Abstract
We report the use of a sulfonated biarylphosphine ligand (sSPhos) to promote the chemoselective modification of cysteine containing proteins and peptides with palladium reagents in aqueous medium. The use of sSPhos allowed for the isolation of several air-stable and water-soluble mono- and bis-palladium reagents, which were used in an improved protocol for the rapid S-arylation of cysteines under benign and physiologically relevant conditions. The cosolvent-free aqueous conditions were applied to the conjugation of a variety of biomolecules with affinity tags, heterocycles, fluorophores, and functional handles. Additionally, bis-palladium reagents were used to perform macrocyclization of peptides bearing two cysteine residues.
Graphical abstract
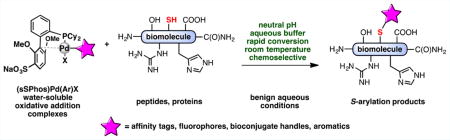
Methods for the chemical modification of amino acids serve as fundamental tools for research at the interface of chemistry and biology and play an important role as enabling technologies for the pharmaceutical and materials sciences. Important applications include the preparation of probe molecules for the study of protein function,1 the development of new protein–drug conjugates,2 nanomedicines with unique therapeutic properties,3 and the incorporation of biological macromolecules in functional hybrid materials.4 Ideally, broadly applicable protocols to modify amino acids for bioconjugation are processes that are chemoselective, proceed at a high rate under mild reaction conditions (i.e., aqueous solvents/buffers, pH 6–8, temperature <37 °C), and are tolerant to the functional group diversity present in complex biomolecules. In addition, such a process should forge stable chemical bonds in a modular manner that provides access to structural diversity.5,6 The preparation of protein bioconjugates dates back over a century;7 however, few reports exist which meet all of these specifications.
With these criteria in mind, we previously reported a palladium-mediated method that can be used for the reliable and rapid S-arylation of cysteine residues in peptides and proteins.8 This method is highly chemoselective for cysteine among other nucleophilic residues, as confirmed by MS/MS analysis, and can be used without incorporation of unnatural amino acids. One notable limitation of this procedure, however, was the necessary addition of an organic cosolvent (N,N-dimethylformamide, dimethyl sulfoxide, or acetonitrile) to dissolve the organometallic reagents. These organic cosolvents have the potential to denature sensitive biomolecules, limiting the scope and utility of our approach. To address this limitation, we sought an improved, cosolvent-free protocol for bioconjugation employing modified, water-soluble palladium complexes.
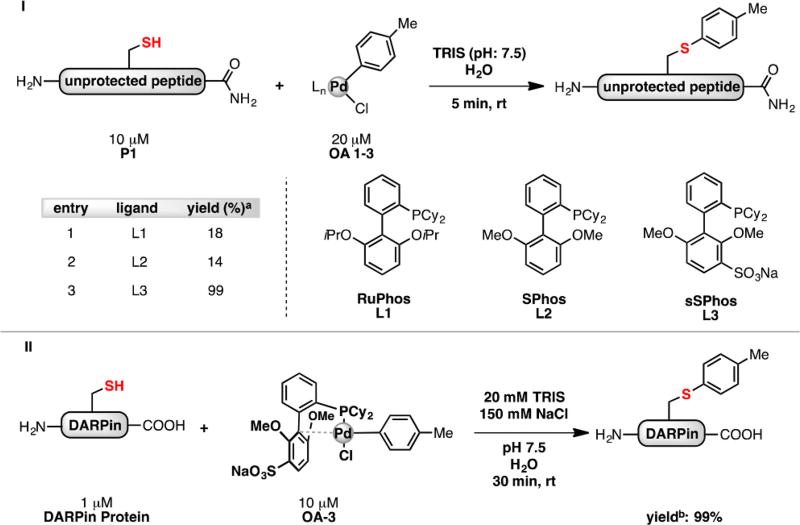
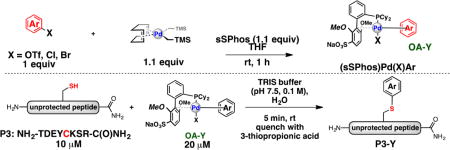
Our group reported the synthesis of a sulfonated ligand, sodium 2′-dicyclohexylphosphino-2,6-dimethoxy-1,1′-biphenyl-3-sulfonate hydrate (sSPhos), which has been utilized in the context of Suzuki–Miyaura couplings in water.9 We hypothesized that palladium(II) complexes supported by this ligand might be sufficiently water-soluble to serve as arylation reagents for cysteine-containing biomolecules under completely aqueous conditions. To test this hypothesis, both a model peptide and a designed ankyrin repeat protein (DARPin), each containing a single cysteine residue, were exposed to an sSPhos supported palladium reagent in a completely aqueous environment (Figure 1). We chose the DARPin protein to ensure the comparison in ligand-enhanced reactivity directly paralelled our previous study.8 In both cases, the product of cysteine arylation was formed rapidly at room temperature with high chemoselectivity. In contrast, use of the complexes supported by RuPhos and SPhos ligands, under these cosolvent-free conditions, provided <20% yield. These results are consistent with the idea that the sulfonate group of sSPhos allows the palladium reagent to be more soluble in water and, therefore, able to react with cysteine residues more efficiently.
