Abstract
Introduction
Cell plasticity is crucial in cloning to allow an efficient nuclear reprogramming and healthy offspring. Hence, cells with high plasticity, such as multipotent mesenchymal stem cells (MSCs), may be a promising alternative for horse cloning. In this study, we evaluated the use of bone marrow-MSCs (BM-MSCs) as nuclear donors in horse cloning, and we compared the in vitro and in vivo embryo development with respect to fibroblasts.
Materials and methods
Zona-free nuclear transfer was performed using BM-MSCs (MSC group, n=3432) or adult fibroblasts (AF group, n=4527). Embryos produced by artificial insemination (AI) recovered by uterine flushing and transferred to recipient mares were used as controls (AI group).
Results
Blastocyst development was higher in the MSC group than in the AF group (18.1% vs 10.9%, respectively; p<0.05). However, pregnancy rates and delivery rates were similar in both cloning groups, although they were lower than in the AI group (pregnancy rates: 17.7% [41/232] for MSC, 12.5% [37/297] for AF and 80.7% [71/88] for AI; delivery rates: 56.8% [21/37], 41.5% [17/41] and 90.1% [64/71], respectively). Remarkably, the gestation length of the AF group was significantly longer than the control (361.7±10.9 vs 333.9±8.7 days), in contrast to the MSC group (340.6±8.89 days). Of the total deliveries, 95.2% (20/21) of the MSC-foals were viable, compared to 52.9% (9/17) of the AF-foals (p<0.05). In addition, the AF-foals had more physiological abnormalities at birth than the MSC-foals; 90.5% (19/21) of the MSC-delivered foals were completely normal and healthy, compared to 35.3% (6/17) in the AF group. The abnormalities included flexural or angular limb deformities, umbilical cord enlargement, placental alterations and signs of syndrome of neonatal maladjustment, which were treated in most cases.
Conclusion
In summary, we obtained 29 viable cloned foals and found that MSCs are suitable donor cells in horse cloning. Even more, these cells could be more efficiently reprogrammed compared to fibroblasts.
Keywords: equine, cloning, MSC, SCNT
Introduction
Nuclear transfer (NT) efficiency is based on the donor cell reprogramming capability to a totipotent state, commanded by the recipient oocyte.1 Consequently, cell plasticity is crucial to guarantee the progression of embryo development and to have healthy offspring after cloning.
Notable differences in cloning efficiencies are regularly observed in different mammalian species when cells obtained from different tissues are used. Skin fibroblasts are the most common cells used, but cells derived from liver,2 kidney,3 granulosa,4,5 and mesenchymal stem cells (MSCs) have also been tested. In the last years, cloning with MSCs has been evaluated in different farm animal species, such as porcine,6–11 bovine,12,13 caprine,14 ovine,15 and equine.16 Although higher blastocyst rates were observed by using MSCs instead of fibroblasts in most reports, only porcine and bovine offspring has been obtained from MSC cloning procedures.6,7,11,13 The developmental ability of embryos reconstructed using MSCs has been associated with their epigenetic, imprinting and pluripotency gene expression profile, which were similar to in vitro fertilized embryos.7,12 In the horse, MSC-derived embryos also showed higher blastocyst rates than fibroblast-derived embryos, but no offspring was obtained.16 However, this was the only report that evaluated equine MSCs’ potential as nuclear donors, and only a single MSC line derived from the umbilical cord was used in this case.16
Among many types of stem cells, MSCs are multipotent stem cells which are easy to maintain in culture and have the capacity to differentiate into several cell types. In the horse, MSCs have been isolated from different tissues including bone marrow (BM-MSC),17,18 umbilical cord,19–21 adipose tissue22 and blood,23 and are mostly used in regenerative medicine.24–26 Moreover, mesenchymal-like progenitors were also derived from equine-induced pluripotent stem cells (iPSCs).27 Equine MSCs have been shown to express some pluripotency markers, including OCT4 and NANOG,28 and to differentiate in adipocytes, chondrocytes and osteocytes, which demonstrated their multipotency in vitro.16
Until now, healthy foals were born only by using fetal and adult fibroblasts as nuclear donors,5,16,29–32 with high rates of embryonic losses and postnatal mortality as typical outcomes, though. The most common defects observed in cloned foals included limb deformities, umbilical abnormalities and failure of passive transfer,16,31,33–35 probably due to inefficient reprogramming of the donor cell.
In this study, we report the birth of foals cloned by MSCs for the first time. We evaluated the efficiency of BM-MSCs as nuclear donors and compared both in vitro and in vivo developmental potential of MSC-derived embryos compared to fibroblast-derived embryos. Moreover, we focused on evaluating the clinicopathologic status of the foals in order to determine the effect of the nuclear donor cell on clones.
Materials and methods
Reagents
All chemicals were obtained from Sigma Chemical Company (St Louis, MO, USA), except indicated otherwise.
Animal care and use of research animals
This study was carried out following the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. The protocols involving animal manipulations were approved by the Institutional Committee for the Care and Use of Experimental Animals of the San Martin National University, Buenos Aires, Argentina (CICUAE-UNSAM, Permit Number: 001/16).
Oocyte collection, in vitro maturation and preparation for enucleation
Both cumulus–oocyte complex collection from the ovaries and in vitro maturation were performed as previously described by Olivera et al.16 Briefly, the cumulus–oocyte complexes were matured in vitro in bicarbonate-buffered TCM-199 (31100-035, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 1 μL/mL insulin-transferrin-selenium (51300-044, Thermo Fisher Scientific), 10% fetal bovine serum (FBS; 10499-044, Thermo Fisher Scientific), 100 mM cysteamine (M-9768), 1 mM sodium pyruvate (P2256), 2% antibiotic–antimycotic (ATB; penicillin, streptomycin and amphotericin B; 15240-096, Thermo Fisher Scientific) and 0.1 mg/mL of follicle-stimulating hormone (NIH-FSH-P1; Folltropin®, Bioniche, Belleville, ON, Canada) in 5% CO2 and humidified air at 39°C for 21–24 h. Mature oocytes were denuded of cumulus cells with hyaluronidase solution (H4272, 1 mg/mL in Tyrode’s albumin lactate pyruvate medium buffered with N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid36). After that, the zona pellucida of the oocytes was removed by incubation in 1.5 mg/mL pronase (p-8811) for 3–8 min at 35°C in order to proceed to zona-free enucleation.
