Key Points
Question
Does auditing and sharing of best practice across institutions lead to optimization of radiation dose in computed tomographic scans?
Findings
In this study, after auditing and sharing of best practices the mean effective dose for chest and abdominal CT decreased by 19% and 25%, respectively, with the percentage of CT scans exceeding benchmarks decreasing by 48% and 54%, respectively. Mean effective dose in head CT scans varied less following auditing and sharing best practices, with some institutions increasing and some decreasing mean head CT doses.
Meaning
Sharing best practices on CT dose optimization with professionals from different institutions can lower radiation doses and potentially improve practice.
This study assesses changes in computed tomography radiation doses across 5 University of California medical centers after implementation of institutional-level audit and collaborative efforts to share best practices.
Abstract
Importance
Radiation doses for computed tomography (CT) vary substantially across institutions.
Objective
To assess the impact of institutional-level audit and collaborative efforts to share best practices on CT radiation doses across 5 University of California (UC) medical centers.
Design, Setting, and Participants
In this before/after interventional study, we prospectively collected radiation dose metrics on all diagnostic CT examinations performed between October 1, 2013, and December 31, 2014, at 5 medical centers. Using data from January to March (baseline), we created audit reports detailing the distribution of radiation dose metrics for chest, abdomen, and head CT scans. In April, we shared reports with the medical centers and invited radiology professionals from the centers to a 1.5-day in-person meeting to review reports and share best practices.
Main Outcomes and Measures
We calculated changes in mean effective dose 12 weeks before and after the audits and meeting, excluding a 12-week implementation period when medical centers could make changes. We compared proportions of examinations exceeding previously published benchmarks at baseline and following the audit and meeting, and calculated changes in proportion of examinations exceeding benchmarks.
Results
Of 158 274 diagnostic CT scans performed in the study period, 29 594 CT scans were performed in the 3 months before and 32 839 CT scans were performed 12 to 24 weeks after the audit and meeting. Reductions in mean effective dose were considerable for chest and abdomen. Mean effective dose for chest CT decreased from 13.2 to 10.7 mSv (18.9% reduction; 95% CI, 18.0%-19.8%). Reductions at individual medical centers ranged from 3.8% to 23.5%. The mean effective dose for abdominal CT decreased from 20.0 to 15.0 mSv (25.0% reduction; 95% CI, 24.3%-25.8%). Reductions at individual medical centers ranged from 10.8% to 34.7%. The number of CT scans that had an effective dose measurement that exceeded benchmarks was reduced considerably by 48% and 54% for chest and abdomen, respectively. After the audit and meeting, head CT doses varied less, although some institutions increased and some decreased mean head CT doses and the proportion above benchmarks.
Conclusions and Relevance
Reviewing institutional doses and sharing dose-optimization best practices resulted in lower radiation doses for chest and abdominal CT and more consistent doses for head CT.
Introduction
Despite a steady increase in the use of computed tomography (CT) in the past 2 decades, there are few concrete standards for radiation doses. The American College of Radiology and other organizations promote performing CT scans with radiation exposures that are as low as reasonably achievable. However, in the absence of explicit definitions of achievable standards, radiation doses from CT examinations vary widely both within and across institutions. This variability suggests opportunity for improvement.
In recent years, numerous strategies were developed and adopted to lower CT doses. These involve a range of technological approaches, such as reducing tube current (mA) and peak kilovoltage, using automatic exposure control, tailoring radiation dose to patient size, and adopting iterative reconstruction. Most of these strategies affect image quality, which can impair the radiologist’s ability to identify imaging findings, and reduce comfort with reviewing the images. Therefore, lowering CT radiation doses generally involves tradeoffs for the interpreting radiologists. The determination on how to optimize dose—keeping the radiation dose as low as possible while retaining the diagnostic accuracy—tends to be made independently by individual physicians and institutions, contributing to the observed variation. Variation also arises from differences in the priority that radiologists place on dose optimization and their beliefs regarding how it may impact diagnostic accuracy.
Achieving dose reduction requires buy-in and coordination among diverse individuals including radiologists, CT technologists, and medical physicists. Furthermore, dose reduction requires dose auditing to determine if protocols are leading to unnecessarily high CT doses. Duong et al suggest that reviewing all protocols at a single institution could require the full-time effort of a dedicated individual for a full year. Obtaining dose reports, such as from the American College of Radiology Dose Index Registry (DIR) may be useful to compare dose levels to local and national averages, but requires infrastructure, radiologist buy-in, registry costs, and a willingness to compare doses across several specific protocols.
A barrier to dose reduction is that individuals and institutions have difficulty finding the right doses that balance diagnostic accuracy and patient radiation exposure. Such decisions may be best made using a consensus approach in which physicians share experiences and preferences across institutions. The University of California (UC) Office of the President funded a 3-year project to standardize and optimize radiation dose from CT across the 5 UC medical centers at Davis, Irvine, Los Angeles, San Diego, and San Francisco. The project strategy was to collectively define metrics, assess radiation doses, and move toward dose standardization. This article presents the results of our efforts using a combination of facility-level audit and collaborative efforts to share best practices.
