Key Points
Question
Does intensive blood pressure control provide better renoprotection for nondiabetic chronic kidney disease?
Findings
In this systematic review including 9 randomized clinical trials with 8127 patients and a median follow-up of 3.3 years, intensive and standard blood pressure control provided similar effects. However, nonblack patients and those with higher levels of proteinuria showed a trend of lower risk of kidney disease progression with intensive blood pressure–lowering treatments.
Meaning
Targeting blood pressure below the current standard is not consistently warranted, but may benefit nonblack patients or those with heavy proteinuria.
Abstract
Importance
The optimal blood pressure (BP) target remains debated in nondiabetic patients with chronic kidney disease (CKD).
Objective
To compare intensive BP control (<130/80 mm Hg) with standard BP control (<140/90 mm Hg) on major renal outcomes in patients with CKD without diabetes.
Data Sources
Searches of PubMed, MEDLINE, Embase, and Cochrane Library for publications up to March 24, 2016.
Study Selection
Randomized clinical trials that compared an intensive vs a standard BP target in nondiabetic adults with CKD, reporting changes in glomerular filtration rate (GFR), doubling of serum creatinine level, 50% reduction in GFR, end-stage renal disease (ESRD), or all-cause mortality.
Data Extraction and Synthesis
Random-effects meta-analyses for pooling effect measures. Meta-regression and subgroup analyses for exploring heterogeneity.
Main Outcomes and Measures
Differences in annual rate of change in GFR were expressed as mean differences with 95% CIs. Differences in doubling of serum creatinine or 50% reduction in GFR, ESRD, composite renal outcome, and all-cause mortality were expressed as risk ratios (RRs) with 95% CIs.
Results
We identified 9 trials with 8127 patients and a median follow-up of 3.3 years. Compared with standard BP control, intensive BP control did not show a significant difference on the annual rate of change in GFR (mean difference, 0.07; 95% CI, −0.16 to 0.29 mL/min/1.73 m2/y), doubling of serum creatinine level or 50% reduction in GFR (RR, 0.99; 95% CI, 0.76-1.29), ESRD (RR, 0.96; 95% CI, 0.78-1.18), composite renal outcome (RR, 0.99; 95% CI, 0.81-1.21), or all-cause mortality (RR, 0.81; 95% CI, 0.64-1.02). Intensive BP control reduced mortality (RR, 0.78; 95% CI, 0.61-0.99) in sensitivity analysis when the study populations were strictly restricted to those without diabetes. Nonblacks and patients with higher levels of proteinuria showed a trend of lower risk of kidney disease progression with intensive BP control.
Conclusions and Relevance
Targeting BP below the current standard did not provide additional benefit for renal outcomes compared with standard treatment during a follow-up of 3.3 years in patients with CKD without diabetes. However, nonblack patients or those with higher levels of proteinuria might benefit from the intensive BP-lowering treatments.
This meta-analysis compares intensive blood pressure control (<130/80 mm Hg) with standard blood pressure control (<140/90 mm Hg) on major renal outcomes in patients with chronic kidney disease without diabetes.
Introduction
Chronic kidney disease (CKD) is a global epidemic, and it leads to higher risks of dialysis, cardiovascular morbidity, and mortality.1,2,3 The prevalence of CKD varies from 8% to 16% worldwide, with nondiabetic CKD accounts for most of the CKD population.4,5,6,7 The development and progression of nondiabetic CKD are closely interrelated to hypertension, and blood pressure (BP) control is able to decrease the risk of decline in renal function and cardiovascular mortality.7,8,9,10,11 However, the optimal BP target for preventing kidney disease progression remain debated.
Major guidelines suggest a target of BP of less than 140/90 mm Hg for patients with nondiabetic CKD,12,13 and some suggest a further reduction to achieve a BP of less than 130/80 mm Hg for those with proteinuria.8,14 Previous randomized clinical trials (RCTs) and systematic reviews have examined the renoprotective effects of an intensive BP control in patients with nondiabetic CKD but reported conflicting results.15,16,17,18,19,20 Recently, the Systolic Blood Pressure Intervention Trial (SPRINT) reported that intensive BP control did not significantly reduce the risk of dialysis or declined renal function in nondiabetic patients with CKD, but rather increased the risk of acute kidney injury.21 In this systematic review and meta-analysis, we synthesized results from RCTs to evaluate the effects of intensive BP-lowering treatment on major renal outcomes and mortality in nondiabetic adults with CKD, and also assessed effect modification by proteinuria.
Methods
Data Sources and Literature Searches
We conducted electronic literature searches of PubMed, MEDLINE, Embase, and the Cochrane Library from the earliest available date of indexing through March 24, 2016. We also hand-searched the reference lists of identified publications for additional studies. The detailed study protocol and search strategies are provided in the eAppendix 1 in the Supplement.