Nuclear donor cell culture
NT was performed with two different nuclear donor cells: fibroblasts and BM-MSCs. In both cases, they were obtained with the owner’s consent and by minimizing animal sufferings or stress. For fibroblast culture, skin biopsies were obtained from four different female horses of Polo Argentino breed as previously reported.16 BM-MSCs were isolated from the bone marrow aspirates of the sternum of four different horses of Polo Argentino breed (three female and one male). To achieve this, horses were kept under sedation with 3 mL Xilacina 100 (Richmont Vet Pharma, Buenos Aires, Argentina) and 0.5 mL Turbogesic (Ekinos, Buenos Aires, Argentina), and local anesthesia was provided with 2% lidocaine (20 mL). Bone marrow aspirates were collected with a Jamshidi needle by giving pressure over the bone (~300 mL). Bone marrow aspirate was then stored in a blood bag treated with sodium citrate and shipped to the lab at 8°C within 3 h. Once at the lab, the aspirate was passed and filtered, and then washed with 20 mL of DMEM containing 10% FBS. The medium containing the cells was then plated onto 150 mm adherent dishes. Both fibroblasts and BM-MSCs were cultured in DMEM with 10% FBS, 1% ATB and 1 μL/mL of ITS in 5% CO2 in humidified air at 39°C. After 4–7 days, they were subcultured and expanded until freezing. Cycle arrest of donor cells was induced by growth to confluence in 0.5% FBS for 2–3 days before NT.
Characterization of isolated MSCs
In order to confirm that MSC cells were obtained after bone marrow processing, we evaluated the expression of typical horse mesenchymal markers by flow cytometry and their chondrogenic, adipogenic and osteogenic differentiation capabilities. First, the MSCs were trypsinized and resuspended in blocking buffer (Dulbecco’s PBS/bovine serum albumin 0.1%+10% FBS) with specific antibodies diluted 1/100 for 30 min at room temperature. The mesenchymal markers used were CD29 (561795, BD Pharmingen, Franklin Lakes, NJ, USA), CD44 (A14749, Thermo Fisher Scientific), CD90 (555595, BD) and CD105 (560819, BD), and we included CD34 (555821, BD) as a negative control of the hematopoietic linage to ensure the quality of our samples. Following washing with Dulbecco’s PBS, cell analysis was performed with a BD Accuri cytometer. These analyses were done in two different BM-MSC lines randomly selected for this purpose. Multilineage differentiation of the MSC cells was performed as previously described.16
Oocyte enucleation, NT and embryo reconstruction
Oocyte enucleation and NT were performed as previously described.16 Briefly, each zona-free ooplast was individually attached to a single donor cell (fibroblast or BM-MSC) by using 1 mg/mL phytohemagglutinin (L8754) dissolved in TCM-199. A double direct current pulse of 1.2 kV/cm (30 μs, separated by 0.1 s) was given to allow membrane fusion. The fusion medium used was 1 mg/mL polyvinyl alcohol, 0.3 M mannitol, 0.05 mM CaCl2 and 0.1 mM MgSO4. After fusion, the reconstructed embryos were individually placed in 5 μL droplets of DMEM/F12 for 2.5 h to allow nuclear reprogramming. Chemical activation was performed with 8.7 μM ionomycin (I24222, Thermo Fisher Scientific) in Tyrode’s albumin lactate pyruvate medium buffered with HEPES for 4 min followed by culture in 1 mM 6-dimethylaminopurine (D2629) and 5 mg/mL cycloheximide (C7698) in drops of DMEM/F12 for 4 h.
Embryo culture
Three zona-free reconstructed embryos of each experimental group were cultured together in microwells in 100 μL droplets of DMEM/F12 containing 1% ATB and 10% FBS in the presence of a humidified gas mixture (5% CO2, 5% O2, 90% N2) at 39°C. Cleavage was evaluated 72 h after activation when half of the medium was renewed. After 7 and 8 days of culture, blastocyst formation was assessed. The blastocysts were transferred to synchronized mares either on day 7 or on day 8.
Artificial insemination
The in vivo embryos used as controls were produced by artificial insemination (AI) during the two breeding seasons (2014–2015 and 2015–2016, Southern Hemisphere). The donor mares’ cycles were followed by transrectal touch and echography until a follicle larger than 35 mm was observed. At that time, ovulation was induced with 1 mg/mL deslorelin (Deslogest; Over, Santa Fe, Argentina) and the insemination was performed with 500×106/mL spermatozoa. At day 8 post-insemination, blastocysts were recovered by uterus flushing. The flushing solution was passed through a filter that did not allow blastocysts to pass through. These embryos were then washed with holding medium and transferred to recipient mares as described in the following section.
Embryo transfer to recipient mares and clone birth
Non-surgical embryo transfers to recipients were performed in 2014–2015 and 2015–2016 in Buenos Aires, Argentina, during spring season. For NT embryos, one, two or three blastocysts were transferred per recipient mare, whereas for in vivo generated embryos, one blastocyst was transferred per recipient mare. Mare examination, estrus synchronization and embryo transfer were performed as previously described by our group.16 Pregnancies were diagnosed by transrectal ultrasonography 7–15 days after embryo transfer. During pregnancy, fetal movements, placental quality, umbilical abnormalities and heart rate were monitored. In addition, the quality of amniotic fluid was analyzed in order to detect fetal suffering as soon as possible. Between 20 and 30 days before expected parturition, the pregnant mares were transported to an equine hospital (KAWELL, Equine Rehabilitation Center, Solís, Argentina) to give birth.
