [PubMed] [Google Scholar]
- 6.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143(12):870-880. [DOI] [PubMed] [Google Scholar]
- 7.Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian Society of Transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5(2):218-227. [DOI] [PubMed] [Google Scholar]
- 8.Puius YA, Snydman DR. Prophylaxis and treatment of cytomegalovirus disease in recipients of solid organ transplants: current approach and future challenges. Curr Opin Infect Dis. 2007;20(4):419-424. [DOI] [PubMed] [Google Scholar]
- 9.Limaye AP, Kirby KA, Rubenfeld GD, et al. . Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001;29(3):541-547. [DOI] [PubMed] [Google Scholar]
- 11.Cook CH, Martin LC, Yenchar JK, et al. . Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31(7):1923-1929. [DOI] [PubMed] [Google Scholar]
- 12.von Müller L, Klemm A, Weiss M, et al. . Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006;12(10):1517-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. 2008;36(12):3145-3150. [DOI] [PubMed] [Google Scholar]
- 14.Chiche L, Forel J-M, Roch A, et al. . Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37(6):1850-1857. [DOI] [PubMed] [Google Scholar]
- 15.Jaber S, Chanques G, Borry J, et al. . Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005;127(1):233-241. [DOI] [PubMed] [Google Scholar]
- 16.Papazian L, Fraisse A, Garbe L, et al. . Cytomegalovirus: an unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84(2):280-287. [DOI] [PubMed] [Google Scholar]
- 17.Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. 1990;97(1):18-22. [DOI] [PubMed] [Google Scholar]
- 18.Coisel Y, Bousbia S, Forel JM, et al. . Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One. 2012;7(12):e51340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stéphan F, Méharzi D, Ricci S, Fajac A, Clergue F, Bernaudin JF. Evaluation by polymerase chain reaction of cytomegalovirus reactivation in intensive care patients under mechanical ventilation. Intensive Care Med. 1996;22(11):1244-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998;26(5):1076-1082. [DOI] [PubMed] [Google Scholar]
- 21.Desachy A, Ranger-Rogez S, François B, et al. . Reactivation of human herpesvirus type 6 in multiple organ failure syndrome. Clin Infect Dis. 2001;32(2):197-203. [DOI] [PubMed] [Google Scholar]
- 22.Razonable RR, Fanning C, Brown RA, et al. . Selective reactivation of human herpesvirus 6 variant A occurs in critically ill immunocompetent hosts. J Infect Dis. 2002;185(1):110-113. [DOI] [PubMed] [Google Scholar]
- 23.Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. 2009;37(8):2350-2358. [DOI] [PubMed] [Google Scholar]
- 24.Prösch S, Wendt CE, Reinke P, et al. . A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272(2):357-365. [DOI] [PubMed] [Google Scholar]
- 25.Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006;34(3):842-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1β triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. 2006;80(18):9151-9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka S, Toh Y, Minagawa H, Mori R, Sugimachi K, Minamishima Y. Reactivation of cytomegalovirus in patients with cirrhosis: analysis of 122 cases. Hepatology. 1992;16(6):1409-1414. [DOI] [PubMed] [Google Scholar]
- 28.Ho M. Cytomegalovirus infection in patients with bacterial sepsis. Clin Infect Dis. 1998;26(5):1083-1084. [DOI] [PubMed] [Google Scholar]
- 29.Smith PD, Saini SS, Raffeld M, Manischewitz JF, Wahl SM. Cytomegalovirus induction of tumor necrosis factor-alpha by human monocytes and mucosal macrophages. J Clin Invest. 1992;90(5):1642-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews DM, Andoniou CE, Granucci F, Ricciardi-Castagnoli P, Degli-Esposti MA. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat Immunol. 2001;2(11):1077-1084. [DOI] [PubMed] [Google Scholar]
- 31.Freeman RB., Jr The ‘indirect’ effects of cytomegalovirus infection. Am J Transplant. 2009;9(11):2453-2458. [DOI] [PubMed] [Google Scholar]
- 32.Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care. 2009;13(3):R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forster MR, Trgovcich J, Zimmerman P, et al. . Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice. Antiviral Res. 2010;85(3):496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalil AC, Mindru C, Florescu DF. Effectiveness of valganciclovir 900 mg versus 450 mg for cytomegalovirus prophylaxis in transplantation: direct and indirect treatment comparison meta-analysis. Clin Infect Dis. 2011;52(3):313-321. [DOI] [PubMed] [Google Scholar]
- 35.Vincent JL, Moreno R, Takala J, et al. . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure: on behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707-710. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. [DOI] [PubMed] [Google Scholar]
- 37.Chase JG, Pretty CG, Pfeifer L, et al. . Organ failure and tight glycemic control in the SPRINT study. Crit Care. 2010;14(4):R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vincent JL, de Mendonça A, Cantraine F, et al. . Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study: working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793-1800. [DOI] [PubMed] [Google Scholar]
- 39.Sedý JR, Spear PG, Ware CF. Cross-regulation between herpesviruses and the TNF superfamily members. Nat Rev Immunol. 2008;8(11):861-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172(4):363-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffith DM, Lewis S, Rossi AG, et al. ; RECOVER Investigators . Systemic inflammation after critical illness: relationship with physical recovery and exploration of potential mechanisms. Thorax. 2016;71(9):820-829. [DOI] [PubMed] [Google Scholar]
- 42.Lowance D, Neumayer HH, Legendre CM, et al. ; International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group . Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. N Engl J Med. 1999;340(19):1462-1470. [DOI] [PubMed] [Google Scholar]
- 43.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant. 2008;8(1):69-77. [DOI] [PubMed] [Google Scholar]
- 44.Winston DJ, Yeager AM, Chandrasekar PH, Snydman DR, Petersen FB, Territo MC; Valacyclovir Cytomegalovirus Study Group . Randomized comparison of oral valacyclovir and intravenous ganciclovir for prevention of cytomegalovirus disease after allogeneic bone marrow transplantation. Clin Infect Dis. 2003;36(6):749-758. [DOI] [PubMed] [Google Scholar]
- 45.Reinke P, Prösch S, Kern F, Volk HD. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl Infect Dis. 1999;1(3):157-164. [DOI] [PubMed] [Google Scholar]
- 46.Ruell J, Barnes C, Mutton K, et al. . Active CMV disease does not always correlate with viral load detection. Bone Marrow Transplant. 2007;40(1):55-61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Study Drug Adjustment for Renal Impairment
eTable 2. Patient Baseline Characteristics