Abstract
Objective
Depressive and anxiety symptoms could affect the quality of life and prognostic outcomes in chronic kidney disease (CKD) patients, but only a few studies focus on the interventions to manage or prevent these symptoms in CKD patients. Therefore, this study was conducted to compare the efficacy and acceptability of agomelatine versus paroxetine in treating depressive and anxiety symptoms in CKD patients.
Methods
CKD stage 2–4 patients with depressive and anxiety symptoms were included. The first patient was randomized in April 2013 and the last clinic visit occurred in March 2017. The included patients were randomly assigned to receive paroxetine 20–40 mg/day or agomelatine 25–50 mg/day. The treatment was continued for 12 weeks. The Hamilton Depression Rating Scale (HDRS) (17-item) and Hamilton Anxiety Rating Scale (HARS) were the primary outcome measures, and the response rate, remission rate, and Activities of Daily Living (ADL) scale were the secondary outcome measures. Meanwhile, the adverse events were recorded during the whole treatment period.
Results
At baseline and week 4, both groups had similar average HDRS and HARS scores. But at week 8 and 12, compared to the patients receiving paroxetine, the patients receiving agomelatine had significantly lower average HDRS scores (p=0.002, p=0.001, respectively) and HARS scores (p<0.00001, p<0.00001, respectively). At week 12, the patients receiving agomelatine had a non-significantly lower average ADL score, and non-significantly higher response and remission rates. The adverse events in both groups were mild and transient.
Conclusion
These results demonstrated that the agomelatine had some advantages over par-oxetine in treating CKD stage 2–4 patients with depressive and anxiety symptoms, and future studies are needed to further explore its efficacy and acceptability.
Keywords: chronic kidney disease, CKD, paroxetine, agomelatine
Introduction
Chronic kidney disease (CKD) is a highly prevalent disease and estimated to affect 8%–16% of the global population.1 There are five stages of CKD according to the glomerular filtration rate (GFR) test. Stage 5 of CKD is defined as a GFR of <15 mL/min with marked kidney failure and dialysis requirement. It is also termed end-stage-kidney-disease (ESKD). The CKD patients in stage 5 usually require renal replacement therapy, which mainly involves transplantation or dialysis. But both therapy methods are associated with a cumulative number of hospitalizations and hospital days, which definitely contribute to the excessive costs.2 Approximately 500,000 individuals develop ESKD every year worldwide.3 Accordingly, as the leading cause of ESKD,4 CKD is a global challenge.
Depression is a debilitating mental disease caused by many factors and can affect a person’s behavior, thoughts, and feelings.5–7 It could impinge on self-management ability and then reduce treatment adherence. Previous studies reported that depression was an independent risk factor for poor outcomes in patients with impaired renal function.8,9 Lee et al reported that depressive and anxiety symptoms could affect the quality of life (QoL) and prognostic outcomes in CKD patients, including ESKD patients.10 Indeed, CKD places a considerable economic burden on individuals and often compromises their QoL, which results in a high level of depression and anxiety.11 A study showed that depressive and anxiety symptoms could affect approximately 25% of CKD patients.12 Moreover, a single-center study found that 71% of hemodialysis patients met the criteria for clinical anxiety.13 Thus, early recognition and treatment of depressive and anxiety symptoms in CKD patients is of great importance for better outcomes.
However, up to now, only a few studies have focused on the interventions to manage or prevent depressive and anxiety symptoms in CKD patients. The number of studies on the association between outcomes and depressive and anxiety symptoms in the CKD population is even less. Thus, rigorous research is urgently needed to establish effective therapy methods to treat these mental health problems. Recently, a randomized trial reported that agomelatine had some advantages over paroxetine in treating depressive and anxiety symptoms in patients with type 2 diabetes mellitus.14 Considering the high prevalence of CKD in diabetes patients,15 we hypothesize that agomelatine could be more effective than paroxetine in treating depressive and anxiety symptoms in CKD patients. Therefore, we conducted this randomized trial to validate this assumption.