Study Selection
We included RCTs comparing different BP targets in primarily nondiabetic CKD patients older than 18 years. Included studies had to report at least 1 of the outcomes: changes in glomerular filtration rate (GFR), doubling of serum creatinine level, 50% reduction in GFR, end-stage renal disease (ESRD), or all-cause mortality. Studies reporting outcomes from nondiabetic CKD subgroups were included. Eligible studies had to be published as full-length articles in peer-reviewed journals.
Data Extraction and Quality Assessment
Two investigators (W.-C.T. and H.-Y.W.) independently extracted relevant information from the included studies and evaluated the methodological quality of eligible trials by using the Cochrane Collaboration’s tool for assessing risk of bias.22 Disagreements between the 2 investigators were resolved by discussion.
Outcomes
Comparing the intensive BP-lowering treatment with the standard BP-lowering treatment during the in-trial follow-up period, our outcomes of interest were the annual rate of change in GFR, doubling of serum creatinine level, or 50% reduction in GFR, ESRD, and all-cause mortality. We also analyzed the composite renal outcome of the doubling of serum creatinine level, 50% reduction in GFR, or ESRD. ESRD was defined as the need for dialysis therapy or kidney transplantation.
Data Synthesis and Analysis
Categorical variables are presented as frequencies or percentages, and continuous variables are presented as mean values unless stated otherwise. The pooled estimates of effect measures and 95% CIs of comparisons between the intensive and standard BP-lowering treatments were calculated using both the fixed-effect model and the DerSimonian and Laird random-effects model.22 The effect size of continuous outcome (annual rate of change in GFR; milliliters per minutes per 1.73 m2 per year) was expressed as mean difference with 95% CI. We used estimation and imputation methods to reconstruct the missing values for annual rate of change in GFR as recommended in the Cochrane Handbook (eAppendix 2 in the Supplement).22 Effect sizes of binary outcomes (doubling of serum creatinine level or 50% reduction in GFR, ESRD, composite renal outcome, and all-cause mortality) were expressed as risk ratios (RRs) with 95% CIs. In consideration of between-study variance, we used the random-effects model as the primary analyses.23
Publication bias was examined using the funnel plot method and Egger regression asymmetry test.24,25 Heterogeneity of treatment effects across studies were assessed by I2 and the Cochrane Q-test.22 Meta-regression and subgroup analyses were performed to explore potential sources of heterogeneity and assess the associations between variables and intervention effects. We conducted meta-regression using mixed-effects model to assess the influences of mean age, race, mean baseline GFR, targeted systolic BP, study sample size, or the method of GFR measurement. Subgroup analysis was performed when a covariate was significant in the meta-regression. Owing to the wide range, the level of proteinuria was not suitable to be assessed as a study-level covariate in meta-regression or subgroup analyses. To determine whether the level of proteinuria influenced the effects of intensive BP-lowering treatment, we extracted available subpopulation data from each study and pooled their results for ESRD or annual rate of change in GFR. To assess the robustness of our meta-analyses, we undertook sensitivity analyses by omitting studies with imputed missing data, or studies that did not totally exclude diabetic patients. To compare with previous meta-analysis,19 we also carried out a sensitivity analysis by including the posttrial follow-up data of the Modification of Diet in Renal Disease (MDRD) study26 and the African American Study of Kidney Disease and Hypertension (AASK).27 A 2-sided P ≤ .05 was considered statistically significant. Statistical analyses were performed with R software (version 3.2.4; R Foundation for Statistical Computing).
Results
The flowchart in eFigure 1 in the Supplement shows the literature search process. Of the 1158 articles retrieved initially, 328 were excluded due to duplicate publication and 816 were excluded on the basis of titles and abstracts. Of the 14 that underwent full-text evaluation, 10 articles met the inclusion criteria.
Study Characteristics and Quality Assessment
There were 9 RCTs from 10 eligible articles, which enrolled a total of 8127 participants. The clinical and methodological characteristics of each study are summarized in Table 1 and Table 2. The median length of in-trial follow-up was 3.3 years (range, 1.6-7.0 years). The median age of the participants was 55 years, with men accounting for 61%. Six studies included mostly whites; 2, mostly blacks; and 1, mostly Asians. Most of the studies excluded all patients with diabetes. The Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients (JATOS)30 and the MDRD15 study excluded patients with poorly controlled diabetes, and a minor percentage of their study population had diabetes (12% and 5%, respectively). Included studies had similar baseline BP between the intensive and standard treatment groups, and the achieved difference in mean systolic BP varied from 4 to 13 mm Hg at the end of the trial. The risk of bias of included studies is summarized in eFigures 2 and 3 in the Supplement. The main causes of potential bias were open-label design, inadequate allocation concealment, and lack of blinding.
