Abstract
Rhizoleucinoside (1), a unique rhamnolipid-amino alcohol hybrid was isolated from the rhizobial symbiont bacteria Bradyrhizobium sp. BTAi1. 1 features a rare rhamnolipid core attached to an unprecedented leucinol moiety. Its structure and absolute configuration were determined by spectroscopic analysis, tandem mass spectrometry, chemical degradation and application of the Marfey’s method. 1 possesses moderate cytotoxicity to BV-2 murine microglia and highly aggressive proliferating immortalized (HAPI) rat microglia cells.
Graphical Abstract
Rhamnolipids are a group of biosurfactants produced by bacteria and their amphiphilic property originates from their glycolipidic structures.1 Rhamnolipids were first isolated from the opportunistic human pathogen Pseudomonas aeruginosa.2 More recent studies have expanded the bacteria taxa known to produce rhamnolipids to include new families such as Acinetobacter calcoaceticus from the family Moraxellaceae,3 different orders such as Pseudoxanthomonas sp. from the order Xanthomonadales,4 and even different classes such as Burkholderia spp. from the class Betaproteobacteria.5,6 Despite the production of rhamnolipids over disparate taxonomic groups, their biological function is still unclear. Studies suggest rhamnolipids might play multifunctional roles such as promoting the uptake of water insoluble substrates,7 immune modulators,8 antimicrobials,9 and insecticides.10 The common core structures are composed of one or two L-rhamnoses linked to one or two β-hydroxy fatty acids (C10-C16) through a glycosidic bond. β-Hydroxy fatty acids are linked through ester bonds while di-saccharides are linked through α-1,2 glycosidic linkages.1
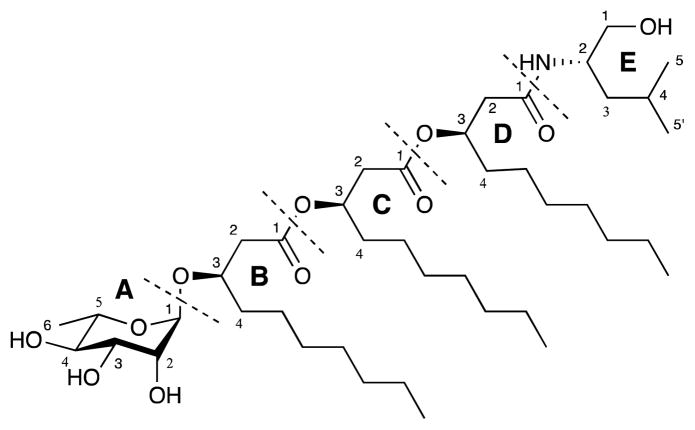
In the study presented here, an unprecedented rhamnolipid-amino alcohol hybrid rhizoleucinoside (1) (Figure 1), featuring a rare Rha-C10-C10-C10 glycolipid core attached to a terminal leucinol moiety, was isolated from Bradyrhizobium sp. BTAi1 (ATCC BAA-1182). Bradyrhizobium species are symbiotic bacteria that fix nitrogen for their leguminous host plants.11 Although the whole genome of this strain has been sequenced,11 secondary metabolites have not been previously reported for this organism. To the best of our knowledge, this is the first example of an amino alcohol-containing rhamnolipid as well as the first rhamnolipid isolated from a bacterium belonging to the class Alphaproteobacteria.
Figure 1.
Structure of rhizoleucinoside (1).
1 was isolated as an amorphous, colorless solid, [α]23.2D −13.7 (c 0.1, MeOH) from culture extracts of Bradyrhizobium sp. BTAi1. The molecular formula of 1 was established as C42H79NO11 on the basis of the HRESIMS ion at m/z 774.5730 [M + H]+ (calcd for C42H80NO11, 774.5726) and its sodium adduct ion m/z 796.5556 [M + Na]+. The IR spectrum showed a broad absorbance for OH and NH (~3320 cm−1) and sharp absorbances for C=O (1736 cm−1 for ester and 1646 cm−1 for amide) and C-O (1042 cm−1).
