Abstract
This study uses the National Inpatient Sample database to assess the association between rates of inferior vena cava filter placement in the United States and the 2010 US FDA device safety warning.
The effectiveness of inferior vena cava filter (IVCF) insertion in reducing venous thromboembolism (VTE)-associated morbidity and mortality is uncertain. Nevertheless, IVCF placement rates in the United States have been rapidly increasing and are 25-fold higher than in Europe. Prompted by the report by Nicholson et al in this journal of high prevalence of fracture and embolization, with potentially life-threatening sequelae of the Bard Recovery and Bard G2 IVC filters, the US Food and Drug Administration (FDA) issued a device safety warning on August 9, 2010, after reviewing 921 adverse events (ie, device migration, fracture, thrombosis) reported over a 5-year period. We sought to assess the nationwide utilization rates of IVCF placement in the United States and the impact of this FDA advisory. We also evaluated VTE-related hospitalization rates during the same period to determine whether any change in IVCF utilization could be accounted for by changes in VTE-related hospitalizations. Temple University waived the requirement for institutional review board approval.
Methods
The National Inpatient Sample (NIS) database was used to identify all patients in the United States who underwent IVCF implantation from January 2005 to December 2014. The NIS is the largest publically available all-payer health care database, which contains clinical and hospital specific information on approximately 8 million US hospital discharges each year. We used the International Classification of Diseases, Ninth Revision, Clinical Modification, code for IVCF (procedure code 38.7) to identify all patients older than 18 years who underwent IVCF implantation. All patients with a diagnosis of VTE (proximal lower extremity or inferior vena caval deep vein thrombosis: codes 453.2, 453.41 or pulmonary embolism: codes 415.1, 415.11, 415.13, 415.19) were also identified during the study period. We determined the rates of IVCF implantation, and VTE-related hospitalizations per 100 000 US population, using annual census data provided by the US Census Bureau. Temporal trends were assessed using Cochran-Armitage test (SPSS software, version 22.0; IBM Corp).
Results
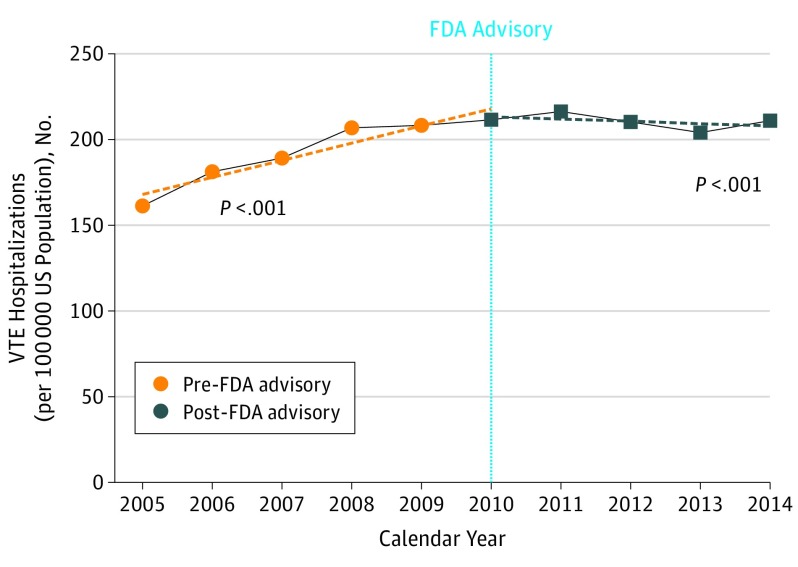
An estimated 1 131 274 patients underwent IVCF placement over the 10-year study period (Figure 1). There was a 22.2% increase in the rate of IVCF placement from 45.2 per 100 000 US population in 2005 to 55.1 per 100 000 in 2010 (P < .001). Following the FDA advisory, there was a 29.0% decrease in the rate of IVCF placement from 55.1 per 100 000 in 2010 to 39.1 per 100 000 in 2014 (P < .001). The rate of VTE-related hospitalizations remained steady between 2010 (211.6 per 100 000) and 2014 (211.3 per 100 000) (Figure 2).
Figure 1. Effect of the US Food and Drug Administration (FDA) Advisory on Trends in Inferior Vena Cava (IVC) Filter Placement in the United States, 2005-2014.
Figure 2. Trends in Venous Thromboembolism (VTE)-Related Hospitalizations in the United States, 2005-2014.
FDA indicates US Food and Drug Administration.
Discussion
This nationwide study demonstrates that more than 1.1 million IVCFs were placed in the United States between 2005 and 2014. Although there was a steady increase in IVCF use from 2005 to 2010, we note a significant decrease in IVCF use following the FDA advisory. As shown in Figure 2, the temporal trends in VTE hospitalizations do not explain the significant reduction in IVCF implantation rates. It has been previously suggested that the decline in the rates of IVCF placement in the United States was a result of decreased reimbursement for this procedure by the way of bundling the associated Current Procedural Terminology (CPT) codes. However, we observed a decline in IVCF use starting in 2010, which was 2 years prior to bundling of IVCF-related CPT codes. It is likely, however, that the reduction in IVCF reimbursement will have an added effect in further reducing the IVCF use across the United States. Another notable variable that changed during course of the study period was introduction of novel oral anticoagulant (NOAC) medications for prevention and treatment of VTE. However, the impact of NOAC use on utilization of IVCFs in the United States is not clear at this time.
Prior studies have reported on the impact of various FDA advisories on clinical practice. Kim et al found a 71% reduction in the use of gadolinium-enhanced magnetic resonance studies among patients with chronic kidney disease in the 2 years following the 2006 FDA advisory describing the risk of nephrogenic systemic sclerosis. Similarly, a 24% reduction in the use of antidepressant medications was seen in young adults following the 2003 FDA advisory regarding increased risk of suicide with these medications among young patients.
Despite the significant reduction in IVCF use following the FDA advisory, implantation rates across the United States remain high. Given the short- and long-term complications associated with IVCF placement, the use of these devices should be mostly reserved for those patients with an absolute indication like active bleeding. Because the rate of IVCF implantation in 5 large European countries is less than 3 per 100 000 population, we believe that the appropriate implantation rate in the United States should be similar to or lower than the rate observed in Europe.
References
- 1.Wang SL, Lloyd AJ. Clinical review: inferior vena cava filters in the age of patient-centered outcomes. Ann Med. 2013;45(7):474-481. [DOI] [PubMed] [Google Scholar]
- 2.Nicholson W, Nicholson WJ, Tolerico P, et al. . Prevalence of fracture and fragment embolization of Bard retrievable vena cava filters and clinical implications including cardiac perforation and tamponade. Arch Intern Med. 2010;170(20):1827-1831. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration Inferior vena cava (IVC) filters: initial communication: risk of adverse events with long term use. Posted online August 9, 2010. https://wayback.archive-it.org/7993/20161022180008/http:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm221676.htm. Accessed May 25, 2017.
- 4.Glocker RJ, TerBush MJ, Hill EL, et al. . Bundling of reimbursement for inferior vena cava filter placement resulted in significantly decreased utilization between 2012 and 2014. Ann Vasc Surg. 2017;38:172-176. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Fonda JR, Lawler EV, Gagnon D, Kaufman JS. Change in use of gadolinium-enhanced magnetic resonance studies in kidney disease patients after US Food and Drug Administration warnings: a cross-sectional study of Veterans Affairs Health Care System data from 2005-2008. Am J Kidney Dis. 2010;56(3):458-467. [DOI] [PubMed] [Google Scholar]
- 6.Lu CY, Zhang F, Lakoma MD, et al. . Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;348:g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]