Methods
CKD patients’ recruitment
This study was approved by the Ethics Committees of the Second Affiliated Hospital of Chongqing Medical University. The first patient was randomized in April 2013 and the last clinic visit occurred in March 2017. Patients who met the following inclusion/exclusion criteria were recruited to this study. The inclusion criteria included: i) adults aged >18 years without suicidal ideation; ii) CKD stage 2–4; iii) Hamilton Depression Rating Scale (HDRS) (17-item) score of ≥17 and Hamilton Anxiety Rating Scale (HARS) score of ≥7; iv) patients were willing to receive random assignment; and v) patients were able to provide written informed consent. The exclusion criteria included: i) renal transplant recipient; ii) gastrointestinal bleeding in the 3 months prior to randomization or significant hepatic dysfunction; iii) use of anti- depressants or psychotherapy 1 month prior to randomization; iv) alcohol or substance abuse or dependence; v) present or past physical or other mental disorders; vi) dementia or a Mini-Mental State Examination score of <23; and vii) women in gestational and lactational period.
Intervention methods
This is a randomized single blind clinical trial. The included patients were randomly assigned to receive paroxetine or agomelatine using a computer-generated random number sequence. The agomelatine was given before bed time, starting with 25 mg/day and could escalate with 5 mg increments to a maximum of 50 mg/day within 2 weeks, if needed. The paroxetine was given in the morning, starting with 20 mg/day and could escalate with 5 mg increments to a maximum of 40 mg/day within 2 weeks, if needed. The treatment was continued for 12 weeks. The patients were not blinded to the randomization, but the investigators in charge of scales’ evaluation and data analysis did not know the drug regimen of the patients.
Evaluation indicators
At baseline, week 4, week 8, and week 12, the depressive and anxiety symptoms of patients were assessed using HDRS and HARS, respectively. Higher HDRS and HARS scores indicated more severe depressive and anxiety symptoms, respectively. At week 8 and week 12, the response rate and remission rate were calculated. Response was defined as a 50% reduction in HDRS scores, and remission was defined as an HDRS score ≤7. At baseline and week 12, the Activities of Daily Living (ADL) scale was used to assess the daily living ability of CKD patients. Higher ADL scores indicated worse daily living ability. The HDRS and HARS scores were the primary outcome measures, and the response rate, remission rate, and ADL score were the secondary outcome measures. In addition, adverse events were recorded during the whole treatment period.
Statistical analysis
Student’s t-test and Chi-squared test were used to assess the differences between the two groups according to the demographic and baseline clinical variables. The number of patients meeting response and remission criteria in the two groups was compared with Chi-squared test at week 8 and week 12. The ADL scores in the two groups at week 12 were compared with Student’s t-test. The repeated measures analysis of variance (ANOVA) was used to investigate the group differences according to HDRS and HARS scores at four time points. All procedures were conducted with SPSS 19.0 (IBM Corporation, Armonk, NY, USA), were 2-tailed, and significance was set at p-value <0.05.
Results
CKD patients’ baseline data
At first, there were 132 patients who met the inclusion/exclusion criteria. Among these patients, 108 patients (81.8%) agreed to sign the informed consent and 24 patients (18.2%) refused to participate. Nine of the 108 patients were excluded because they did not complete the interview. Finally, 99 patients were randomly assigned to receive paroxetine (n=49) or agomelatine (n=50). Due to the potential ethical problems, there was no placebo control group. The demographic variables of participants, such as age, body mass index, and sex ratio, were similar between the two groups. There were also no significant differences according to baseline clinical variables, such as CKD stage, disease duration of CKD, HDRS and HARS scores. The detailed information is shown in Table 1.
Table 1.