The 1H (Table 1) and HSQC NMR spectra of 1 in DMSO-d6 showed a methine proton at δH 4.62 bound to a di-oxygenated carbon at δc 100.2, four methine protons between 3.15 and 3.53 ppm correlating with oxygenated sp3 carbons between 69.1 and 78.8 ppm, three exchangeable protons between 4.48 and 4.69 ppm, and one methyl group (δH 1.08/δc 18.2). gCOSY and zTOCSY NMR spectra revealed a spin system (Figure 2) comprising anomeric proton H-A1 (δH 4.62), H-A2 (δH 3.53), H-A3 (δH 3.35), H-A4 (δH 3.15), H-A5 (δH 3.44) and H-A6 (δH 1.08), suggesting the presence of a 6-deoxyhexosyl subunit. This subunit was designated here as subunit A. Subunit A was determined to be rhamnopyranosyl by High Performance Anion Exchange Chromatography coupled with Pulsed Amperometric Detection (HPAEC-PAD) analysis of the hydrolyzed 1 in comparison with monosaccharide standards. The absolute stereochemistry of subunit A was determined after complete hydrolysis of 1. By measurement of optical rotation, rhamnose purified from 1 hydrolysates was determined to be L-rhamnose [α]20D +7.9 (c 0.1, H2O).12 The diequatorial coupling between anomeric proton H-A1 and H-A2 (J1,2 = 1.3 Hz)13 indicated the glycosidic bond of L-rhamnopyranosyl moiety was in the α conformation.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data of 1 in DMSO-d6
| no. | δ (ppm), type | δ H (ppm) (mult, J (Hz)) | 1H-1H COSY | 1H-13C HMBC |
|---|---|---|---|---|
| A1 | 100.2, CH | 4.62, d (1.3) | A2 | A2, A5, B3 |
| A2 | 71.2, CH | 3.53, m | A1, A2, A2-OH | A3, A4 |
| A2-OH | 4.65, d (5.6) | A2 | A3 | |
| A3 | 71.0, CH | 3.35, m | A2, A4, A3-OH | |
| A3-OH | 4.48, d (6.1) | A3 | A2 | |
| A4 | 72.3, CH | 3.15, m | A3, A5, A4-OH | |
| A4-OH | 4.69, d (4.8) | A4 | A4 | |
| A5 | 69.2, CH | 3.44, m | A4, A6 | A3, A4, |
| A6 | 18.2, CH3 | 1.08, d (6.2) | A5 | A5 |
| B1 | 170.3, qC | |||
| B2 | 39.0, CH2 | 2.41, dd (14.0, 7.5), 2.46 | B3 | B1, B3 |
| B3 | 74.3, CH | 3.85, m | B2, B4 | A1, B1, B4 |
| B4 | 33.8, CH2 | 1.43, m | B3 | |
| B5-B9 | 22.5–31.6, CH2 | 1.19–1.27, m | ||
| B10 | 14.5, CH3 | 0.84, m | ||
| C1 | 169.4, qC | |||
| C2 | 40.6, CH2 | 2.45, m, 2.53, m | C3 | C1, C3 |
| C3 | 70.3, CH | 5.04, m | C2, C4 | B1 |
| C4 | 33.5, CH2 | 1.52, m | C3 | |
| C5-C9 | 22.5–31.6, CH2 | 1.18–1,25, m | ||
| C10 | 14.5, CH3 | 0.84, m | ||
| D1 | 168.7, qC | |||
| D2 | 40.4, CH2 | 2.29, dd (14.0, 5.5), 2.33. dd (14.0, 7.9) | D3 | D1, D3 |
| D3 | 71.8, CH | 5.08, m | D2, D4 | C1 |
| D4 | 33.5, CH2 | 1.46, m | D3 | |
| D5-D9 | 22.5–31.6, CH2 | 1.17–1.24, m | ||
| D10 | 14.5, CH3 | 0.84, m | ||
| E1 | 64.2, CH2 | 3.16, m, 3.27, m | E1-OH | |
| E1-OH | 4.56, t (5.5) | E1 | E1, E2 | |
| E2 | 49.0, CH | 3.76, m | E2-NH | |
| E2-NH | 7.54, d (8.7) | E2 | E2 | |
| E3 | 40.3, CH2 | 1.23, m | ||
| E4 | 24.7, CH | 1.53, m | ||
| E5 | 22.2, CH3 | 0.79, d (6.5) | E4 | E4 |
| E5′ | 23.8, CH3 | 0.84, d | E4 | E4 |
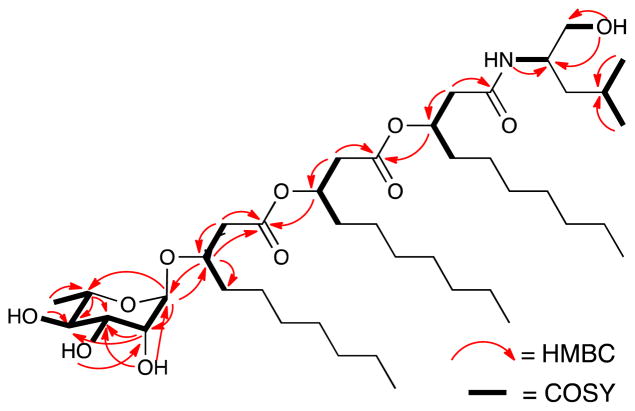
Figure 2.