Methods
Data Collection
The University of California, San Francisco, Committee on Human Research approved the study and waived informed consent. We collected radiation doses on diagnostic CT examinations performed between October 1, 2013, and December 31, 2014, at 12 imaging facilities associated with the UC medical centers that use 34 scanners from GE Medical Systems, Neurologica, Philips, Siemens, and Toshiba. Images were uploaded from facilities to a single server using eXposure (Radimetrics, Bayer), a software tool for tracking patient radiation exposure. Raw dose metrics from digital imaging and communication in medicine tags were collected directly from CT scanners or picture archiving and communication system. The method for calculating effective dose from these metrics has been described. Data were deidentified, electronically sent to a server at UCSF, and downloaded for analysis. We evaluated doses for the CTs of the chest, abdomen (all examinations including abdomen or pelvis), and head, which account for over 80% of all CT imaging performed at the medical centers.
Dose Audit
In April 2014, we provided collaborators at each medical center with a summary of their doses compared with the other medical centers using the 2 National Quality Forum (NQF) frameworks for reporting CT radiation dose. The audit summarized several NQF suggested dose metrics including effective dose, dose length product, CT volumetric dose index, and size-specific dose estimate. We assembled affinity groups of medical physicists and technologists and radiologists, including the radiology section heads for the anatomic areas of chest, abdomen, and neuroradiology. We convened webinar meetings for each affinity group to review methodological questions on the creation and interpretation of the audit reports prior to the in-person meeting.
In-Person Meeting
In late April 2014, we invited the chest, abdomen, and neuroradiology section chiefs and the medical physicists and radiology technologists from the 5 medical centers to participate in a 1.5-day, in-person meeting to review the dose audits and share best practices. Radiologists were asked to come prepared to share their imaging strategies for common clinical questions (such as assessment for pulmonary embolism, renal stones, and headache), and to share representative images. Technologists were asked to explain how the decisions to image using different strategies were operationalized (ie, how a patient in the emergency department with a given symptom would be imaged under various strategies). For example, at one of the University of California medical centers, a lead technologist oversees the protocol assignment for all patients to ensure imaging protocols are consistent, whereas at another University of California medical center, rotating radiology residents oversee protocol assignment. A sampling of best practices discussed at the meeting is included in the Box. The meeting included lectures, sharing of images so that participants could discuss trade-offs between image quality and dose levels, large group discussions, and small breakout sessions to discuss variations in doses across sites, experience with dose optimization, and opportunities for standardization. The radiology section chiefs were asked to share their experiences with dose optimization (both positive and negative), their decision-making processes around dose reduction, and specific protocols for the most common imaging indications. During the meeting, there were 4 breakout sessions of anatomic area-specific affinity groups, composed of radiologists, physicists, medical technologists, and a note taker. Each group was asked to focus on specific indications for imaging, the protocols used for those indications, and to discuss when multiple phase imaging could be reduced.
Sample Best Practices Discussed at In-Person Meeting.
Streamline and control process for creating and updating protocols
-
Invest the time to optimize protocols to reduce dose and maximize diagnostic value
Use helical and wide aperture scanning whenever possible, including for brain imaging
Use iterative reconstruction whenever possible, including for brain imaging
Optimize peak kilovoltage selection wherever possible, including for brain imaging
Designate a single person (such as the lead technologist) to oversee assignment of patients to specific protocols
Use intuitive protocol names to allow greater standardization and assessment of doses over time
-
Reduce use of multiphase computed tomography (CT) where possible, for example:
Avoid noncontrast phase for chest CT for suspected pulmonary embolism
Use only single (portal venous) phase CT for abdominal pain, trauma, suspected pancreatitis, or appendicitis
-
Reduce subsequent phase doses where possible in multiphase studies, for example:
Reduce dose for noncontrast and excretory phase scans for triple phase urogram for hematuria
Reduce dose for noncontrast scan for 4 phase liver examination
Reduce dose for noncontrast scan for a renal mass
-
Reduce anatomic regions being scanned, for example:
Do not include the pelvis to assess a renal mass
Do not include the pelvis for a triple phase urogram
-
Use the lowest possible doses for low-dose studies, for example:
Low-dose CT lung cancer screening examinations can have the dose further reduced
Single-phase noncontrast scans for suspected nephrolithiasis can have the dose lowered further
-
Lowering dose by 10% will have minimal impact on diagnosis
Continue reducing dose by small increments (eg, 10%) until affecting diagnostic image quality
Use low-dose studies wherever possible (for assessment of hydrocephalus, or pectus excavatum)
Each medical center was asked to generate a list of specific changes that they would make after the meeting and the logistics of sharing the plans with faculty and staff were discussed. Travel and accommodations were paid by the UC grant. Overall, 13 of 14 invited radiologists, 8 medical physicists, 5 radiology technologists, and 9 project coordinators and clinical administrators attended.