Baseline characteristics of CKD patients
| Variables | Paroxetine | Agomelatine | p-value |
|---|---|---|---|
| n | 49 | 50 | – |
| Age (years) | 51.43 (9.38) | 52.60 (9.55) | 0.54 |
| Education (years) | 8.31 (3.08) | 8.22 (2.89) | 0.87 |
| Duration of CKD (years) | 2.79 (0.85) | 2.75 (0.81) | 0.81 |
| BMI (kg/m2) | 22.98 (3.21) | 22.61 (3.53) | 0.58 |
| Female/male | 20/29 | 18/32 | 0.62 |
| Single household (Y/N) | 12/37 | 14/36 | 0.69 |
| Smoking (Y/N) | 15/34 | 21/29 | 0.24 |
| Alcohol abuse (Y/N) | 5/44 | 9/41 | 0.27 |
| Diabetes mellitus (Y/N) | 30/19 | 28/22 | 0.60 |
| CKD by stage (2/3/4) | 12/27/10 | 10/29/11 | 0.86 |
| HDRS scores | 23.61 (2.86) | 23.62 (2.95) | 0.98 |
| HARS scores | 18.73 (3.06) | 18.46 (3.54) | 0.68 |
| ADL scores | 28.20 (5.40) | 28.06 (6.84) | 0.91 |
Note: Data shown as mean (standard deviation) unless indicated otherwise.
Abbreviations: CKD, chronic kidney disease; BMI, body mass index; HDRS, Hamilton Depression Rating Scale; HARS, Hamilton Anxiety Rating Scale; ADL, Activities of Daily Living.
Depressive symptoms
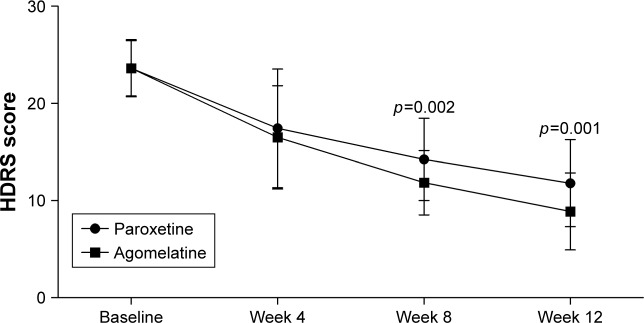
As shown in Figure 1, there was a significant improvement in depressive symptoms over time in both groups. The significant effect of time (p<0.00001) indicated that both paroxetine and agomelatine could effectively treat depressive symptoms in CKD stage 2–4 patients. The significant effect of group × time interaction (p=0.012) indicated that the improvements were significantly different between the two groups. At baseline and week 4, both groups had similar average HDRS scores. But at week 8 and 12, compared to the patients receiving paroxetine, patients receiving agomelatine had significantly lower average HDRS scores (p=0.002, p=0.001, respectively). These results showed that compared to paroxetine, agomelatine was more effective in treating depressive symptoms in CKD stage 2–4 patients.
Figure 1.
HDRS scores in the two groups at baseline, week 4, week 8, and week 12.
Abbreviation: HDRS, Hamilton Depression Rating Scale.
At week 8, 16 patients (32.6%) and three patients (6.1%) in the paroxetine group met response and remission criteria, respectively; 20 patients (40%) and six patients (12%) in agomelatine group met response and remission criteria, respectively. There was no significant group difference in response rates (p=0.44) or remission rates (p=0.48, Fisher’s exact test) at week 8. At week 12, 20 patients (40.8%) and 13 patients (26.5%) in the paroxetine group met response and remission criteria, respectively; 27 patients (54%) and 18 patients (36%) in the agomelatine group met response and remission criteria, respectively. There was no significant group difference in response rates (p=0.30) or remission rates (p=0.31) at week 12. These results showed that compared to paroxetine, agomelatine could obtain non-significantly higher response and remission rates.