Table 1. Baseline Characteristics of Participants in Studies Included in the Systematic Review.
| Source | Country | Inclusion Criteria | Patient No. | CKD, % | Age, y | Female, % | Race, % |
|---|---|---|---|---|---|---|---|
| Klahr et al,15 (MDRD) 1994a | USA | Study A: GFR 25-55; study B: GFR 13-24; proteinuria level <10 g/d | A: 585; B: 255 | 100 | 52 | 40 | White, 85 |
| Toto et al,28 1995 | USA | HN; serum Cr 1.6-7.0; GFR ≤70; proteinuria ≤2 g/d | 77 | 100 | 56 | 37 | Black, 75 |
| Schrier et al,29 2002 | USA | ADPKD; LVH; CrCl >30; proteinuria ≤3 g/d | 75 | 100 | 41 | 45 | NA |
| Wright et al,16 (AASK) 2002 | USA | African Americans; GFR 20-65; proteinuria ≤2.5 g/d | 1094 | 100 | 55 | 39 | Black, 100 |
| Ruggenenti et al,17 (REIN-2) 2005 | Italy | Proteinuria 1-3 g/d and GFR <45, or proteinuria >3 g/d and GFR <70 | 338 | 100 | 54 | 26 | NA |
| Hayashi et al,30 (JATOS) 2010 | Japan | Serum Cr <1.5 | 4418 | 57 | 74 | 64 | Asian, 100 |
| Schrier et al,31 (HALT-PKD) 2014 | USA | ADPKD; GFR >60; proteinuria ≤0.5 g/d (Study A) | 558 | 100 | 37 | 49 | White, 93 |
| Wright et al,21 (SPRINT) 2015a | USA | GFR ≥20; proteinuria <1 g/d | 9361 | 28 | 68 | 36 | Black vs white, 31/58 |
Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; Cr, creatinine (mg/dL); CrCl, creatinine clearance (mL/min/1.73 m2); GFR, glomerular filtration rate (mL/min/1.73 m2); HALT-PKD, Halt Progression of Polycystic Kidney Disease; HN, hypertensive nephrosclerosis; HTN, hypertension; JATOS, Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients; LVH, left ventricular hypertrophy; MDRD, Modification of Diet in Renal Disease; NA, not available; REIN-2, Ramipril Efficacy In Nephropathy 2; SPRINT, Systolic Blood Pressure Intervention Trial.
Characteristics of the entire study population were provided.
Table 2. Characteristics of Included Studies in the Systematic Review.
| Source | Causes of CKD | GFR | Follow-up, y | Antihypertensive Regimens | Blood Pressure, mm Hg | Study End Points | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Target | Achieved | |||||||||
| Baseline | Method | Intensive | Standard | Intensive | Standard | ||||||
| Klahr et al,15 1994 (MDRD)a | GN, PKD | A: 38.6, B: 18.5 | 125I IOT clearance | 2.2 | ACEI with or without diuretic; CCB or other antihypertensive drugs as needed | 131/80 | MAP <92 | MAP <107 | 126/77 | 134/81 | Rate of change in GFR |
| Toto et al,28 1995 | HN | 38.3 | 125I IOT clearance | 3.4 | Enalapril vs placebo; diuretic, β-blocker, vasodilators, α-blocker, as needed | 123/76 | DBP 65-80 | DBP 85-95 | 133/81 | 138/87 | Rate of decline in GFR |
| Schrier et al,29 2002 | ADPKD | 83.0 | 24-h CrCl | 7.0 | Enalapril vs amlodipine | 143/96 | <120/80 | 135-140/85-90 | MAP 90 ± 5 | MAP 101 ± 4 | Change in GFR |
| Wright et al,16 2002 (AASK) | HN | 45.7 | 125I IOT clearance | 3.8 | Ramipril vs amlodipine vs metoprolol | 151/96 | MAP <92 | MAP 102-107 | 128/78 | 141/85 | Rate of change in GFR |
| Ruggenenti et al,17 2005 (REIN-2) | NA | 35.0 | Iohexol clearance | 1.6 | Ramipril + felodipine vs ramipril | 137/84 | <130/80 | DBP <90 | 130/80 | 134/82 | ESRD |
| Hayashi et al,30 2010 (JATOS) | NA | 48.8 | Japanese MDRD equation | 2.0 | Efonidipine; plus ACEI, ARB, diuretic, or β-blocker, as needed | 172/89 | SBP <140 | SBP 140-160 | NA | NA | Change in GFR; doubled Cr or ESRD |
| Schrier et al,31 2014 (HALT-PKD) | ADPKD | 91.5 | CKD-EPI equation | 5.7 | Lisinopril + telmisartan vs lisinopril + placebo | 127/80 | 95-110/60-75 | 120-130/70-80 | Difference: SBP, 13.4/DBP, 9.3 | Annual % of change in kidney volume | |
| Wright et al,21 2015 (SPRINT)a | HTN | 47.9 | MDRD | 3.3 | All major classes of antihypertensive drugs were acceptable | 140/78 | SBP <120 | SBP <140 | SBP 121.5 | SBP 134.6 | 50% Reduction in GFR or ESRD |
Abbreviations: ACEI, angiotensin converting enzyme inhibitors; ADPKD, autosomal dominant polycystic kidney disease; ARB, angiotensin receptor blocker; AASK, African American Study of Kidney Disease and Hypertension; CCB, calcium channel blocker; CKD, chronic kidney disease; CKD-EPI, CKD Epidemiology Collaboration; Cr, creatinine (mg/dL); CrCl, creatinine clearance (mL/min/1.73 m2); DBP, diastolic blood pressure; ESRD, end-stage renal disease; GFR, glomerular filtration rate (mL/min/1.73 m2); GN, glomerulonephritis; HALT-PKD, Halt Progression of Polycystic Kidney Disease; HN, hypertensive nephrosclerosis; HTN, hypertension; 125I, iodine-125; IOT, iothalamate; JATOS, Japanese Trial to Assess Optimal Systolic Blood Pressure in Elderly Hypertensive Patients; MAP, mean arterial pressure; MDRD, Modification of Diet in Renal Disease; NA, not available; REIN-2, Ramipril Efficacy In Nephropathy 2; SBP, systolic blood pressure; SPRINT, Systolic Blood Pressure Intervention Trial.