Evaluation of Impact
To evaluate the impact of the dose audit and in-person meeting on medical center doses, we compared the distribution in effective dose during the 12-week baseline period and a 12-week period following the in-person meeting, calculating percentage change in dose following the meeting. We used previously published diagnostic reference levels as benchmarks for each anatomic area, defined as the 75th percentile of radiation dose across all medical centers. Diagnostic reference levels are generally considered as dose levels that should not be exceeded without good reason, although these were not explicitly used in this way during the time of the study. We calculated the percentage of scans exceeding benchmarks at baseline and after the in-person meeting. For all comparisons, we excluded CT examinations within 12 weeks immediately following the meeting, which was considered an implementation period for medical centers to make the changes identified at the meeting. We conducted sensitivity analyses varying the length of the implementation period between 1 week and 12 weeks to see if conclusions were robust to this choice.
To visually evaluate trends in imaging over time and changes potentially related to provision of audit feedback and the in-person meeting, we graphed lines of dose over the entire study period. We fit a nonparametric cubic smoothing spline to the data, with a smoothing parameter of 50 using PROC GPLOT in SAS statistical software (version 9.3, SAS Inc). No period was excluded from graphing. The identification of the medical centers in the tables (using a combination of letters and numbers) was done so that medical center size could not be used to link the doses with a particular medical center.
Results
From October 1, 2013, through December 31, 2014, 158 274 diagnostic CT scans were performed including 29 594 during the 12-week period prior to providing audits and the in-person meeting; 30 423 scans during the 12-week implementation period; and 32 839 in the 12 weeks after the audit and in-person meeting (Table 1). Most CT scans were of the abdomen and head. There was variation across anatomic areas regarding which site had the highest dose.
Table 1. Number of Computed Tomography Scans Contributed by Each Medical Center During the Entire Data Collection Period, and During the Periods Used for Analysisa.
| Variable | Complete Data Collection Period (60-Week Period) | 12 Weeks | ||
|---|---|---|---|---|
| Before Audit and Meeting | Implementation | After Audit and Meeting | ||
| All anatomic areas, all medical centersb | 158 274 | 29 594 | 30 423 | 32 839 |
| Chest | ||||
| All medical centers | 35 788 | 6927 | 6963 | 7394 |
| UC A | 2888 | 566 | 531 | 576 |
| UC B | 5751 | 1148 | 1047 | 1172 |
| UC C | 7603 | 1233 | 1514 | 1606 |
| UC D | 8190 | 1688 | 1554 | 1629 |
| UC E | 11 356 | 2292 | 2317 | 2411 |
| Abdomen | ||||
| All medical centers | 65 370 | 11 953 | 12 621 | 13 709 |
| UC A | 8302 | 1562 | 1655 | 1635 |
| UC B | 12 577 | 2461 | 2248 | 2605 |
| UC C | 14 223 | 2129 | 2744 | 3164 |
| UC D | 12 960 | 2536 | 2575 | 2694 |
| UC E | 17 308 | 3265 | 3399 | 3611 |
| Head | ||||
| All medical centers | 57 116 | 10 714 | 10 839 | 11 736 |
| UC A | 8932 | 1507 | 1685 | 1986 |
| UC B | 12 627 | 2466 | 2352 | 2560 |
| UC C | 9012 | 1447 | 1720 | 1865 |
| UC D | 9764 | 1873 | 1913 | 1899 |
| UC E | 16 781 | 3421 | 3169 | 3426 |
Abbreviation: UC, University of California.
Data are given as numbers.
University of California medical centers are designated A to E to for confidentiality.