Evaluation of clinical status and scoring of abnormalities
Foals were analyzed for different pathologies after birth. In order to standardize grading of severity of the abnormalities, a different score was used for each abnormaility.33 One of the pathologies was a congenital articular contracture of the carpus involving the joint capsule that shows fibrosis in a position of marked flexion. To classify this pathology, limbs were scored as follows: 0=straight limbs; 1=slight joint retraction corrected without treatment within days after birth; 2=moderate changes (valgus or tendon contracture) treated by application of bandages, splints and antibiotic oxytetracycline, corrected in less than a month; 3=moderate changes requiring long treatment or surgery; 4=severe contraction not correctable but compatible with life; and 5=severe contraction not correctable and incompatible with life. Another pathology was the syndrome of neonatal maladjustment (SNM). After 48 h of birth, the presence of signs of hypoxic ischemic encephalopathy was evaluated and the following scores were assigned: 0=clinically normal foals; 1=slightly weak foals with prolonged time to stand and suckle, but these signs were overcome without treatment; 2=weak foals without the ability to stand and suckle, but these signs were resolved within 24–72 h with palliative treatment; 3=markedly maladjusted foals with hypoxic signs that were recovered with intensive care; 4=markedly maladjusted foals with hypoxic signs that were recovered with intensive care; however, slight alterations persisted compatible with life but not with the sporty performance; and 5=severely maladjusted and the foal died. At birth, the umbilical cord associated with the placenta, the external umbilical opening and the internal remnants were also evaluated by transabdominal ultrasonography. The umbilicus was scored as follows: 0=normal and broke without assistance; 1=slightly enlarged (2–3 cm); 2=moderately enlarged (>3 cm) and ligatures at parturition were required; 3=markedly enlarged (>3 cm) and surgical removal required; and 4=omphalocele. In addition, anatomic characteristics of the placenta were evaluated at foals’ birth and the pathologies related to this organ were scored as follows: 0=normal placenta; 1=placenta >12% of the foal weight or <8% and/or with slight changes in its structure; 2=placenta >12% of the foal weight or <8% and/or with moderate changes in its structure (edema, villi, abnormal color, secretions, tissue folds); 3=placenta >12% of the foal weight or <8% and/or with big changes in its structure (edema, villi, abnormal color, secretions, tissue folds); and 4=placental retention.
Neonatal hospitalization
All the cloned foals were hospitalized in a preventive manner. After birth, oxygen therapy was given and the colostrum of the mother was measured. In those cases where the colostrum was <15° Brix, 1 L of colostrum from the hospital stock was given to the neonates. Moreover, a preventive glycerin enema was also supplied and the neonates were under control until they eliminated meconium. Three hours after birth, laboratory studies were performed, including blood count, creatinine, lactate, blood gases and electrolytes. Twelve hours after birth, the glutaraldehyde test was performed to evaluate passive transfer, and a clot formation in <5 min was taken as a reference value. In case it coagulated in >5 min, parenteral plasma was transferred to the foal in a preventive manner until correct immunization was achieved. Depending on the status of the foal, veterinary treatment was established and the discharge was assigned when the foal’s conditions were suitable for field management.
Confirmation of clones
Once the foals were born, the genome of each foal was analyzed in order to confirm that they were clones. To achieve this, 15 loci of the foal and the respective donor animal were compared in the Laboratorio de Genética Aplicada Sociedad Rural Argentina, Buenos Aires, Argentina. The evaluated loci were AHT4, AME, ASB2, HMS3, HMS7, HTG4, LEX33, HMS2, AHT5, ASB17, ASB23, HMS6, HTG10, LEX3 and VHL20.
Statistical analysis
Statistical analysis for the results of in vitro and in vivo embryo development was performed using chi-square test or nonparametric Fisher’s exact test. Gestational periods, weight at birth and days of hospitalization were compared by one-way analysis of vairance test. In all cases, the software used was Statistix version 0.8, and differences were considered significant at p<0.05.
Results
Confirmation of the mesenchymal status of the bone marrow–derived cells
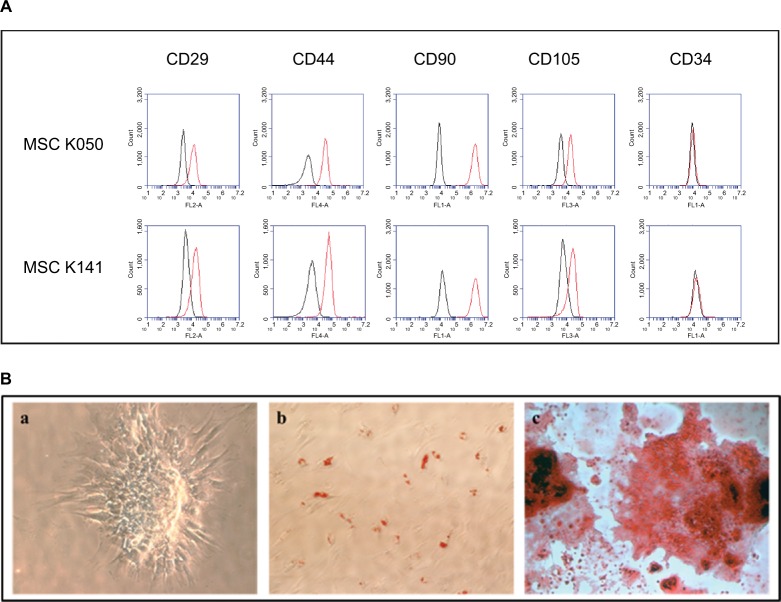
After immunofluorescence cell staining and flow cytometry, we observed that the isolated cells were positive for the four MSC surface markers, including CD29, CD44, CD90 and CD105, and negative for the hematopoietic cell marker CD34 (Figure 1A). Moreover, we assessed the differentiation capability of these cells into osteocytes, adipocytes and chondrocytes, confirming their multipotent capability (Figure 1B). Both these experiments and the adherence of the cells to plastic suggested that the isolated cells were of mesenchymal origin instead of hematopoietic origin.
Figure 1.
Evaluation of the mesenchymal status of the bone marrow–derived cells.
Notes: (A) Characterization of cell surface markers CD29, CD44, CD90 and CD105 of mesenchymal origin and CD34 of hematopoietic origin by flow cytometry in two different MSC lines. The histograms show cell fluorescence intensity on the X-axis and cell frequency distribution on the Y-axis. The bone marrow isolated cells were positive for the four mesenchymal markers and negative for the hematopoietic marker. (B) Evaluation of multipotent capability of bone marrow–derived cells into (a) chondrogenic, (b) adipogenic and (c) osteogenic lineages. The isolated cells were able to differentiate into these three lineages.
Abbreviation: BM-MSC, bone marrow–derived mesenchymal stem cell.