Characteristics of the entire study population were provided.
Effects of Intensive BP-Lowering Treatments on Kidney Disease Progression
During the in-trial follow-up period, there were 194 patients whose serum creatinine level doubled or GFR declined by 50%, 314 with ESRD, 306 with composite renal outcomes, and 276 deaths. The Figure shows the pooled estimates for all study outcomes. Compared with the standard BP-lowering strategy, intensive BP lowering did not show a significant difference on the annual rate of change in GFR (mean difference, 0.07; 95% CI, −0.16 to 0.29 mL/min/1.73 m2/y) (Figure, A), doubling of serum creatinine level or 50% reduction in GFR (RR, 0.99; 95% CI, 0.76-1.29) (Figure, B), ESRD (RR, 0.96; 95% CI, 0.78-1.18) (Figure, C), composite renal outcome (RR, 0.99; 95% CI, 0.81-1.21) (Figure, D), or all-cause mortality (RR, 0.81; 95% CI, 0.64-1.02) (Figure, E). The funnel plots and the Egger regression asymmetry test indicated no significant publication bias for any outcome (eFigure 4 in the Supplement). There was no statistical heterogeneity for any outcomes (I2 = 0%; P > .05) (Figure).
Figure. Pooled Estimates Comparing Intensive Blood Pressure Control With Standard Blood Pressure Control on the Study Outcomes.
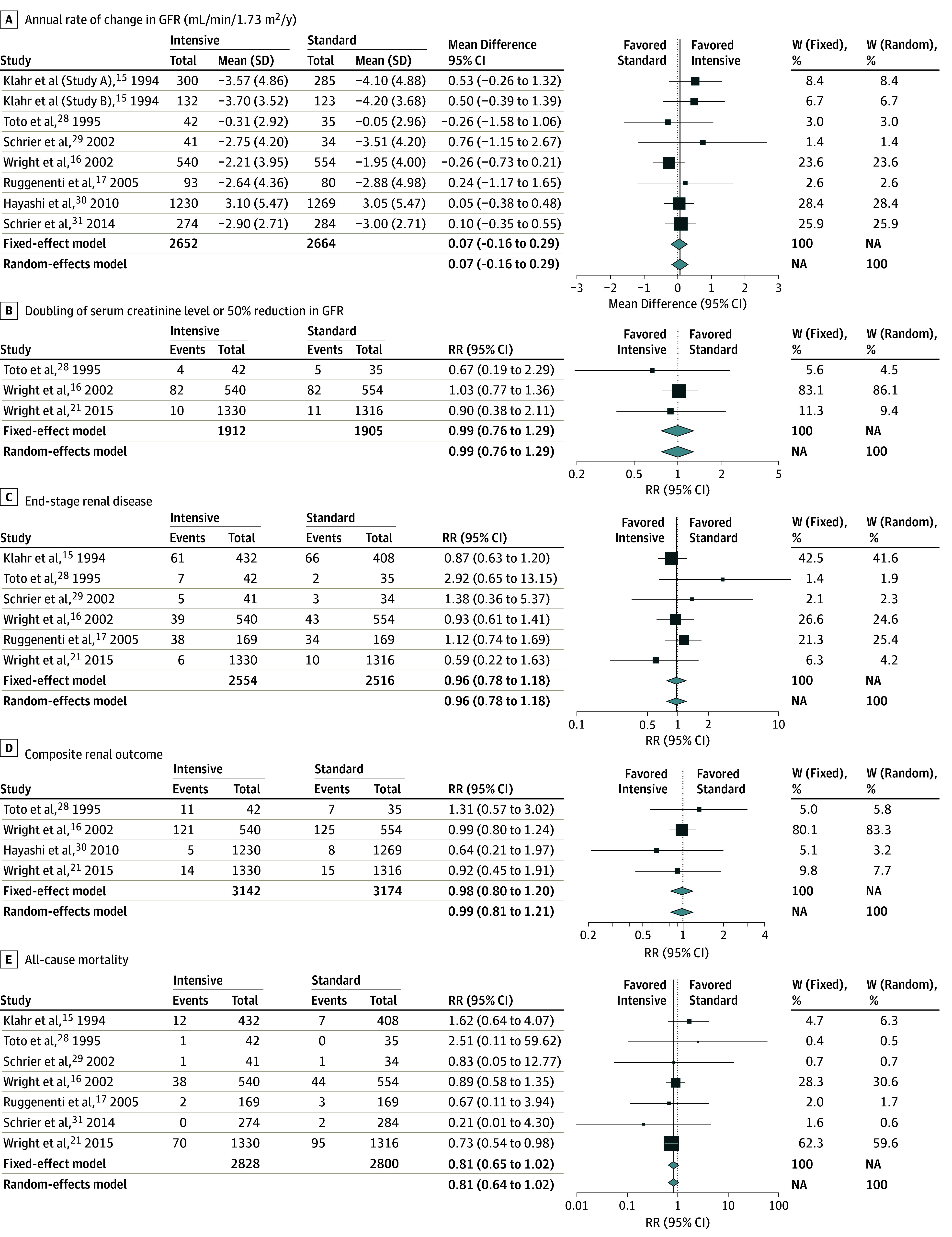
A, Heterogeneity: I2 = 0%; τ2 = 0; P = .67. B, Heterogeneity: I2 = 0%; τ2 = 0; P = .78. C, Heterogeneity: I2 = 0%; τ2 = 0; P = .53. D, Heterogeneity: I2 = 0%; τ2 = 0; P = .79. E, Heterogeneity: I2 = 0%; τ2 = 0; P = .66. For study outcomes C and E, Klahr et al15 reported information from their study A and study B together. GFR indicates glomerular filtration rate; W, weight.
Results were similar after omitting studies with imputed missing data for the annual rate of change in GFR (mean difference, 0.09; 95% CI, −0.38 to 0.55 mL/min/1.73 m2/y) (eFigure 5 in the Supplement). In sensitivity analyses omitting results of the JATOS and MDRD studies, which enrolled a small percentage of diabetic patients, results were also similar except for a reduced mortality in patients treated