Change in Mean Dose
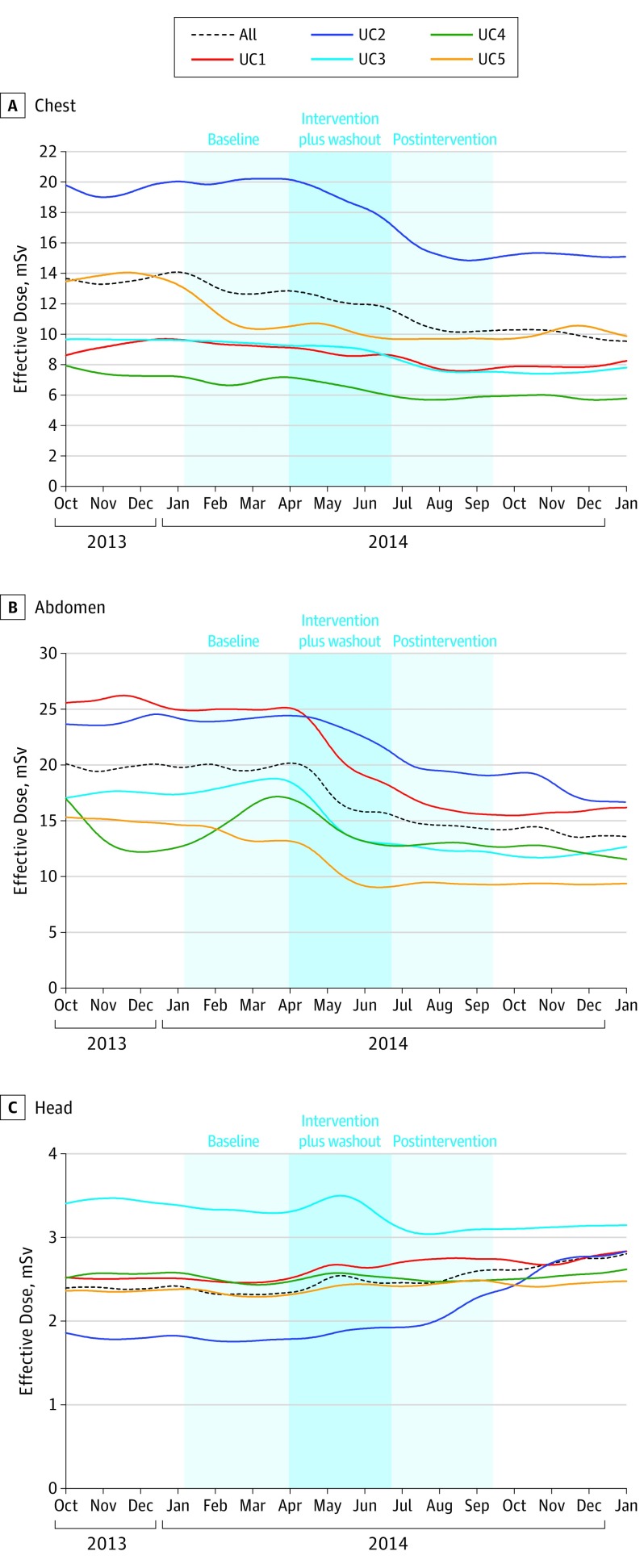
The mean effective dose for the chest and abdomen changed considerably following the audit and in-person meeting (Table 2) (Figure). For chest, there was a considerable 18.9% reduction in mean effective dose across medical centers (decreased from 13.2 to 10.7 mSv), reflecting a change in mean effective dose ranging from 3.8% to 23.5% across the centers. The largest decline occurred at the medical center with highest dose at baseline (UC2 in Table 2 decreased from 20.4 mSv to 15.6 mSv). For abdomen, there was a 25.0% reduction in mean effective dose across medical centers (decreased from 20.0 to 15.0 mSv), reflecting a change in mean effective dose ranging from 10.8% to 34.7% across centers. The largest decline occurred at the medical center with highest dose at baseline (UC1 decreased from 24.8 to 16.2 mSv). For both chest and abdomen, reductions in median dose were also considerable: 29% reduction for chest and 34% for abdomen. Variability of doses was reduced, as indicated by narrower interquartile ranges (Table 2).
Table 2. Distribution of Effective Dose Before and After Audit Reports and In-Person Meeting With Percent Change in Mean Effective Dose With 95% CI and P Value for Change, and 25% and 75% Interquartile Range Across and Within Medical Centers, by Anatomic Area.
| Variable | Effective Dose, mSv | Change in Mean Dose | ||||||
|---|---|---|---|---|---|---|---|---|
| Before Audit and In-Person Meeting | After Audit and In-Person Meeting | |||||||
| Mean | Median (25th-75th) | IQR | Mean | Median (25th-75th) | IQR | % (95% CI) | P Valuea | |
| Chest | ||||||||
| All medical centersb | 13.2 | 10.8 (5.7 to 17.0) | 11.3 | 10.7 | 8.4 (5.4 to 13.4) | 8.0 | −18.9 (−18.0 to −19.8) | <.001 |
| UC 1 | 9.3 | 7.9 (5.8 to 11.1) | 5.4 | 7.7 | 6.3 (5.1 to 8.3) | 3.3 | −17.2 (−33.8 to 0.0) | .05 |
| UC 2 | 20.4 | 17.4 (14.0 to 24.6) | 10.7 | 15.6 | 13.2 (10.3 to 17.1) | 6.7 | −23.5 (−18.0 to −28.8) | <.001 |
| UC 3 | 9.4 | 9.1 (5.9 to 11.2) | 5.2 | 7.7 | 6.9 (5.5 to 8.6) | 3.1 | −18.1 (−42.1 to 5.4) | .34 |
| UC 4 | 7.9 | 6.3 (3.7 to 10.2) | 6.5 | 7.6 | 5.9 (3.6 to 9.3) | 5.6 | −3.8 (−21.5 to 15.2) | >.99 |
| UC 5 | 11.3 | 8.6 (5.3 to 14.9) | 9.7 | 9.8 | 7.7 (5.5 to 12.6) | 7.1 | −13.3 (−1.8 to −24.8) | .01 |
| Abdomen | ||||||||
| All medical centers | 20.0 | 17.1 (10.7 to 25.5) | 14.8 | 15.0 | 12.8 (8.4 to 18.2) | 9.8 | −25.0 (−24.3 to −25.8) | <.001 |
| UC 1 | 24.8 | 20.6 (13.9 to 31.1) | 17.2 | 16.2 | 13.5 (9.9 to 18.6) | 8.7 | −34.7 (−29.6 to −39.6) | <.001 |
| UC 2 | 24.5 | 21.8 (17.1 to 29.8) | 12.7 | 19.9 | 17.2 (13.8 to 23.8) | 10.0 | −18.8 (−14.3 to −22.9) | <.001 |
| UC 3 | 18.3 | 15.1 (10.5 to 23.3) | 12.8 | 12.5 | 11.0 (8.2 to 14.7) | 6.5 | −31.7 (−23.3 to −40.2) | <.001 |
| UC 4 | 15.8 | 12.9 (7.7 to 19.3) | 11.6 | 14.1 | 11.8 (7.4 to 17.3) | 9.9 | −10.8 (−3.3 to −18.8) | <.001 |