Key 1H-1H COSY and HMBC correlations of 1.
The 1H, gCOSY and HSQC NMR spectra of 1 also showed one oxygenated methylene (δH Ha 3.16/Hb 3.27/δc 64.2), one methine (δH 3.76/δc 49.0) bound to a secondary amine at δH 7.54, one aliphatic methylene (δH 1.23/δc 40.3), one aliphatic methine (δH 1.53/δc 24.7) and two aliphatic methyl groups (δH 0.79/δc 22.2 and δH 0.84/δc 23.8). This subunit was designated as subunit E. The gCOSY and zTOCSY NMR spectra revealed a spin system along the hydroxyl methylene H-E1 (δH Ha 3.16/Hb 3.27), amine bound methine H-E2 (δH 3.76), H-E3 (δH 1.23), H-E4 (δH 1.53) and the two methyls H-E5 (δH 0.79) and H-E5′ (δH 0.84), suggesting the presence of a leucinol subunit. Correlation between the amine proton (δH 7.54) and carbonyl carbon (δc 168.7) in the HMBC spectrum indicated that the leucinol subunit connected to the rest of 1 through an amide bond. The chirality of leucinol purified from 1 hydrolysates was determined by reaction with Marfey’s reagent and comparison with the retention time of its FDAA derivative with L-leucinol and D-leucinol standards FDAA derivatives. Marfey’s method has been previously used to determine the stereochemistry of amino alcohols such as alaninol and valinol.14 To further validate this method for leucinol, L-leucinol and D-leucinol standards were derivatized with Marfey’s reagent and analyzed by reversed phase HPLC. By comparison of the HPLC retention time, the amino alcohol subunit in 1 was determined to be L-leucinol.
In addition to the resonances belonging to rhamnopyranosyl and leucinol subunits, 1H and 13C NMR spectra showed three sets of resonances sharing highly similar structural features. The 13C NMR spectrum showed three carbonyl carbons at δc 168.7, δc 169.4 and δc 170.3, three oxygenated methine carbons at δc 70.3, δc 71.8 and δc 74.3, three α-methylene carbons at δc 39.0, δc 40.4 and δc 40.6, and three long aliphatic chains with highly overlapped resonances, consistent with three β-hydroxyl fatty acid subunits designated here as B, C and D. The gCOSY and zTOCSY spectra revealed partial features of the spin systems. For subunit B, a partial spin system was observed along α-methylene protons B-H2 (δH Ha 2.41/Hb 2.46), oxygenated methine B-H3 (δH 3.85), aliphatic methylene B-H4 (δH 1.43) and a group of methylene resonances between δH 1.12 ppm and δH 1.32 ppm. Similarly, two other spin systems were assigned along C-H2 (δH Ha 2.45/Hb 2.53), oxygenated methine C-H3 (δH 5.04) and aliphatic methylene C-H4 (δH 1.52) for subunit C; and D-H2 (δH Ha 2.29/Hb 2.33), oxygenated methine D-H3 (δH 5.08) and aliphatic methylene D-H4 (δH 1.46) for subunit D, respectively. HMBC correlations from B-H3 to A-C1 and from A-H1 to B-C3 indicated that the α-rhamnopyranosyl group was connected to subunit B via a glycosidic bond. The ester bond between subunit C and B was deduced from HMBC correlations B-H2 and C-H3 to the carbonyl carbon at δc 170.3. Similarly, the ester bond between subunit D and C was deduced from HMBC correlations C-H2 and D-H3 to carbonyl carbon at δc 169.4. The amide proton (δH 7.54) from subunit E and α-methylene protons D-H2 (δH Ha 2.29/Hb 2.33) showed HMBC correlations to the carbonyl carbon at δc 168.7, providing the connectivity between subunits D and E.