with intensive BP-lowering strategy (RR, 0.78; 95% CI, 0.61-0.99) (eTable 1 in the Supplement). Sensitivity analysis, including the posttrial follow-up data of the MDRD and the AASK studies, demonstrated a significantly lower risk of ESRD for the intensive BP-lowering strategy (RR, 0.91; 95% CI, 0.85-0.99) (eFigure 6 in the Supplement).
Meta-regression and Subgroup Analyses
Table 3 lists the results of univariable meta-regression analyses for exploring potential sources of between-study heterogeneity. Meta-regression showed that the annual rate of decline in GFR with intensive BP control tended to be faster among blacks compared with nonblacks (β value, −0.44; 95% CI, −0.96 to 0.07 mL/min/1.73 m2/y; P = .09) (Table 3). Subgroup analyses (eFigure 7 in the Supplement) showed a trend of faster decline in GFR for intensive BP control among studies including mostly blacks (mean difference, −0.26; 95% CI, −0.70 to 0.18 mL/min/1.73 m2/y), and a slower decline in GFR among studies with nonblacks (mean difference, 0.18; 95% CI, −0.08 to 0.45; P for interaction = .09).
Table 3. Univariable Meta-regression for Effects of Intensive Blood Pressure Control on Annual Rate of Change in Glomerular Filtration Rate (GFR).
| Covariates | Studies, No. | R2, %a | P Value of Qmodel | β (95% CI) |
|---|---|---|---|---|
| Age, mean, <55 vs ≥55 y | ||||
| <55 | 5 | 0.0 | .12 | 0.37 (−0.09 to 0.83) |
| ≥55 | 3 | |||
| Race, black vs nonblack | ||||
| Blackb | 2 | 0.0 | .09 | −0.44 (−0.96 to 0.07) |
| Nonblack | 6 | |||
| SBP target, <120 vs ≥120 mm Hg | ||||
| <120 | 3 | 0.0 | .87 | 0.04 (−0.46 to 0.54) |
| ≥120 | 5 | |||
| Baseline GFR, mean, <40 vs ≥40 mL/min/1.73 m2 | ||||
| <40 | 4 | 0.0 | .18 | −0.38 (−0.95 to 0.18) |
| ≥40 | 4 | |||
| Study sample size, <500 vs ≥500 patients | ||||
| <500 | 4 | 0.0 | .40 | 0.28 (−0.38 to 0.95) |
| ≥500 | 4 | |||
| Method of GFR measurement, direct measurement vs estimation equation | ||||
| Direct measurement | 5 | 0.0 | .81 | −0.06 (−0.52 to 0.40) |
| Estimation equation | 3 |
Abbreviation: SBP: systolic blood pressure.
