Abstract
This data analysis simulates the annual price of evolocumab under the outcome-based contracts proposed by Amgen and puts estimates in the context of the available evidence on the cost-effectiveness of PCSK9 inhibitors.
The approval of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab was based on the 61% reduction in the surrogate outcome of low-density lipoprotein cholesterol shown in clinical trials, which, based on the low-density lipoprotein hypothesis, was expected to translate into a 36% to 39% reduction in the risk of cardiovascular events. Under these conditions, Kazi et al and Arrieta et al predicted PCSK9 inhibitors would be cost-effective at an annual price of $4250 to $4500, and the Institute for Clinical and Economic Review recommended an annual price of $2177, estimates considerably lower than the price Amgen set for evolocumab($14 000). The results of the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, powered to evaluate the efficacy of evolocumab in preventing cardiovascular events, were recently published. In the FOURIER trial, evolocumab was associated with a 27% and 21% reduction in the risk of myocardial infarction (MI) and stroke, respectively. There was no reduction in cardiovascular death or all-cause mortality. Following the publication of these results, which failed to meet the expectations generated by previous predictions, Amgen announced its willingness to engage in contracts where the cost of evolocumab would be refunded for patients who have an MI or a stroke while using the drug. In this article, I simulate the annual price of evolocumab under the outcome-based contracts proposed by Amgen, and put my estimates in the context of the available evidence on the cost-effectiveness of PCSK9 inhibitors.
Methods
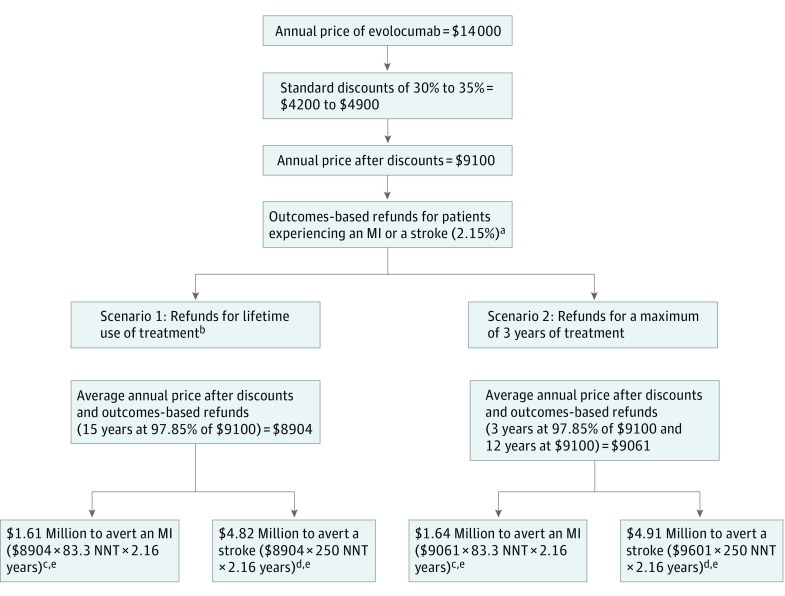
To conduct this analysis, I used an estimate recently released by Amgen of the standard industry discounts for evolocumab. To estimate outcomes-based refunds, I simulated the number of patients that would qualify for outcomes-based refunds based on the rates of MI and stroke derived from the FOURIER trial. I computed these calculations for 2 scenarios: (1) assuming refunds would equal lifetime evolocumab expenditures; and (2) assuming refunds would be limited to a maximum of 3 years of evolocumab therapy. Finally, I calculated the cost of averting an MI and a stroke as the product of the annual price of evolocumab after the application of standard industry discounts and outcomes-based refunds, as well as the number needed to treat estimated from the FOURIER trial.