| UC 5 | 14.2 | 10.9 (7.2 to 18.2) | 11.0 | 9.6 | 7.8 (6.0 to 11.5) | 5.5 | −32.4 (−23.9 to −40.8) | <.001 |
| Head | ||||||||
| All medical centers | 2.4 | 2.2 (1.6 to 3.0) | 1.4 | 2.5 | 2.6 (1.9 to 3.0) | 1.1 | 4.0 (3.6 to 4.4) | <.001 |
| UC 1 | 2.5 | 2.5 (2.0 to 2.9) | 0.9 | 2.8 | 2.7 (2.4 to 3.0) | 0.7 | 12.0 (5.6 to 15.6) | <.001 |
| UC 2 | 1.8 | 1.6 (1.4 to 1.9) | 0.5 | 2.1 | 1.9 (1.6 to 2.4) | 0.8 | 16.7 (12.8 to 24.4) | <.001 |
| UC 3 | 3.4 | 3.4 (3.1 to 3.6) | 0.5 | 3.1 | 3.0 (2.8 to 3.2) | 0.4 | −8.8 (−3.2 to −12.0) | <.001 |
| UC 4 | 2.5 | 2.5 (2.3 to 2.7) | 0.4 | 2.5 | 2.6 (2.3 to 2.8) | 0.5 | 0.0 (−4.8 to 7.2) | >.99 |
| UC 5 | 2.3 | 2.0 (1.7 to 2.6) | 0.9 | 2.4 | 2.2 (1.9 to 2.7) | 0.9 | 4.3 (0.4 to 13.0) | .01 |
Abbreviations: IQR, interquartile range; mSv, millisievert; UC, University of California.
Calculated P values represent test for change in means preaudit and postaudit reports and in-person meeting.
University of California (UC) medical centers are designated 1 to 5 for confidentiality. This numbering is not the same convention as used in Table 1.
Figure. Weekly Effective Dose Over Time, by Anatomic Area and Medical Center, During the 60 Weeks of Data Collection From October 1, 2013, to December 31, 2015.
For head CT, doses across medical centers became more similar over time, although the direction of changes varied (Figure). The medical center with the highest dose at baseline (UC3) showed an 8.8% reduction in dose (3.4 mSv to 3.1 mSv), whereas the medical center with the lowest dose at baseline (UC2), demonstrated a 16.7% increase in dose (1.8 mSv to 2.1 mSv). Head CT doses varied less over time, as reflected in the decline in interquartile range from 1.4 mSv before the audit and in-person meeting to 1.1 mSv after.
Change in Proportion of CT Examinations Exceeding Benchmarks
The average proportions of CT examinations exceeding benchmarks at baseline were 21%, 24%, and 24% for chest, abdomen, and head, respectively. Following the intervention, reductions in the proportion of scans that exceeded benchmarks were 48% for chest scans and 54% for abdomen scans (Table 3). All medical centers demonstrated considerable reductions in the proportions of chest and abdomen scans exceeding benchmarks, ranging from a 17% reduction to an 80% reduction. Overall, the proportion of head CT examinations that exceeded the benchmark decreased 1%, but the direction of change in proportion of high-dose examinations varied. The medical center with the highest proportion of examinations above the benchmark at baseline (UC3, where 82% of examinations exceeded the benchmark) demonstrated the largest decrease (34%) following the audit and in-person meeting; 82% of examinations exceed the benchmark at baseline compared with 54% after the meeting, reflecting a 34% reduction.