R2 indicated the proportion of between-study variance explained by the model. P ≤ .05 indicated a between-group difference of the effects of intensive blood pressure control for the covariate. The annual rate of decline in GFR was significantly slower for intensive control group if the regression coefficient (β) was significantly greater than zero and vice versa.
Influence of the Level of Proteinuria on Effects of Intensive BP-Lowering Treatments
Only the annual rate of change in GFR and ESRD could be assessed by different levels of proteinuria. Overall, the effects of intensive BP control were not significantly different among patients with different levels of proteinuria (eTables 2 and 3 in the Supplement). However, there was a trend for intensive BP control to slow the rate of decline in GFR level among patients with proteinuria higher than 1 g/d (mean difference, 0.75; 95% CI, −0.40 to 1.89 mL/min/1.73 m2/y; P for interaction = .15), and a trend of lower risk for ESRD among those with proteinuria level higher than 0.5 g/d (RR, 0.92; 95% CI, 0.70-1.21; P for interaction = .43).
Adverse Events of Intensive BP Control
Three studies28,29,30 did not report data on adverse events, and 1 study21 did not present data on adverse events for the CKD subgroup. The 3 studies reporting the risk of hypotension and associated symptoms had inconsistent results.15,16,31 The pooled estimates of 2 studies with a total of 1652 patients showed that there was an increased risk of dizziness for intensive BP-lowering treatments (RR, 1.13; 95% CI, 1.05-1.22),16,31 but Klahr et al15 reported that events of hypotension were not significantly different between BP-lowering strategies. Wright et al16 also reported that there was no significant difference in syncope between the intensive and standard BP-lowering groups (6.3% vs 5.2%). One study31 reported that the intensive and the standard BP-lowering groups had similar risk of acute kidney injury (5.8% vs 4.6%), and 2 studies17,31 reported that intensive BP-lowering treatments did not increase the risk of serious adverse events.
Discussion
In this systematic review and meta-analysis of nondiabetic adults with CKD, there were no differences in renal outcomes comparing intensive and standard BP-lowering strategies during a median follow-up of 3.3 years. However, intensive BP control tended to reduce mortality, and nonblacks or patients with higher levels of proteinuria showed a trend of lower risk of kidney disease progression with intensive BP-lowering treatments. There was no clear evidence that intensive BP control increased the risk of adverse events, except for the symptom of dizziness. These estimates are fairly robust and changed little in sensitivity analyses.
Strengths of This Study
This systematic review provides up-to-date information and included more than 8000 patients and more than 800 events of kidney disease progression. Five study outcomes were analyzed to evaluate effects of intensive BP-lowering treatments, and all showed similar results. We followed a standard protocol, used a comprehensive search strategy, and applied rigorous methods to assess the robustness of study results, including meta-regression and subgroup analyses.
Results in Relation to Other Studies and Reviews
Our study results are consistent with those of previous meta-analyses. In a systematic review of 19 RCTs with a mean follow-up of 3.8 years, Xie et al32 reported that the intensive BP strategy reduced the risk of cardiovascular events in patients with hypertension but not the risk for ESRD or all-cause mortality. However, the meta-analysis by Xie et al32 included mostly patients without CKD and did not report renal outcomes for the CKD subgroup. By including updated evidence such as the SPRINT study,21 our main analyses revealed that intensive BP control offered no additional benefit on the 4 renal outcomes but a trend to reduce mortality. The sensitivity analysis showed a lower mortality in patients treated with intensive BP-lowering strategy when the study populations were strictly restricted to those without diabetes.
In a meta-analysis of 5308 CKD patients, Lv et al19 reported that intensive BP-lowering reduced the risk of a composite kidney failure outcome by 17% and reduced the risk of ESRD by 18%. This meta-analysis also demonstrated that intensive BP-lowering reduced the risk of kidney failure by 27% in patients with proteinuria and concluded that proteinuria is an effect modifier (P = .006).19 The meta-analysis by Lv et al19 included posttrial follow-up data from the MDRD33 and the AASK27 trials. Including the posttrial cohort data in the meta-analysis increased the number of events and statistical power but might also introduce biases because patients may not have adhered to assigned BP targets during the posttrial follow-up period. In addition, the systematic review by Lv et al19 enrolled children and patients with diabetes. Because the pathogenesis and clinical course in pediatric patients and those with diabetic kidney disease are different from nondiabetic adults with CKD,7,12,34,35,36 pooling results might not clarify the effects of intensive BP control. To maintain the pooled evidence in the highest quality, we included only data from nondiabetic adults during the trial phase, and showed that the intensive and standard BP control provided similar effects during a follow-up of 3.3 years. We also noted a trend of better renal outcomes for intensive BP control among patients with higher levels of proteinuria, but this finding did not reach statistical significance during this timeframe of follow-up.