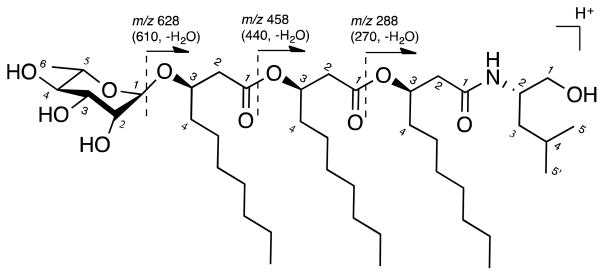
The length of each fatty acid chain for subunits B, C and D could not be resolved by NMR data alone. To address this issue, tandem mass spectrometry (MS/MS) was performed on the precursor ion m/z 774.5674 [M + H]+. As illustrated in Figure 3, fragment ions at m/z 628.5108 and m/z 610.5000 were detected as loss of the rhamnose residue (−146 Da) and with additional loss of H2O (−164 Da), respectively, indicating fragmentation across the glycosidic bond. Minor fragment ions at m/z 458.3804 and m/z 440.3707 were detected as loss of one terminal fatty acyl chain (C10H18O2, −170 Da) from product ions m/z 628.5108 and m/z 610.5000, respectively. Similarly, fragment ions m/z 288.2508 and m/z 270.2414 were detected as further loss of a terminal fatty acyl chain (C10H18O2, −170 Da). The N-(3-hydroxydecanoyl)-leucinol (m/z 288.2508) and its dehydrated ion were left with no further visible fragmentations. From these data, all subunits B, C and D in 1 have C10 backbones.
Figure 3.
Fragments of 1 by ESI-MS/MS.
To determine the absolute configuration of the oxygenated methines, the 3-hydroxydecanoic acids were isolated following complete hydrolysis of 1. The optical rotation of the 1:1:1 mixture was measured as [α]25D −18.2 (c 0.1. CHCl3), which is in agreement with the reported optical rotation of (R)-3-hydroxydecanoic acid ([α]25D −17.5(c 0.9 CHCl3).15 Therefore, all three 3-hydroxyldecanoic acid subunits possess the R-configuration, in agreement with other bacterial rhamnolipids.1
1 showed cytotoxicity with IC50 of 6.9 μM in BV-2 (murine microglia) and IC50 of 22 μM in HAPI (rat microglia). 1 was also tested for antimicrobial activities against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa PAO1, but no visible growth inhibition was observed.
In summary, we identified a novel rhamnolipid-amino alcohol hybrid rhizoleucinoside (1) from Bradyrhizobium sp. BTAi1. Its uniqueness lies in the incorporation of an amino alcohol moiety and the unprecedented rhamnolipid core structure that includes three sequential β-hydroxydecanoic acids. While the ecological role of this molecule requires further investigation, evidence suggests its possible role in establishing the symbiosis between plant host and bacterium. The first step to forming the symbiotic relationship between rhizobial bacteria and plant involves invasion of root cells.16 Legume immune systems can be triggered during the infection but become rapidly suppressed.17 Recent studies18,19 have suggested that Nod factors (NFs), a group of signal molecules widely produced by rhizobium, may be responsible for plant immunity suppression. Interestingly, as a unusual phenomenon, Bradyrhizobium sp. BTAi1 is reported to lack the genes nodABC, which are essential for the production of NFs.11 Alhede et al20 postulates that rhamnolipids produced by the pathogen P. aeruginosa function as a shield to thwart the immune responses during infections of a human host. Further investigation will be required to determine if rhizoleucinoside plays a role for Bradyrhizobium sp. BTAi1 in suppressing the host plant defense system and facilitating the establishment of symbiosis.