Results
After the application of standard industry discounts, which average 30% to 35% for evolocumab, the annual price of evolocumab is $9100 (65% of $14 000). If the contracts announced by Amgen were applied to a population comparable to the FOURIER trial sample, payers would be refunded the cost of evolocumab in 2.15% of the patients. This would be equivalent to the application of a 2.15% price reduction across patients, which would translate into an annual price of $8904 (Figure [Scenario 1]). These estimates are, however, too optimistic because they assume the manufacturer would refund lifetime expenditures in PCSK9 inhibitors. More likely, refunds would be limited to just a few years of therapy. For instance, if refunds were limited to 3 years of therapy, the average annual price of evolocumab would be $9061 (Figure [Scenario 2]). This scenario is also more realistic because, with the high turnover characteristic of the insurance market, payers would unlikely get refunds for patients who switch plans. Under these conditions, it would take $1.6 million to prevent a single MI and $4.9 million to avert a single stroke. Putting these estimates into the cost-effectiveness context is challenging because the FOURIER trial did not directly measure quality–adjusted life years and because previous cost-effectiveness studies assumed evolocumab would be associated with a greater reduction in the risk of cardiovascular events than the FOURIER trial found. Even if the results from these studies are too favorable, my estimates for the annual price of evolocumab after discounts and outcomes-based refunds double the prices at which these studies predicted evolocumab would be cost effective ($4250-$4500).
Figure. Estimated Annual Price of Evolocumab After the Application of Standard Industry Discounts and Outcomes-Based Refunds.
MI indicates myocardial infarction; NNT, number needed to treat.
aIf outcomes-based contracts were applied to a population comparable to the FOURIER sample, payers would be refunded the cost of evolocumab in 2.15% of the patients treated (estimate from the FOURIER trial for the combined risk of MI and stroke in the evolocumab group at 12 months follow-up).
bLength of therapy was assumed 15 years because it is the difference between the US average life expectancy and the baseline age of the participants in the FOURIER trial.
cNumber needed to treat to prevent an MI was calculated using the incidence of MI in the evolocumab and placebo groups reported in the FOURIER trial (1/[0.046 − 0.034]).
dNumber needed to treat to prevent a stroke was calculated using the incidence of stroke in the evolocumab and placebo groups reported in the FOURIER trial (1/[0.019 − 0.015]).
eThe cost of averting an MI or a stroke was calculated as the product of the average annual price of treatment, the number needed to treat, and 2.16, because the average follow-up in the FOURIER trial was 26 months (2.16 years).
Discussion
As payers consider engaging in outcomes-based contracts for pharmaceuticals, they will have to negotiate whether these contracts will be applied in lieu of current discounts or as a complement. For outcomes-based contracts to replace current discounts, manufacturers will have to bear an amount of risk that is at least financially comparable to current discounts. Additionally, payers will have to make sure outcomes-based contracts capture the evidence available on the value of the therapy under negotiation. Otherwise, there is a risk that outcomes-based contracts will become the latest complicated artifact used in the reimbursement of pharmaceuticals without providing any additional value to the current mechanisms.
References
- 1.Sabatine MS, Giugliano RP, Wiviott SD, et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500-1509. [DOI] [PubMed] [Google Scholar]
- 2.Kazi DS, Moran AE, Coxson PG, et al. . Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743-753. [DOI] [PubMed] [Google Scholar]
- 3.Arrieta A, Page TF, Veledar E, Nasir K. Economic Evaluation of PCSK9 inhibitors in reducing cardiovascular risk from health system and private payer perspectives. PLoS One. 2017;12(1):e0169761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute for Clinical and Economic Review Final Report. PCSK9 Inhibitors for Treatment of High Cholesterol: Effectiveness, Value, and Value-Based Price Benchmarks. https://icer-review.org/wp-content/uploads/2016/01/Final-Report-for-Posting-11-24-15-1.pdf. Published November 24, 2015. Accessed June 12, 2017.
- 5.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713-1722. doi: 10.1056/NEJMoa1615664 [DOI] [PubMed] [Google Scholar]
- 6.Beasley D, Humer C. Reuters. Amgen discounts cholesterol drug, but payers want more. http://uk.reuters.com/article/us-heart-amgen-pricing-idUKKBN16O2P2. Published March 18, 2017. Accessed March 18, 2017.