Compared with whites, blacks with hypertension are more prone to develop CKD and progress to ESRD, and this is likely to involve a complex interaction between biological and socioeconomic factors.37,38,39 Previous studies have reported that the kidney protection with antihypertensive therapy is less favorable in blacks than in whites.18,40 Similarly, we found a trend that only nonblacks gained additional kidney protection from intensive BP lowering. However, statistical power in our meta-analysis to address effects in blacks is relatively limited because there were only 2 RCTs among the black population.
Limitations
Our study has several limitations. First, there was between-study variability owing to different patient characteristics and trial designs among included studies. The causes of CKD (hypertension, glomerulonephritis, polycystic kidney disease, or other causes) and the types of BP target (systolic BP, diastolic BP, or mean arterial pressure) varied across included studies. In spite of the efforts in meta-regression and subgroup analyses, we could only partly explain the influences of race or proteinuria on intervention effects. The number of included studies limited power for further exploration with multivariable meta-regression or multilevel subgroup analyses. Second, achieved BP could result in unblinding of the included trials. Nevertheless, the objective nature of the outcome measures reduced the possible impact of the lack of blinding. Third, most of the included studies had a follow-up time shorter than 4 years because we only included data during the trial phase. The length of follow-up might not have been long enough to distinguish outcome differences among the overall study population. Fourth, this systematic review included information from published studies only. Although funnel plots and Egger test did not suggest publication bias, such bias could still exist owing to the relatively low power of these statistical tests. Finally, this study was designed to evaluate nondiabetic patients with CKD and focused on renal outcomes. Considering the competing risks between ESRD and death, further studies are needed to evaluate the cardioprotective effects of intensive BP-lowering treatments in nondiabetic CKD patients.
Conclusions
Targeting BP below the current standard did not provide additional benefit for renal outcomes compared with standard treatment during a follow-up of 3.3 years in patients with CKD without diabetes. However, nonblack patients or those with higher levels of proteinuria might benefit from the intensive BP lowering, and the risk of adverse events are mostly similar among different BP targets.
eAppendix 1. Study Protocol and Search Strategies
eAppendix 2. Estimations and Imputations for Missing Data
eReferences. References for eAppendix 1 and eAppendix 2
eFigure 1. Summary of Study Identification and Selection
eFigure 2. Summary for Risk of Bias of Included Studies
eFigure 3. Risk of Bias Graph of Included Studies
eFigure 4. Funnel Plots, Contour-enhanced Funnel Plots, and Egger’s Regression Asymmetry Test for Assessment of Publication Bias
eFigure 5. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate by Omitting Studies with Imputed Missing Data
eFigure 6. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control on End-stage Renal Disease by Including the Post-trial Follow-up Data of Randomized Clinical Trials
eFigure 7. Forest Plots of Subgroup Analysis Assessing Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate, by Different Race Subgroups
eTable 1. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control after Omitting Studies Which Did Not Exclude Diabetic Subjects
eTable 2. Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate among Patients with Different Levels of Proteinuria
eTable 3. Effects of Intensive Blood Pressure Control on End-stage Renal Disease among Patients with Different Levels of Proteinuria
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305. [DOI] [PubMed] [Google Scholar]
- 2.Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine (Baltimore). 2016;95(11):e3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu HY, Peng YS, Chiang CK, et al. Diagnostic performance of random urine samples using albumin concentration vs ratio of albumin to creatinine for microalbuminuria screening in patients with diabetes mellitus: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(7):1108-1115. [DOI] [PubMed] [Google Scholar]
- 4.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173-2182. [DOI] [PubMed] [Google Scholar]
- 5.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260-272. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815-822. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS. Clinical practice. Nondiabetic kidney disease. N Engl J Med. 2002;347(19):1505-1511. [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease; Improving Global Outcomes (KDIGO) Blood Pressure Work Group . KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):337-414. [Google Scholar]
- 9.Kidney Disease Outcomes Quality Initiative (K/DOQI) . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5)(suppl 1):S1-S290. [PubMed] [Google Scholar]
- 10.Fukuma S, Shimizu S, Niihata K, et al. Development of quality indicators for care of chronic kidney disease in the primary care setting using electronic health data: a RAND-modified Delphi method [published online May 4, 2016]. Clin Exp Nephrol. 2016. doi: 10.1007/s10157-016-1274-8 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Uchida S, Kawamura T, Kuwahara M, Nangaku M, Iino Y; PROTECT-CKD Study Group . Prospective randomized study of the tolerability and efficacy of combination therapy for hypertensive chronic kidney disease: results of the PROTECT-CKD study. Clin Exp Nephrol. 2015;19(5):925-932. [DOI] [PubMed] [Google Scholar]
- 12.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta K, Quinn RR, Zarnke KB, et al. ; Canadian Hypertension Education Program . The 2014 Canadian Hypertension Education Program recommendations for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2014;30(5):485-501. [DOI] [PubMed] [Google Scholar]
- 14.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159-2219. [DOI] [PubMed] [Google Scholar]
- 15.Klahr S, Levey AS, Beck GJ, et al. ; Modification of Diet in Renal Disease Study Group . The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877-884. [DOI] [PubMed] [Google Scholar]
- 16.Wright JT Jr, Bakris G, Greene T, et al. ; African American Study of Kidney Disease and Hypertension Study Group . Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421-2431. [DOI] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Perna A, Loriga G, et al. ; REIN-2 Study Group . Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939-946. [DOI] [PubMed] [Google Scholar]
- 18.Ku E, Gassman J, Appel LJ, et al. BP control and long-term risk of ESRD and mortality. J Am Soc Nephrol. 2017;28(2):671-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185(11):949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Upadhyay A, Earley A, Haynes SM, Uhlig K. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154(8):541-548. [DOI] [PubMed] [Google Scholar]
- 21.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. Updated March 2011. The Cochrane Collaboration, 2011. http://handbook.cochrane.org. Accessed July 16, 2016.