Table 3. Computed Tomography Examinations Exceeding Benchmarks Before and After Audit Reporting and In-Person Meeting, and Percent Change in Proportion of Examinations Exceeding Benchmarks by Anatomic Area.
| Variablea | Examinations Exceeding Benchmarks, No. (%) | Change, % (95% CI) | |
|---|---|---|---|
| Before Audit and Meeting | After Audit and Meeting | ||
| Chest | |||
| All medical centers | 6927 (21) | 7394 (11) | −48 (−47 to −49) |
| UC 1 | 1148 (6) | 1172 (2) | −67 (−64 to −70) |
| UC 2 | 2292 (46) | 2411 (21) | −54 (−52 to −56) |
| UC 3 | 566 (4) | 576 (3) | −25 (−21 to −29) |
| UC 4 | 1233 (6) | 1606 (5) | −17 (−15 to −19) |
| UC 5 | 1688 (16) | 1629 (9) | −44 (−42 to −46) |
| Abdomen | |||
| All medical centers | 11 953 (24) | 13 709 (11) | −54 (−53 to −55) |
| UC 1 | 2461 (35) | 2605 (13) | −63 (−61 to −65) |
| UC 2 | 3265 (34) | 3611 (20) | −41 (−39 to −43) |
| UC 3 | 1562 (20) | 1635 (5) | −75 (−73 to −77) |
| UC 4 | 2129 (14) | 3164 (9) | −36 (−34 to −38) |
| UC 5 | 2536 (10) | 2694 (2) | −80 (−78 to −82) |
| Head | |||
| All medical centers | 10 714 (24) | 11 736 (24) | −1 (0 to −1) |
| UC 1 | 2466 (21) | 2560 (27) | 29 (27 to 31) |
| UC 2 | 3421 (10) | 3426 (14) | 40 (38 to 42) |
| UC 3 | 1507 (82) | 1986 (54) | −34 (−32 to −36) |
| UC 4 | 1447 (12) | 1865 (11) | 8 (6 to 10) |
| UC 5 | 1873 (15) | 1899 (18) | 20 (18 to 22) |
Abbreviation: UC, University of California.
University of California medical centers are designated 1 to 5 for confidentiality.
The results were largely the same when we varied the length of time for the implementation period.
Doses changed markedly around the time of the intervention (Figure) and the timing of the change was faster for chest and abdomen than head imaging. For seasonality comparison, no drop in effective radiation dose occurred during the same time period the following year across the UC medical centers.
Discussion
We found a considerable decrease in average radiation doses and the proportion of chest and abdomen CT examinations that exceeded benchmarks after providing radiologists and medical physicists with quantitative institutional feedback summarizing CT radiation doses and an in-person meeting to share best practices. Feedback followed the NQF format for radiation dose measures. The same intervention resulted in reduced variation in doses for head CT, but no shift in average doses. Using the methodology outlined by Berrington de Gonzalez et al and Miglioretti et al, we estimate that the average reduction in abdominal CT dose of 3 mSv that we found, if applied to all abdominal CTs performed in the US, would result in the aversion of approximately 12 000 future cancers nationally per year. These findings indicate a benefit to reviewing summary institutional radiation doses, providing feedback to radiology practices, and bringing professionals together to discuss strategies for improvement.
The difference in the impact of the feedback on CT doses for chest and abdomen compared with head was unexpected. The audit and meeting appeared to bring the 2 medical centers with the highest and lowest baseline head CT doses closer to the doses of other institutions. However, average doses did not consistently decline, possibly because of less opportunity for head CT dose reduction if doses are already optimal. Another possibility is that doses for head are lower than other anatomic areas, making dose reduction a lower priority for the medical centers, as well as neuroradiologists.
Previous studies demonstrated dose optimization using quality improvement techniques for specific CT examination types, such as coronary imaging. Another study brought radiologists and radiology residents together to discuss dose-reduction techniques and reduced doses within specific protocols, such as for renal mass CT. Our work expands on these studies, because our effort covered most imaging study types rather than focusing on specific imaging indications. Relatively few studies have covered optimizing doses across all areas in the United States, but several studies in Europe focused on optimizing doses across larger groups of patients and indications.
Our study follows the Six Sigma philosophy framework outlined by Feld and Stone to promote organizational actions and decisions: define, measure, analyze, improve and control (DMAIC). The DMAIC strategy aims to develop commitment from institutions and ensure that management personnel are actively involved in the quality improvement process. During the first year of our collaboration, we defined the metrics we would assess and strategies to summarize doses at the institutional level. During the second year, we analyzed doses and created opportunities for medical centers to improve by sharing best practices. The month of the in-person meeting, we provided dose audits to the institutions, and discussed how each center could best contribute to the meeting. In the months following the meeting, we reassessed doses to determine how the in-person meeting and audit review affected medical center doses.