Anxiety symptoms
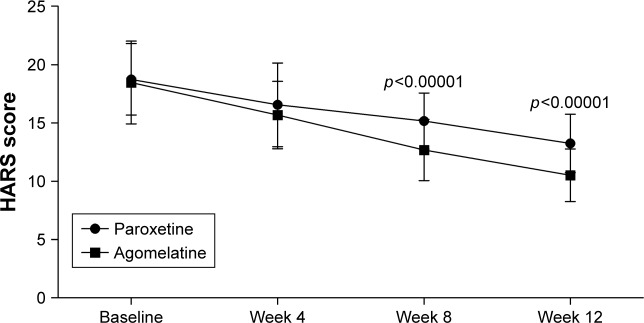
As shown in Figure 2, there was a significant improvement in anxiety symptoms over time in both groups. The significant effect of time (p<0.00001) indicated that both paroxetine and agomelatine could effectively treat anxiety symptoms in CKD stage 2–4 patients. The significant effect of group × time interaction (p=0.001) indicated that the improvements were significantly different between the two groups. At baseline and week 4, both groups had similar average HARS scores. But at week 8 and 12, compared to the patients receiving paroxetine, the patients receiving agomelatine had significantly lower average HARS scores (p<0.00001, p<0.00001, respectively). These results showed that compared to paroxetine, agomelatine was more effective in treating anxiety symptoms in CKD stage 2–4 patients.
Figure 2.
HARS scores in the two groups at baseline, week 4, week 8, and week 12.
Abbreviation: HARS, Hamilton Anxiety Rating Scale.
Daily living ability
As shown in Figure 3, there was a significant improvement in daily living ability in both groups. Compared to the baseline values, the average ADL scores at week 12 were significantly decreased in paroxetine group (p<0.00001) and agomelatine group (p<0.00001). The difference in ADL scores at week 12 between the two groups was still non-significant (p=0.08), although the agomelatine group had a lower average ADL score. Meanwhile, subgroup analysis found that compared to patients who met response criteria in the paroxetine group, patients who met response criteria in the agomelatine group had a significantly lower average ADL score (p=0.02). The difference in ADL scores in patients failing to meet response criteria in the two groups was non-significant (p=0.88). These results showed that compared to paroxetine, agomelatine was more effective in improving the daily living ability of patients who met response criteria.
Figure 3.
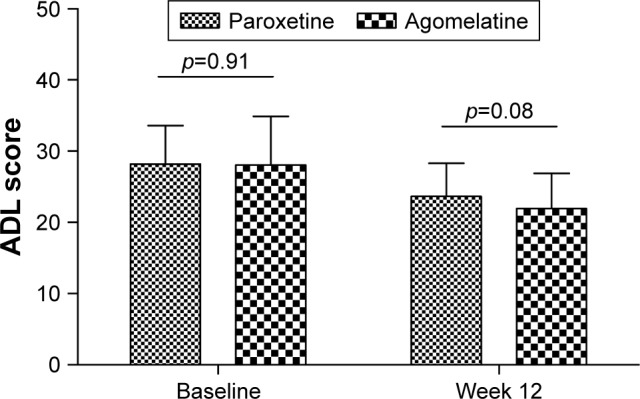
ADL scores in the two groups at baseline and week 12.
Abbreviation: ADL, Activities of Daily Living.
Adverse events
All patients in the two groups successfully completed the trial, and there were no serious adverse events within 12 weeks of treatment. The reported adverse events in the agomelatine group included hyperhidrosis (n=4), nausea (n=2), headache (n=2), vomiting (n=2), dizziness (n=2), diarrhea (n=3), constipation (n=2), and insomnia (n=3). The reported adverse events in the paroxetine group included diarrhea (n=3), constipation (n=2), dry mouth (n=4), nausea (n=4), asthenia (n=3), loss of appetite (n=3), insomnia (n=2), headache (n=3), and dizziness (n=2). These adverse events were transient and went away with continued treatment. There was no significant difference in adverse events between the paroxetine (53.1%) and agomelatine group (40%) (p=0.46). These results indicated that both agomelatine and paroxetine were well tolerated in this study.
Discussion
To our knowledge, this is the first study to compare the efficacy and acceptability of agomelatine and paroxetine in treating CKD stage 2–4 patients with depressive and anxiety symptoms. At the end of the trial, the depressive and anxiety symptoms, and daily living ability were significantly improved in both groups. But compared to paroxetine, agomelatine could yield significantly lower average HDRS and HARS scores, and non-significantly lower average ADL score. The response and remission rates were non-significantly higher in the agomelatine group compared to the paroxetine group. In addition, the number of mild and transient adverse events was similar between the two groups. These results showed that compared to paroxetine, agomelatine was more effective in treating depressive and anxiety symptoms in patients with CKD (stage 2–4).