Supplementary Material
Acknowledgments
Instruments used for chemical analyses were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (grant number 2 P20 GM103430). NMR data was acquired at a research facility supported in part by the National Science Foundation EPSCoR Cooperative Agreement #EPS-1004057. J.C. was supported in part by the China Scholarship Council (File no. 201408330156).
Footnotes
Notes
The authors declare no competing financial interest.
Experimental details, the HRESIMS, MS/MS, NMR spectra and biological activity of 1. The Supporting Information is available free of charge on the ACS Publications website.
References
- 1.Abdel-Mawgoud AM, Lepine F, Déziel E. Appl Microbiol Biotechnol. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soberón-Chávez G, Lepine F, Déziel E. Appl Microbiol Biotechnol. 2005;68:718–725. doi: 10.1007/s00253-005-0150-3. [DOI] [PubMed] [Google Scholar]
- 3.Rooney AP, Price NPJ, Ray KJ, Kuo TM. FEMS Microbiol Lett. 2009;295:82. doi: 10.1111/j.1574-6968.2009.01581.x. [DOI] [PubMed] [Google Scholar]
- 4.Nayak AS, Vijaykumar MH, Karegoudar TB. Int Biodeterior Biodegrad. 2009;63:73–79. [Google Scholar]
- 5.Andrä J, Rademann J, Howe J, Koch MHJ, Heine H, Zähringer U, Brandenburg K. Biol Chem. 2006;387:301–310. doi: 10.1515/BC.2006.040. [DOI] [PubMed] [Google Scholar]
- 6.Dubeau D, Déziel E, Woods DE, Lepine F. BMC Microbiol. 2009;9:1–12. doi: 10.1186/1471-2180-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Itoh S, Suzuki T. Agric Biol Chem. 1972;36:2233–2235. [Google Scholar]
- 8.McClure CD, Schiller NL. J Leukocyte Biol. 1992;51:97–102. doi: 10.1002/jlb.51.2.97. [DOI] [PubMed] [Google Scholar]
- 9.Itoh S, Honda H, Tomita F, Suzuki T. J Antibiot. 1971;24:855–859. doi: 10.7164/antibiotics.24.855. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Kim YC, Lee S, Kim JC, Yun MY, Kim IS. J Agric Food Chem. 2011;59:934–938. doi: 10.1021/jf104027x. [DOI] [PubMed] [Google Scholar]
- 11.Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal L, Fardoux J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z, Mangenot S, Segurens B, Dossat C, Franck WL, Chang WS, Saunders E, Bruce D, Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Verméglio A, Médigue C, Sadowsky M. Science. 2007;316:1307–1312. doi: 10.1126/science.1139548. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis FG, Johnson MJ. J Am Chem Soc. 1949;71:4124–4126. [Google Scholar]
- 13.Zeng YB, Wang H, Zuo WJ, Zheng B, Yang T, Dai HF, Mei WL. Mar Drugs. 2012;10:598–603. doi: 10.3390/md10030598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii K, Ikai Y, Mayumi T, Oka H, Suzuki M, Harada K. Anal Chem. 1997;69:3346–3352. [Google Scholar]
- 15.Cartwright NJ. Biochem J. 1957;67:663–669. doi: 10.1042/bj0670663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray JD. Mol Plant-Microbe Interact. 2011;24:631–639. doi: 10.1094/MPMI-08-10-0181. [DOI] [PubMed] [Google Scholar]
- 17.Gourion B, Berrabah F, Ratet P, Stacey G. Trends Plant Sci. 2015;20:186–194. doi: 10.1016/j.tplants.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara M, Sadowsky MJ. Mol Plant-Microbe Interact. 2014;27:328–335. doi: 10.1094/MPMI-10-13-0312-R. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Cao Y, Tanaka K, Thibivilliers S, Wan J, Choi J, Kang CH, Qiu J, Stacey G. Science. 2013;341:1384–1387. doi: 10.1126/science.1242736. [DOI] [PubMed] [Google Scholar]
- 20.Alhede M, Bjarnsholt T, Jensen PØ, Phipps RK, Moser C, Christophersen L, Christensen LD, van Gennip M, Parsek M, Høiby N, Rasmussen TB, Givskov M. Microbiology. 2009;155:3500–3508. doi: 10.1099/mic.0.031443-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.