- 23.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Hoboken, NJ: John Wiley & Sons; 2009. [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991-996. [DOI] [PubMed] [Google Scholar]
- 26.Ku E, Glidden DV, Johansen KL, et al. Association between strict blood pressure control during chronic kidney disease and lower mortality after onset of end-stage renal disease. Kidney Int. 2015;87(5):1055-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appel LJ, Wright JT Jr, Greene T, et al. ; AASK Collaborative Research Group . Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toto RD, Mitchell HC, Smith RD, Lee HC, McIntire D, Pettinger WA. “Strict” blood pressure control and progression of renal disease in hypertensive nephrosclerosis. Kidney Int. 1995;48(3):851-859. [DOI] [PubMed] [Google Scholar]
- 29.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13(7):1733-1739. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi K, Saruta T, Goto Y, Ishii M; JATOS Study Group . Impact of renal function on cardiovascular events in elderly hypertensive patients treated with efonidipine. Hypertens Res. 2010;33(11):1211-1220. [DOI] [PubMed] [Google Scholar]
- 31.Schrier RW, Abebe KZ, Perrone RD, et al. ; HALT-PKD Trial Investigators . Blood pressure in early autosomal dominant polycystic kidney disease. N Engl J Med. 2014;371(24):2255-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387(10017):435-443. [DOI] [PubMed] [Google Scholar]
- 33.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342-351. [DOI] [PubMed] [Google Scholar]
- 34.Wühl E, Trivelli A, Picca S, et al. ; ESCAPE Trial Group . Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639-1650. [DOI] [PubMed] [Google Scholar]
- 35.Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. 2016;9(4):583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckardt KU, Coresh J, Devuyst O, et al. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382(9887):158-169. [DOI] [PubMed] [Google Scholar]
- 37.Wetmore JB, Guo H, Liu J, Collins AJ, Gilbertson DT. The incidence, prevalence, and outcomes of glomerulonephritis derived from a large retrospective analysis. Kidney Int. 2016;90(4):853-860. [DOI] [PubMed] [Google Scholar]
- 38.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363-2370. [DOI] [PubMed] [Google Scholar]
- 39.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902-2907. [DOI] [PubMed] [Google Scholar]
- 40.Flack JM, Neaton JD, Daniels B, Esunge P. Ethnicity and renal disease: lessons from the Multiple Risk Factor Intervention Trial and the Treatment of Mild Hypertension Study. Am J Kidney Dis. 1993;21(4)(suppl 1):31-40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Study Protocol and Search Strategies
eAppendix 2. Estimations and Imputations for Missing Data
eReferences. References for eAppendix 1 and eAppendix 2
eFigure 1. Summary of Study Identification and Selection
eFigure 2. Summary for Risk of Bias of Included Studies
eFigure 3. Risk of Bias Graph of Included Studies
eFigure 4. Funnel Plots, Contour-enhanced Funnel Plots, and Egger’s Regression Asymmetry Test for Assessment of Publication Bias
eFigure 5. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate by Omitting Studies with Imputed Missing Data
eFigure 6. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control on End-stage Renal Disease by Including the Post-trial Follow-up Data of Randomized Clinical Trials
eFigure 7. Forest Plots of Subgroup Analysis Assessing Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate, by Different Race Subgroups
eTable 1. Sensitivity Analysis Assessing Effects of Intensive Blood Pressure Control after Omitting Studies Which Did Not Exclude Diabetic Subjects
eTable 2. Effects of Intensive Blood Pressure Control on the Annual Rate of Change in Glomerular Filtration Rate among Patients with Different Levels of Proteinuria
eTable 3. Effects of Intensive Blood Pressure Control on End-stage Renal Disease among Patients with Different Levels of Proteinuria