In vitro development of zona-free aggregated horse embryos reconstructed with BM-MSC or fibroblasts as nuclear donors
In vitro development of horse cloned embryos reconstructed with BM-MSC or fibroblasts as nuclear donors is summarized in Table 1. A total of 7959 zona-free reconstructed embryos were generated for this experiment, obtaining 1114 blastocysts in total. Both cleavage and blastocyst production were higher when BM-MSCs were used as nuclear donors (p<0.05). Cleavage rates were 85.6% (3875/4527) vs 90.2% (3095/3432), and blastocyst rates were 32.6% (492/1509) and 54.4% (622/1144) for adult fibroblast (AF) and MSC groups, respectively.
Table 1.
In vitro development of ZFREs reconstructed with BM-MSC or fibroblasts as nuclear donors
| Groups | ZFREs | No. of embryos (wells)* | No. of cleaved embryos (%) | Blastocyst production
|
||
|---|---|---|---|---|---|---|
| No. | % per embryo | % per ZFRE | ||||
| AF | 4527 | 1509 | 3875 (85.6)a | 492 | 32.6a | 10.9a |
| MSC | 3432 | 1144 | 3095 (90.2)b | 622 | 54.4b | 18.1b |
Notes:
Values with different superscripts in a column are significantly different (Fisher’s exact test p<0.05).
Embryo aggregation was performed and each well contained three ZFREs.
Abbreviations: ZFREs, zona-free reconstructed embryos; BM-MSCs, bone marrow–derived mesenchymal stem cells; AF, adult fibroblasts; MSC, mesenchymal stem cell.
In vivo development of zona-free aggregated horse embryos reconstructed with BM-MSC or fibroblasts as nuclear donors
After embryo transfer, we examined the pregnancy rates, loss of early and late pregnancies and the number of viable offspring for each group (Table 2). Both AF and MSC cloning groups were compared with the AI group as an in vivo control. As expected, pregnancy rates were significantly lower in AF and MSC groups compared to the AI group: 41/476 (17.7%) and 37/594 (12.5%) vs 71/88 (80.7%) (p<0.05). Moreover, higher embryo loss rates were observed for these groups, especially during the first trimester of gestation: 46% (19/41) of pregnancies were lost in the AF group between 0 and 3 months of gestation and 32.4% pregnancies (12/37) were lost in the MSC group (p=NS). No fetal losses were observed in the in vivo group after 90 days of gestation, although 12.2% (5/41) and 10.8% (4/37) of fetal losses were recorded for the AF and the MSC groups, respectively. A total of 29 foals were born alive between both cloning groups and 63 in the AI control group, giving a cloning efficiency per recipient mare of 3.9% in the AF group, 6.7% in the MSC group and 71.6% in the AI group. Although foal delivery rates were similar between both cloning groups, healthy offspring rates were significantly higher in the MSC group compared to the AF group, with 95.2% (20/21) of the delivered foals being viable in the MSC group vs 52.9% (9/17) in the AF group (p<0.05; Figure 2).
Table 2.
Effect of donor cell type on in vivo development of horse cloned blastocysts
| Groups | Transferred embryos | Recipient mares | Pregnancies (%) | Embryo losses at 0–3 months (%) | Fetal losses at 4–11 months (%) | Deliveries (%) | Viable foals
|
||
|---|---|---|---|---|---|---|---|---|---|
| No. | % per pregnancy | % per delivery | |||||||
| AF | 476 | 232 | 41 (17.7)a | 19 (46)a | 5 (12.2)a | 17/41 (41.5)a | 9 | 22.0a | 52.9a |
| MSC | 594 | 297 | 37 (12.5)a | 12 (32.4)a | 4 (10.8)a | 21/37 (56.8)a | 20 | 54.1b | 95.2b |
| AI | 88 | 88 | 71 (80.7)b | 7 (9.9)b | 0 (0)b | 64/71 (90.1)b | 63 | 88.7c | 98.4b |
Notes:
Values with different superscripts in a column are significantly different (Fisher’s exact test p<0.05).
Abbreviations: AF, adult fibroblasts; MSC, mesenchymal stem cell; AI, artificial insemination embryos as controls.
Figure 2.
Eight cloned polo horses derived from the same MSC line, born in August, September and October 2016.
Abbreviation: MSC, mesenchymal stem cell.
Clinical status of delivered foals
In order to determine the capacity of the fibroblasts or BM-MSCs to generate healthy foals after cloning, we compared the weight at birth, the days of gestation and the clinical status of the delivered clones with those of in vivo foals. These results are summarized in Figure 3 and Table 3. The average weight at birth of the AF foals was statistically higher than the average weight at birth of the MSC foals: 49.6±8.5 kg (n=17) vs 45±5.3 kg (n=21), respectively (p<0.05). Considering that AI Polo Argentino horses are born with 45–55 kg on an average (according to the information obtained from the Argentinian equine hospital Kawell), both cloning groups were in the range of normality (Figure 3). The gestation period in this breed may range between 310 and 365 days. In this study, MSC foals’ pregnancy lasted on an average 340.6±8.9 days (n=21), which correlates with the gestation of the in vivo foals, 333.9±8.7 days on an average (n=64; p=nonsignificant). However, gestation length of the AF foals (n=17) was significantly higher than those of the other two groups, 361.7±10.9 days on an average (p<0.05). In addition, AF clones had more clinical complications and anatomic defects at birth than MSC clones. In the AF group, three of the live-born foals had some degree of flexural or angular limb deformity with score ≥2, whereas none of the live-born foals from the MSC group had this type of defects. The only foal with limb retractions in this group (score=3) also presented serious clinical abnormalities including umbilicus enlargement (score=3), SNM (score=3) and placental disorders (score=3) and died after birth as a consequence of colon hypoplasia. In the AF group, six of the not viable foals also exhibited limb contractions with score ≥2. Marked umbilicus cord enlargements (score ≥3) were also observed in 6 of the 17 fibroblast born foals (35.3%); two of them were viable and required surgery for treatment. The other four foals with this pathology that were not viable also exhibited SNM (scores=5, 5, 3 and 1) and limb retractions (scores=4, 4, 3 and 4) and died after birth. Among the AI foals, one also exhibited SNM with score ≥3 and died after birth (1/64, 1.6%), similar to the MSC group (1/21, 4.7%). Placental abnormalities with score ≥3 were also observed in not viable foals of both cloning groups, with no statistical differences between them.
Figure 3.