CKD was the cause of approximately 900,000 deaths globally in 2013, up from approximately 400,000 deaths globally in 1990.16 The high medical costs and poor prognosis of CKD places great psychological pressure on patients; in addition, many CKD patients have anxiety, pessimism, and despair symptoms.17 Physiological function impairment and negative emotions, such as anxiety and depression, could affect CKD patients’ physical functioning and overall health.18 Therefore, it is important to treat these symptoms during the management of CKD. Unfortunately, few studies have been conducted to investigate effective methods in treating depressive and anxiety symptoms in CKD patients. Pascoe et al found that psychosocial interventions might reduce the depressive symptoms and have some beneficial effects on anxiety symptoms.19 Jhee et al reported that management of vitamin D deficiency might be beneficial for the prevention of depression in CKD patients.20 Another study suggested a protective effect of regular physical activity on major depressive episodes in patients with early stages of CKD.21 Here, we found that the HDRS and HARS scores of CKD patients were significantly decreased after treatment with paroxetine or agomelatine. Future studies are still needed to explore optimal methods to treat depressive and anxiety symptoms in CKD patients.
Hedayati et al conducted a trial to determine whether sertraline could improve depressive symptoms in CKD patients, and found that the 16-item Quick Inventory of Depression Symptomatology-Clinician Rated score changed by −4.2 and −4.1 in the placebo group and sertraline group, respectively.22 The response and remission rates were 25.0% and 14.6%, respectively, in the placebo group, and 32.0% and 15.5%, respectively, in the sertraline group. Their findings did not support the use of sertraline to treat major depressive disorder in patients with non-dialysis-dependent CKD. Both sertraline and paroxetine are selective serotonin reuptake inhibitors. In this study, we found that the average HDRS score changed by -11.8, and the response and remission rates were 40.8% and 26.5%, respectively, in the paroxetine group. Compared to sertraline in the previously mentioned study, paroxetine in this study yielded better efficacy in treating depressive symptoms, which might be mainly due to the following reasons: i) the high average age versus low average age; and ii) patients with stage 3–5 CKD versus patients with stage 2–4 CKD.
Limitations should be mentioned here. Firstly, the number of recruited CKD patients with depressive and anxiety symptoms was relatively small; therefore future large-scale clinical trials are needed to verify and support our conclusions. Secondly, there was no placebo control group. Thirdly, this was a single blind trial; the patients knew which medication they received, which might cause potential bias in our results. Fourthly, only patients with stage 2–4 CKD were included; thus, whether results would be similar among patients with other stages of CKD is unknown. Finally, only short-term efficacy and acceptability of paroxetine and agomelatine were assessed here, thus the long-term efficacy and acceptability should be assessed in future studies.
In conclusion, this randomized controlled single blind trial found that compared to paroxetine, agomelatine could yield significantly better efficacy in treating depressive and anxiety symptoms in CKD stage 2–4 patients. Meanwhile, the incidences of adverse events were comparable between the two groups, and these adverse events were mild and transient. Therefore, agomelatine was preferred over paroxetine in treating these populations and should be explored further.