The primary goal of the in-person meeting for UC radiology professionals was to share strategies on lowering radiation dose without affecting diagnostic performance. It focused on common clinical scenarios, such as imaging patients with pulmonary embolism. We chose the most common indications for imaging (determined by a survey of participants who recorded the indication for imaging for 100 consecutive examinations in each area), and focused on areas with variation among practices. For example, the use of multiphase scanning for the indication of pulmonary embolism varied across medical centers, making it a focus of our discussions.
We did not review or directly compare the hundreds of protocols used across sites because of time and expense. For example, in 1 study, a hospital system created a multidisciplinary committee that met in person and discussed protocol development and optimization over email. The approach required 60 person-hours and $12 000. At another system, a committee reviewed, optimized, and developed standard protocols for the most common CT examinations in their health system. The percentage of outliers in pediatric and adult scans decreased after the first year of protocol distribution, and this trend persisted when a dose-tracking program was implemented. In contrast, our project discussed general approaches for imaging patients and lowering doses, and empowered radiologists to decide where to prioritize their efforts. Our results showed that a more targeted approach, guided by the priorities of the involved clinical sites, could also be effective.
For medical centers to optimize and lower radiation doses, data collection and analysis of their doses is necessary. Our results suggest that assessing doses using the NQF dose measures provides a useful framework. Several commercially available dose-monitoring programs could facilitate this dose assessment. Alternatively, data can be manually collected and assessed with minimal resources, even if an institution lacks electronic dose-collection tools. Including radiation dose metrics in a dedicated and standardized field in electronic health records would greatly facilitate internal dose assessments over time, and comparisons across institutions. The ACR DIR allows registered facilities to compare specific radiation dose indices with regional and national values in specific protocols. Studies incorporating DIR data have successfully reduced doses in specific protocols, such as thoracic CT angiography.
The strengths of our study are its large size and broad inclusion of most CT scan types. The use of a single software tool for data aggregation provided a consistent and uniform source of data from each site.
Limitations
Our study also has several limitations. First, since our design was observational, the temporal change in doses that occurred around the time of our in-person meeting might have happened without our intervention. However, we observed no clear or consistent pattern of dose decline prior to the intervention, nor any other consistent change up to 1 year following the intervention, suggesting our collaborative meeting was causally associated with the dose reduction. A randomized trial would provide more definitive evidence of the association between dose feedback and dose. Discussions at the in-person meeting were broad and open-ended. The meeting was casual, to allow professionals to freely discuss their work. Therefore, we did not record the specific topics, protocols or anatomic sites that were covered most frequently and cannot determine which discussions were most effective. We did not measure diagnostic accuracy and do not know if dose reductions were associated with loss in quality or accuracy. We found in previous work that case mix is broadly similar across the included medical centers, making comparisons across medical centers valid even without our broad anatomic areas. In addition, because each medical center was compared with itself before and after audits, as long as the case mix did not change profoundly over time within a medical center, case differences should not have affected the results.
Conclusions
Providing dose audit feedback and sharing best practices on dose optimization was associated with meaningfully lower radiation doses. Reducing unnecessary variation in radiation dose across hospitals and imaging facilities is a complex but important process for improving patient safety. This study indicated that inclusion of dose audit data can lead to lower and more consistent CT doses. Importantly, bringing health professionals together to discuss best practices after receipt of a site-specific audit report can change the approach to dose management and optimization, which could effectively lower CT doses.
References
- 1.Medicare Part B Imaging Services Rapid spending growth and shift to physician offices indicated need for cms to consider additional management practices [GAO-08-452]. Washington DC; 2008. http://www.gao.gov/new.items/d08452.pdf. Accessed August 8, 2016.