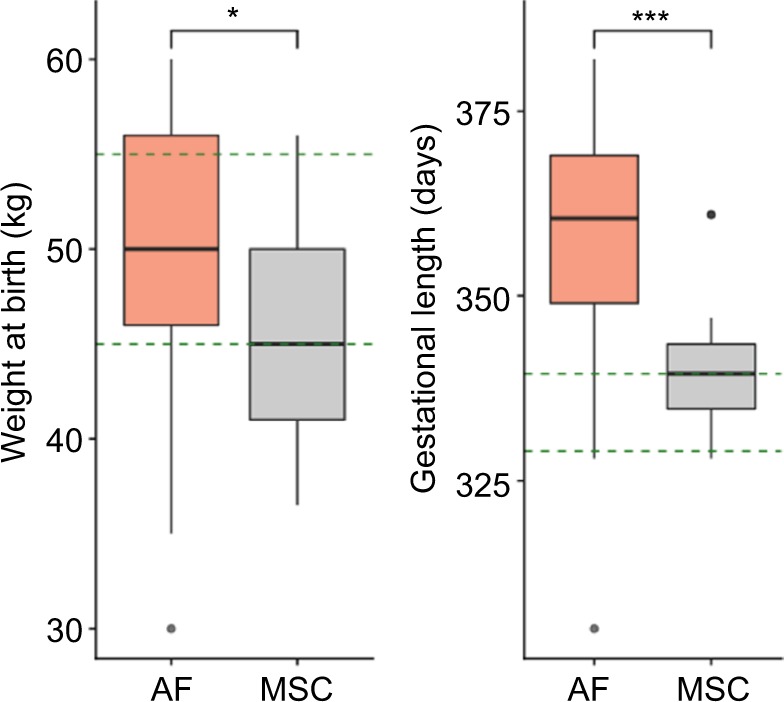
Weight at birth and gestational length of the AF and MSC cloned groups.
Notes: The green dashed lines are delimiting the range of normality according to information obtained from artificial insemination foals. Both weight at birth and gestational length were higher in the AF group compared to the MSC group (Student’s t-test, *p<0.05; ***p<0.001).
Abbreviations: AF, adult fibroblasts; MSC, mesenchymal stem cell.
Table 3.
Clinical status and scoring of abnormalities of delivered cloned foals and in vivo foals
| Groups | Deliveries | Gestational length (mean days ± SD) | Score retractions ≥2 n (%)
|
Score umbilicus ≥3 n (%)
|
Score SNM ≥3 n (%)
|
Score placentas ≥3 n (%)
|
Hospitalization (mean days ± SD)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Viable | Not viable | Viable | Not viable | Viable | Not viable | Viable | Not viable | Viable | Not viable | |||
| AF | 17 | 361.7±10.9a (305–382) | 3 (17.6)a | 6 (35.3)a | 2 (11.8)a | 4 (23.5)a | 0a | 7 (41.2)a | 0 | 2 (11.8)a | 21.2±5.4a | 1.8±1.3a |
| MSC | 21 | 340.6±8.9b (328–361) | 0b | 1 (4.7)b | 0ab | 1 (4.7)a,b | 1 (4.7)a | 1 (4.7)b | 0 | 1 (4.7)a,b | 6.3±4.0b | 1a |
| AI | 64 | 333.9±8.7b (312–363) | 0b | 0b | 0b | 0b | 0a | 1 (1.6)b | 0 | 0b | NA | NA |
Notes:
Values with different superscripts in a column are significantly different (Fisher’s exact test p<0.05).
Abbreviations: SNM, syndrome of neonatal maladjustment; AF, adult fibroblasts; MSC, mesenchymal stem cell; AI, artificial insemination embryos as controls; NA, not applicable.
The hospitalization time was also recorded in order to determine the care required by the neonates of cloning groups. In all cases, mares pregnant with clones were transported to an equine hospital to give birth. Comparing the hospitalized days between both cloning groups, we observed that AF foals needed much more veterinary care than MSC foals (14.3±10.6 vs 6.05±4.1 days, respectively; p<0.05), especially the viable foals (21.2±5.4 vs 6.3±4.0 days, respectively). In contrast, mares pregnant with in vivo derived embryos were not hospitalized and gave birth without special assistance.
Discussion
We demonstrated for the first time the capability of BM-MSCs to generate viable healthy offspring after NT in the horse. In addition to the scientific relevance of studying nuclear reprogramming and horse embryo development by this technique, the interest on cloning has increased to maintain and reproduce high-quality genetic composition of sports animals. For this reason, since the first cloned horse was born,37 researchers have focused on improving this technique in order to increase healthy offspring rates. By using BM-MSCs as nuclear donors, we could reach this goal. We achieved 95% (20/21) of foals born without any cloning defects commonly observed, thus improving the viability rates and their general clinical status.
To the best of our knowledge, this study is the first report on the use of MSCs in equine NT, but their potential as nuclear donors has been demonstrated before in other mammalian species. As previously reported, higher in vitro preimplantation development was observed in bovine,12 porcine38 and goat14 with MSCs as nuclear donors compared to fibroblasts. In the porcine, embryos reconstructed with adipose tissue MSCs (aMSCs) resulted in higher blastocyst rates compared to peripheral blood MSCs or fibroblast-reconstructed embryos,11 which reflects the variability among different MSC sources. On the other hand, another report in the same species showed no differences in in vitro embryo development, but higher quality blastocysts were obtained when MSCs were used instead of fibroblasts.7 This might be related to the gene expression profile of MSC-derived embryos which resulted in being similar to in vivo embryos unlike fibroblast-derived embryos.38 We obtained higher cleavage and blastocyst rates in the MSC group than in the AF group, both by using BM-MSCs in this study and umbilical cord MSCs in a previous report of our group.16 For in vivo embryo development assessment, 617 embryo transfers were achieved among the MSC group, the AF group and the AI control group. As expected, the AI group showed the highest pregnancy rates, and similar pregnancy rates were observed between both cloning groups. Regardless of the cell source we used for cloning, pregnancy loss of the NT-derived embryos was substantial, especially during the first trimester of gestation, which is a common concern when working on this technique. Most studies have also reported high pregnancy loss after cloning, with usually ≤5% of transferred embryos resulting in viable foals.5,16,30,31,37,39–41 However, seven pregnancies from the control group were also lost in the first trimester, which suggests that the greatest vulnerability of pregnancy occurs during this period.