Acknowledgments
Our sincere gratitude is extended to the nurses in the Department of Nephrology for their efforts in this trial.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ene-Iordache B, Perico N, Bikbov B, et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307–e319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- 2.Joyce AT, Iacoviello JM, Nag S, et al. End-stage renal disease-associated managed care costs among patients with and without diabetes. Diabetes Care. 2004;27(12):2829–2835. doi: 10.2337/diacare.27.12.2829. [DOI] [PubMed] [Google Scholar]
- 3.Ojo A. Addressing the global burden of chronic kidney disease through clinical and translational research. Trans Am Clin Climatol Assoc. 2014;125(1):229–246. [PMC free article] [PubMed] [Google Scholar]
- 4.Duran-Salgado MB, Rubio-Guerra AF. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5(3):393–398. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mata DA, Ramos MA, Bansal N, Khan R, Guille C, Di Angelantonio E, Sen S. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373–2383. doi: 10.1001/jama.2015.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen JJ, Zhou CJ, Zheng P, et al. Differential urinary metabolites related with the severity of major depressive disorder. Behav Brain Res. 2017;332:280–287. doi: 10.1016/j.bbr.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen JJ, Zeng BH, Li WW, et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behav Brain Res. 2017;322(Pt A):34–41. doi: 10.1016/j.bbr.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Kop WJ, Seliger SL, Fink JC, et al. Longitudinal association of depressive symptoms with rapid kidney function decline and adverse clinical renal disease outcomes. Clin J Am Soc Nephrol. 2011;6(4):834–844. doi: 10.2215/CJN.03840510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedayati SS, Jiang W, O’Connor CM, et al. The association between depression and chronic kidney disease and mortality among patients hospitalized with congestive heart failure. Am J Kidney Dis. 2004;44(2):207–215. doi: 10.1053/j.ajkd.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, Kim MS, Cho S, Kim SR. Association of depression and anxiety with reduced quality of life in patients with predialysis chronic kidney disease. Int J Clin Pract. 2013;67(4):363–368. doi: 10.1111/ijcp.12020. [DOI] [PubMed] [Google Scholar]
- 11.Theofilou P. Quality of life in patients undergoing hemodialysis or peritoneal dialysis treatment. J Clin Med Res. 2011;3(3):132–138. doi: 10.4021/jocmr552w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stasiak CE, Bazan KS, Kuss RS, Schuinski AF, Baroni G. Prevalence of anxiety and depression and its comorbidities in patients with chronic kidney disease on hemodialysis and peritoneal dialysis. J Bras Nefrol. 2014;36(3):325–331. doi: 10.5935/0101-2800.20140047. [DOI] [PubMed] [Google Scholar]
- 13.Cukor D, Coplan J, Brown C, et al. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52(1):128–136. doi: 10.1053/j.ajkd.2008.02.300. [DOI] [PubMed] [Google Scholar]
- 14.Kang R, He Y, Yan Y, et al. Comparison of paroxetine and agomelatine in depressed type 2 diabetes mellitus patients: a double-blind, randomized, clinical trial. Neuropsychiatr Dis Treat. 2015;11:1307–1311. doi: 10.2147/NDT.S85711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Shen B, Zhuang X, Wang X, Weng W. Investigating factors associated with depressive symptoms of chronic kidney diseases in china with type 2 diabetes. J Diabetes Res. 2017;2017:1769897. doi: 10.1155/2017/1769897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brosnahan G, Fraer M. Management of chronic kidney disease: what is the evidence? South Med J. 2010;103(3):222–230. doi: 10.1097/SMJ.0b013e3181ce0f48. [DOI] [PubMed] [Google Scholar]
- 18.Porter AC, Vijil JC, Jr, Unruh M, Lora C, Lash JP. Health-related quality of life in Hispanics with chronic kidney disease. Transl Res. 2010;155(4):157–163. doi: 10.1016/j.trsl.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascoe MC, Thompson DR, Castle DJ, McEvedy SM, Ski CF. Psychosocial interventions for depressive and anxiety symptoms in individuals with chronic kidney disease: systematic review and meta-analysis. Front Psychol. 2017;8:992. doi: 10.3389/fpsyg.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jhee JH, Kim H, Park S, et al. Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease. PLos One. 2017;12(2):e0171009. doi: 10.1371/journal.pone.0171009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu FX, Zhang XY, Ding XK, Han B. Protective effect of regular physical activity on major depressive episodes in patients with early stages of chronic kidney disease. Ren Failure. 2017;39(1):602–606. doi: 10.1080/0886022X.2017.1361833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hedayati SS, Gregg LP, Carmody T, et al. Effect of sertraline on depressive symptoms in patients with chronic kidney disease without dialysis dependence: the CAST randomized clinical trial. JAMA. 2017;318(19):1876–1890. doi: 10.1001/jama.2017.17131. [DOI] [PMC free article] [PubMed] [Google Scholar]