- 2.Smith-Bindman R, Miglioretti DL, Johnson E, et al. . Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith-Bindman R, Lipson J, Marcus R, et al. . Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith-Bindman R, Moghadassi M, Wilson N, et al. . radiation doses in consecutive CT examinations from five University of California medical centers. Radiology. 2015;277(1):134-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith-Bindman R, Wang Y, Yellen-Nelson TR, et al. . Predictors of CT radiation dose and their effect on patient care: a comprehensive analysis using automated data. Radiology. 2017;282(1):182-193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lukasiewicz A, Bhargavan-Chatfield M, Coombs L, et al. . Radiation dose index of renal colic protocol CT studies in the United States: a report from the American College of Radiology National Radiology Data Registry. Radiology. 2014;271(2):445-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Litmanovich DE, Tack DM, Shahrzad M, Bankier AA. Dose reduction in cardiothoracic CT: review of currently available methods. Radiographics. 2014;34(6):1469-1489. [DOI] [PubMed] [Google Scholar]
- 8.Smith-Bindman R, Boone JM. Introduction to the special issue: radiation dose optimization—improving the safety of CT. J Am Coll Radiol. 2014;11(3):229-230. [DOI] [PubMed] [Google Scholar]
- 9.Rawat U, Cohen SL, Levsky JM, Haramati LB. ACR White Paper-Based Comprehensive Dose Reduction Initiative is associated with a reversal of the upward trend in radiation dose for chest CT. J Am Coll Radiol. 2015;12(12, A):1251-1256. [DOI] [PubMed] [Google Scholar]
- 10.Berrington de González A, Kim KP, Knudsen AB, et al. . Radiation-related cancer risks from CT colonography screening: a risk-benefit analysis. AJR Am J Roentgenol. 2011;196(4):816-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heydari B, Leipsic J, Mancini GBJ, et al. . Diagnostic performance of high-definition coronary computed tomography angiography performed with multiple radiation dose reduction strategies. Can J Cardiol. 2011;27(5):606-612. [DOI] [PubMed] [Google Scholar]
- 12.Fei X, Du X, Li P, Liao J, Shen Y, Li K. Effect of dose-reduced scan protocols on cardiac coronary image quality with 64-row MDCT: a cardiac phantom study. Eur J Radiol. 2008;67(1):85-91. [DOI] [PubMed] [Google Scholar]
- 13.Hara AK, Wellnitz CV, Paden RG, Pavlicek W, Sahani DV. Reducing body CT radiation dose: beyond just changing the numbers. AJR Am J Roentgenol. 2013;201(1):33-40. [DOI] [PubMed] [Google Scholar]
- 14.Duong P-A, Little BP. Dose tracking and dose auditing in a comprehensive computed tomography dose-reduction program. Semin Ultrasound CT MR. 2014;35(4):322-330. [DOI] [PubMed] [Google Scholar]
- 15.Dose Index Registry - American College of Radiology American College of Radiology. http://www.acr.org/Quality-Safety/National-Radiology-Data-Registry/Dose-Index-Registry. 2016. Accessed November 28, 2016.
- 16.National Quality Forum National Quality Forum Measure 0739, Radiation Dose of Computed Tomography (CT) Steward. San Francisco; 2011. http://www.qualityforum.org/QPS/0739. Accessed August 10, 2016.
- 17.2820 NNQFM. Pediatric Computed Tomography (CT) Radiation Dose, Measure 2820. San Francisco, CA; 2016. http://www.qualityforum.org/QPS/2820. Accessed August 10, 2016.
- 18.Keegan J, Miglioretti DL, Gould R, Donnelly LF, Wilson ND, Smith-Bindman R. Radiation dose metrics in CT: assessing dose using the National Quality Forum CT patient safety measure. J Am Coll Radiol. 2014;11(3):309-315. [DOI] [PubMed] [Google Scholar]
- 19.Berrington de González A, Mahesh M, Kim K-P, et al. . Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miglioretti DL, Johnson E, Williams A, et al. . The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Bijl N, Joemai RMS, Mertens BJA, et al. . Effect of dose reduction on image quality and diagnostic performance in coronary computed tomography angiography. Int J Cardiovasc Imaging. 2013;29(2):453-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachiappan AC, Valentin LI, Metwalli ZA, et al. . CT dose reduction workshop: an active educational experience. J Am Coll Radiol. 2015;12(6):610-6.e1. [DOI] [PubMed] [Google Scholar]
- 23.Seuri R, Rehani MM, Kortesniemi M. How tracking radiologic procedures and dose helps: experience from Finland. AJR Am J Roentgenol. 2013;200(4):771-775. [DOI] [PubMed] [Google Scholar]
- 24.Tack D, Jahnen A, Kohler S, et al. . Multidetector CT radiation dose optimisation in adults: short- and long-term effects of a clinical audit. Eur Radiol. 2014;24(1):169-175. [DOI] [PubMed] [Google Scholar]
- 25.Jones E, Parast M, Adams S A framework for effective Six Sigma implementation. Total Qual Manag. 2010. http://www.tandfonline.com/doi/abs/10.1080/14783361003606720. Accessed July 30, 2015.
- 26.Feld KG, Stone WK. Using six-sigma to change and measure improvement. Perform Improv. 2002;41(9):20-26. [Google Scholar]
- 27.Siegelman JRQW, Gress DA. Radiology stewardship and quality improvement: the process and costs of implementing a CT radiation dose optimization committee in a medium-sized community hospital system. J Am Coll Radiol. 2013;10(6):416-422. [DOI] [PubMed] [Google Scholar]
- 28.Goenka AH, Dong F, Wildman B, Hulme K, Johnson P, Herts BR. CT radiation dose optimization and tracking program at a large quaternary-care health care system. J Am Coll Radiol. 2015;12(7):703-710. [DOI] [PubMed] [Google Scholar]
- 29.Zamora DA, Robinson JD, Kanal KM. Targeted CT dose reduction using a novel dose metric and the American College of Radiology Dose Index Registry: application to thoracic CT angiography. AJR Am J Roentgenol. 2016;207(5):1039-1045. [DOI] [PubMed] [Google Scholar]