We observed that the delivery rates between the AF group and the MSC group were not significantly different, as 41.5% (17/41) of the pregnancies reached their term in the AF group and 56.8% (21/37) in the MSC group (p=NS). However, significant differences were obtained in the viability of the delivered foals. Almost all of the MSC-delivered foals were viable and healthy, whereas half of the AF-delivered foals were alive after birth. Differences were also obtained in the weight at birth and in the gestation length (Figure 3). Higher weight at birth in the AF group may be related to longer gestational periods in this group. MSC pregnancies lasted similar to AI embryos, in contrast to AF pregnancies that lasted longer. The normal gestation length is considered to be between 310 and 365 days in Polo Argentino breed. Despite 361 days of gestation on an average in the fibroblast group which is within the range of normality, there were seven foals that were born after longer pregnancy periods (367–382 days); five of them were born alive and two died after birth. These variations in the gestation lengths were not observed in the MSC group since all the pregnancies were in the normal range. We believe that abnormally longer gestational periods are related to placental insufficiency, which could result in a reduction of the nutrient supply to the developing fetus. Moreover, high creatinine levels were observed in these neonatal foals, which revealed the dysfunctional capacity of the placenta as the excretory organ of the fetal foals.42
In addition to higher viability rates, almost all the MSC-derived foals were healthy and did not exhibit any clinico-pathologic defect after birth. In the AF group, in addition to eight neonatal losses, three viable foals presented with limb contraction (scores 2 and 3) and umbilicus enlargement (score 3), which were treated and resolved with specialized care, though. Some of these pathologic disorders were also reported previously in cloned foals,16,31,33–35 and they were directly related to the NT technique. In concordance with our results, cloned piglets derived from MSCs did not exhibit any visible defect,7 which usually could be seen in piglets cloned from fibroblasts.7,43 Moreover, higher live birth rates were obtained from peripheral blood–derived MSCs as nuclear donors than fibroblasts in the same species.11 Similar observations were made in the bovine, as higher offspring rates were obtained when amniotic fluid MSCs (25%) and aMSCs (12.5%) were used as nuclear donors with respect to the overall efficiency in this species.13,44
Another finding that revealed differences in the clinical status between the foals of both cloning groups was the time the neonates spent in hospital with veterinary care after birth. Despite the fact that all pregnant mares gave birth in an equine hospital as a precaution, MSC foals left medical cares within 1 week on an average, whereas viable AF foals required hospitalization for 3 weeks. This information highlights the intensive care assistance the fibroblast-derived foals required after birth in comparison to the MSC-derived foals.
We consider that the differences between the MSC-embryos and the AF-embryos were due to the initial differentiation status of each cell type. We propose that nuclear reprogramming was improved when BM-MSCs were used because of their multipotent capacity. While some articles defend the idea that there is no correlation between the stemness of the cell and the cloning efficiency,45–47 others have demonstrated that the differentiation status influences the developmental potential of the reconstructed embryos. There are many studies that observed higher developmental rates of the embryos when less-differentiated cells were used compared to fully differentiated cells as nuclear donors.6,7,11,13,38,48 Moreover, it has been shown that cells showing high expression of Oct4 are more efficient as NT donor cells,8 which reinforces this statement. On the other hand, cloning with iPSCs as nuclear donors in domestic animals has not been promising, in spite of their pluripotent capacity.16,47,49,50 These differences between the efficiencies of MSCs and iPSCs may be related to the resident expression of the exogenous pluripotent genes oct4, cmyc, klf4 and sox2 in integrative iPSC lines,16,51 the suboptimal culture conditions52 or the difficulties regarding cell cycle synchronization of the iPSCs which could compromise the success of cloning.53 In contrast, MSCs are multipotent stem cells that are easy to maintain in culture and behave similar to fibroblasts in vitro, which facilitates their manipulation and cell cycle synchronization. Not only the multipotent capacity of the MSCs, but also their easy manipulation makes these cells advantageous for cloning. In summary, our study strongly suggests that BM-MSCs are suitable nuclear donor cells and that they can produce healthier cloned horses through NT, as compared with fibroblasts. In addition, further experiments are required to compare other MSC sources for NT, such as aMSCs or peripheral blood MSCs, to strengthen our work. Until now, equine MSCs have been used in regenerative medicine for therapeutic applications,54–56 but our report is the first one that has used these cells as nuclear donors with proper evidence that healthy offspring can be generated. The birth of 29 viable foals in both cloning groups supports our conclusions.
Acknowledgments
The authors thank Lic. Dara Dobler, MV Victoria Arnold, MV Emanuel Galvagno, MV Benjamin Esturla and Dra. Fernanda Ortiz for their technical assistance.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Niemann H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology. 2016;86(1):80–90. doi: 10.1016/j.theriogenology.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Waghmare SK, Estrada J, Reyes L, et al. Gene targeting and cloning in pigs using fetal liver derived cells. J Surg Res. 2011;171(2):e223–e229. doi: 10.1016/j.jss.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 3.Richter A, Kurome M, Kessler B, et al. Potential of primary kidney cells for somatic cell nuclear transfer mediated transgenesis in pig. BMC Biotechnol. 2012;12:84. doi: 10.1186/1472-6750-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polejaeva IA, Chen SH, Vaught TD, et al. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature. 2000;407(6800):86–90. doi: 10.1038/35024082. [DOI] [PubMed] [Google Scholar]
- 5.Lagutina I, Lazzari G, Duchi R, et al. Somatic cell nuclear transfer in horses: effect of oocyte morphology, embryo reconstruction method and donor cell type. Reproduction. 2005;130(4):559–567. doi: 10.1530/rep.1.00772. [DOI] [PubMed] [Google Scholar]
- 6.Jin HF, Kumar BM, Kim JG, et al. Enhanced development of porcine embryos cloned from bone marrow mesenchymal stem cells. Int J dev Biol. 2007;51(1):85–90. doi: 10.1387/ijdb.062165hj. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, He X, Chen L, et al. Bone marrow mesenchymal stem cells are an attractive donor cell type for production of cloned pigs as well as genetically modified cloned pigs by somatic cell nuclear transfer. Cell Reprogram. 2013;15(5):459–470. doi: 10.1089/cell.2013.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Lee WJ, Jeon RH, et al. Development and gene expression of porcine cloned embryos derived from bone marrow stem cells with overexpressing Oct4 and Sox2. Cell Reprogram. 2014;16(6):428–438. doi: 10.1089/cell.2014.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samiec M, Opiela J, LipiNski D, Romanek J. Trichostatin A-mediated epigenetic transformation of adult bone marrow-derived mesenchymal stem cells biases the in vitro developmental capability, quality, and pluripotency extent of porcine cloned embryos. Biomed Res Int. 2015;2015:814686. doi: 10.1155/2015/814686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Z, Cong P, Ji Q, et al. Establishment, differentiation, electroporation and nuclear transfer of porcine mesenchymal stem cells. Reprod Domest Anim. 2015;50(5):840–148. doi: 10.1111/rda.12577. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Vajta G, Xu Y, et al. Production of pigs by hand-made cloning using mesenchymal stem cells and fibroblasts. Cell Reprogram. 2016;18(4):256–263. doi: 10.1089/cell.2015.0072. [DOI] [PubMed] [Google Scholar]
- 12.Nazari H, Shirazi A, Shams-Esfandabadi N, Aafzali A, Aahmadi E. The effect of amniotic membrane stem cells as donor nucleus on gene expression in reconstructed bovine oocytes. Int J Dev Biol. 2016;60(4–6):95–102. doi: 10.1387/ijdb.160010hn. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva CG, Martins CF, Cardoso TC, et al. Production of bovine embryos and calves cloned by nuclear transfer using mesenchymal stem cells from amniotic fluid and adipose tissue. Cell Reprogram. 2016;18(2):127–136. doi: 10.1089/cell.2015.0064. [DOI] [PubMed] [Google Scholar]
- 14.Kwong PJ, Nam HY, Wan Khadijah WE, Kamarul T, Abdullah RB. Comparison of in vitro developmental competence of cloned caprine embryos using donor karyoplasts from adult bone marrow mesenchymal stem cells vs ear fibroblast cells. Reprod Domest Anim. 2014;49(2):249–253. doi: 10.1111/rda.12262. [DOI] [PubMed] [Google Scholar]
- 15.Su X, Ling Y, Liu C, et al. Isolation, culture, differentiation, and nuclear reprogramming of mongolian sheep fetal bone marrow-derived mesenchymal stem cells. Cell Reprogram. 2015;17(4):288–296. doi: 10.1089/cell.2014.0109. [DOI] [PubMed] [Google Scholar]
- 16.Olivera R, Moro LN, Jordan R, et al. In vitro and in vivo development of horse cloned embryos generated with ipscs, mesenchymal stromal cells and fetal or adult fibroblasts as nuclear donors. PLoS One. 2016;11(10):e0164049. doi: 10.1371/journal.pone.0164049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidal MA, Kilroy GE, Johnson JR, Lopez MJ, Moore RM, Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006;35(7):601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Arnhold SJ, Goletz I, Klein H, et al. Isolation and characterization of bone marrow-derived equine mesenchymal stem cells. Am J Vet Res. 2007;68(10):1095–1105. doi: 10.2460/ajvr.68.10.1095. [DOI] [PubMed] [Google Scholar]
- 19.Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. doi: 10.1186/1472-6750-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed SA, Johnson SE. Equine umbilical cord blood contains a population of stem cells that express Oct 4 and differentiate into mesodermal and endodermal cell types. J Cell Physiol. 2008;215(2):329–336. doi: 10.1002/jcp.21312. [DOI] [PubMed] [Google Scholar]
- 21.Hoynowski SM, Fry MM, Gardner BM, et al. Characterization and differentiation of equine umbilical cord-derived matrix cells. Biochem Biophys Res Commun. 2007;362(2):347–353. doi: 10.1016/j.bbrc.2007.07.182. [DOI] [PubMed] [Google Scholar]
- 22.Vidal MA, Kilroy GE, Lopez MJ, Johnson JR, Moore RM, Gimble JM. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet Surg. 2007;36(7):613–622. doi: 10.1111/j.1532-950X.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 23.Koerner J, Nesic D, Romero JD, Brehm W, Mainil-Varlet P, Grogan SP. Equine peripheral blood-derived progenitors in comparison to bone marrow-derived mesenchymal stem cells. Stem Cells. 2006;24(6):1613–1619. doi: 10.1634/stemcells.2005-0264. [DOI] [PubMed] [Google Scholar]
- 24.Smith RKW, Korda M, Blunn GW, Goodship AE. Isolation and implantation of autologous equine mesenchymal stem cells from bone marrow into the superficial digital flexor tendon as a potential novel treatment. Equine Vet J. 2003;35(1):99–102. doi: 10.2746/042516403775467388. [DOI] [PubMed] [Google Scholar]
- 25.Guest DJ, Ousey JC, Smith MR. Defining the expression of marker genes in equine mesenchymal stromal cells. Stem Cells Cloning. 2008;1:1–9. doi: 10.2147/sccaa.s3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilke MM, Nydam DV, Nixon AJ. Enhanced early chondrogenesis in articular defects following arthroscopic mesenchymal stem cell implantation in an equine model. J Orthop Res. 2007;25(7):913–925. doi: 10.1002/jor.20382. [DOI] [PubMed] [Google Scholar]
- 27.Lepage SI, Nagy K, Sung HK, Kandel RA, Nagy A, Koch TG. Generation, characterization, and multilineage potency of mesenchymal-like progenitors derived from equine induced pluripotent stem cells. Stem Cells Dev. 2016;25(1):80–89. doi: 10.1089/scd.2014.0409. [DOI] [PubMed] [Google Scholar]
- 28.Esteves CL, Sharma R, Dawson L, et al. Expression of putative markers of pluripotency in equine embryonic and adult tissues. Vet J. 2014;202(3):533–535. doi: 10.1016/j.tvjl.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Lagutina I, Lazzari G, Duchi R, et al. Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig and sheep. Theriogenology. 2007;67(1):90–98. doi: 10.1016/j.theriogenology.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Gambini A, Jarazo J, Olivera R, Salamone DF. Equine cloning: in vitro and in vivo development of aggregated embryos. Biol Reprod. 2012;87(1):1–9. doi: 10.1095/biolreprod.112.098855. [DOI] [PubMed] [Google Scholar]
- 31.Gambini A, De Stefano A, Bevacqua RJ, Karlanian F, Salamone DF. The aggregation of four reconstructed zygotes is the limit to improve the developmental competence of cloned equine embryos. PLoS One. 2014;9:e110998. doi: 10.1371/journal.pone.0110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi YH, Velez IC, Macías-García B, Hinrichs K. Timing factors affecting blastocyst development in equine somatic cell nuclear transfer. Cell Reprogram. 2015;17(2):124–130. doi: 10.1089/cell.2014.0093. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AK, Clark-Price SC, Choi YH, Hartman DL, Hinrichs K. Physical and clinicopathologic findings in foals derived by use of somatic cell nuclear transfer: 14 cases (2004±2008) J Am Vet Med Assoc. 2010;236(9):983–990. doi: 10.2460/javma.236.9.983. [DOI] [PubMed] [Google Scholar]
- 34.Choi YH, Norris JD, Velez IC, Jacobson CC, Hartman DL, Hinrichs K. A viable foal obtained by equine somatic cell nuclear transfer using oocytes recovered from immature follicles of live mares. Theriogenology. 2013;79(5):791–796. doi: 10.1016/j.theriogenology.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Hall V, Hinrichs K, Lazzari G, Betts DH, Hyttel P. Early embryonic development, assisted reproductive technologies, and pluripotent stem cell biology in domestic mammals. Vet J. 2013;197(2):128–142. doi: 10.1016/j.tvjl.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Bavister B, Yanagimachi R. The effects of sperm extracts and energy sources on the motility and acrosome reaction of hamster spermatozoa in vitro. Biol Reprod. 1977;16(2):228–237. doi: 10.1095/biolreprod16.2.228. [DOI] [PubMed] [Google Scholar]
- 37.Galli C, Lagutina I, Crotti G, et al. Pregnancy: a cloned horse born to its dam twin. Nature. 2003;424(6949):635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- 38.Kumar BM, Jin HF, Kim JG, et al. Differential gene expression patterns in porcine nuclear transfer embryos reconstructed with fetal fibroblasts and mesenchymal stem cells. Dev Dyn. 2007;236(2):435–446. doi: 10.1002/dvdy.21042. [DOI] [PubMed] [Google Scholar]
- 39.Woods GL, White KL, Vanderwall DK, et al. A mule cloned from fetal cells by nuclear transfer. Science. 2003;301(5636):1063. doi: 10.1126/science.1086743. [DOI] [PubMed] [Google Scholar]
- 40.Vanderwall DK, Woods GL, Aston KI, et al. Cloned horse pregnancies produced using adult cumulus cells. Reprod Fertil Dev. 2004;16(7):675–679. doi: 10.1071/rd04025. [DOI] [PubMed] [Google Scholar]
- 41.Wonyou L, Kilyoung S, Inhyung L, Hyungdo S, Byeong CL, Seongchan Y. Cloned foal derived from in vivo matured horse oocytes aspirated by the short disposable needle system. J Vet Sci. 2015;16(4):509–516. doi: 10.4142/jvs.2015.16.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cummins C, Carrington S, Fitzpatrick E, Duggan V. Ascending placentitis in the mare: a review. Ir Vet J. 2008;61(5):307–313. doi: 10.1186/2046-0481-61-5-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt M, Kragh PM, Li J, et al. Pregnancies and piglets from large white sow recipients after two transfer methods of cloned and transgenic embryos of different pig breeds. Theriogenology. 2010;74(7):1233–1240. doi: 10.1016/j.theriogenology.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 44.Heyman Y, Chavatte-Palmer P, LeBourhis D, Camous S, Vignon X, Renard JP. Frequency and occurrence of late-gestation losses from cattle cloned embryos. Biol Reprod. 2002;66(1):6–13. doi: 10.1095/biolreprod66.1.6. [DOI] [PubMed] [Google Scholar]
- 45.Berg D, Li C, Asher G, Wells D, Oback B. Red deer cloned from antler stem cells and their differentiated progeny. Biol Reprod. 2007;77(3):384–394. doi: 10.1095/biolreprod.106.058172. [DOI] [PubMed] [Google Scholar]
- 46.Oback B. Cloning from stem cells: different lineages, different species, same story. Reprod Fertil Dev. 2009;21(1):83–94. doi: 10.1071/rd08212. [DOI] [PubMed] [Google Scholar]
- 47.Secher J, Liu Y, Petkov S, et al. Evaluation of porcine stem cell competence for somatic cell nuclear transfer and production of cloned animals. Anim Reprod Sci. 2017;178:40–49. doi: 10.1016/j.anireprosci.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Faast R, Harrison S, Beebe L, McIlfatrick S, Ashman R, Nottle M. Use of adult mesenchymal stem cells isolated from bone marrow and blood for somatic cell nuclear transfer in pigs. Cloning Stem Cells. 2006;8(3):166–173. doi: 10.1089/clo.2006.8.166. [DOI] [PubMed] [Google Scholar]
- 49.Fan N, Chen J, Shang Z, et al. Piglets cloned from induced pluripotent stem cells. Cell Res. 2013;23(1):162–166. doi: 10.1038/cr.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du X, Feng T, Yu D, et al. Barriers for deriving transgene-Free pig iPS cells with episomal vectors. Stem Cells. 2015;33(11):3228–3238. doi: 10.1002/stem.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 52.Hall V, Hyttel P. Breaking down pluripotency in the porcine embryo reveals both a premature and reticent stem cell state in the inner cell mass and unique expression profiles of the naive and primed stem cell states. Stem Cells Dev. 2014;23(17):2030–2045. doi: 10.1089/scd.2013.0502. [DOI] [PubMed] [Google Scholar]
- 53.Yuan Y, Lee K, Park K, et al. Cell cycle synchronization of leukemia inhibitory factor (LIF)-dependent porcine-induced pluripotent stem cells and the generation of cloned embryos. ABBV Cell Cycle. 2014;13(18):1265–1276. doi: 10.4161/cc.28176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;8(9):e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broeckx S, Zimmerman M, Crocetti S, et al. Regenerative therapies for equine degenerative joint disease: a preliminary study. PLoS One. 2014;9(1):e85917. doi: 10.1371/journal.pone.0085917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geburek F, Roggel F, van Schie HTM, et al. Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial. Stem Cell Res Ther. 2017;8(1):129. doi: 10.1186/s13287-017-0564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]