Figure 1.
Effect ligand imposes on efficiency of palladium-mediated unprotected cysteine bioconjugation under ambient aqueous conditions. a Yield determined by LC-MS analysis. b Yield determined by deconvolution of mass spectrum.
In our previous report, the palladium reagents were prepared in a nitrogen-filled glovebox, presenting a potential barrier for adoption by laboratories without routine access to a glovebox. To ameliorate this situation, we investigated the preparation of several sSPhos-ligated palladium complexes employing a benchtop protocol, without the exclusion of moisture or oxygen from the reaction mixture (see Supporting Information, SI, for details). Under these conditions, it was found that a mixture of aryl halide (ArX), ligand, and [(1,5-COD)Pd-(CH2TMS)2] dissolved in tetrahydrofuran (THF) afforded the desired palladium complexes in excellent yields after 1 h of stirring (Table 1) and subsequent filtration. These easily purified arylation reagents, sSPhosPd(X)Ar, are air-stable and stored under ambient atmosphere and temperature for over 12 months without displaying noticeably diminished reactivity.
Table 1.
Preparation of Air-Stable, Storable Palladium Reagents of Interest in Chemical Biologya and Subsequent S-Arylation of an Unprotected Peptideb
| ||||
|---|---|---|---|---|
|
| ||||
| name | Ar | X | isolated yield of OA (%) | S-arylation (%) |
| OA-20 |
|
OTf | 94 | 99 |
| OA-21 |
|
Br | 99 | 99 |
| OA-22 |
|
Cl | 86 | 99 |
| OA-23 |
|
Br | 82 | 99 |
| OA-24 |
|
Cl | 94 | 99 |
| OA-25 |
|
Br | 99 | 99 |
Reported isolated yields are an average of two runs after purification.
S-Arylation conversions determined by LC-MS analysis of the crude mixture; see SI for details.
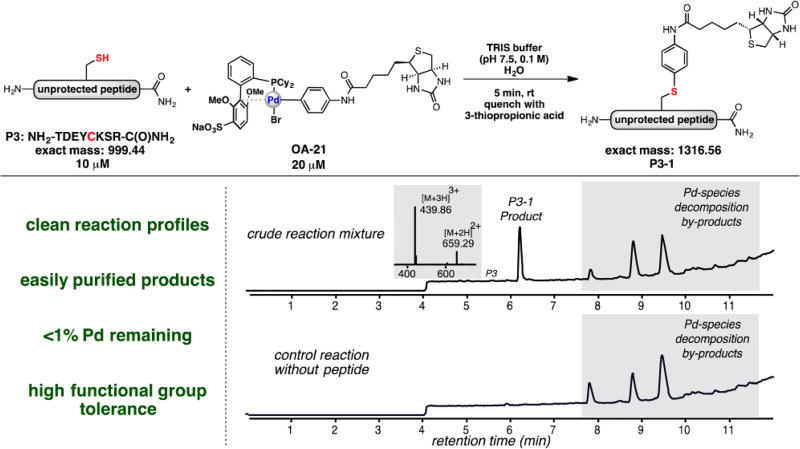
In order to showcase the utility of this process for application in chemical biology, several types of biologically relevant arylation reagents were isolated in high yield using this procedure (Table 1) including examples that contain a fluorescein dye (OA-20), a biotin labeling reagent (OA-21), and an aldehyde or an alkyne as bioconjugation handles for further diversification (OA-22,23). Additionally, a heterocycle-containing reagent (OA-24) and one containing a fluoroaromatic group (OA-25) were prepared in good to excellent yield. Each of these reagents, regardless of complexity, displayed similar reaction profiles with biomolecules containing cysteine (Table 1, Figure 2), highlighting the generality of this palladium-mediated S-arylation approach. Additionally, P3-1 was generated on a larger scale, 8 mg of starting peptide, and subsequent purification by reversed-phase HPLC provided an isolated yield of 81% (see SI for details) indicating that these modified peptides can be obtained in measurable quantities with high efficiency.
Figure 2.
LC-MS spectra depicting the reaction of an unprotected peptide with an sSPhos-ligated palladium biotin complex (OA-21) and the control reaction mixture without addition of peptide. All palladium reagents allowed for rapid transformation of biomolecules exhibiting similar reaction profiles and near-quantitative conversion. a Yield determined by LC-MS analysis of the crude mixture; see SI for details.
Peptide macrocyclization is a burgeoning area in chemical biology and in the development of new therapeutics.10,11 Compared to their linear counterparts, peptide macrocycles have been shown to exhibit enhanced cell permeability,12 increased target binding affinity,13 and resistance to proteolytic degradation.14 As such, several methods have been developed to bridge natural and unnatural amino acid residues along a peptide chain.15 Notably, many of these methods utilize the same cross-linker structures with few examples reported that introduce structural diversity at a late stage.16 Methods that allow a divergent array of cross-linker structures to be used would better equip researchers to investigate structure–activity relationships and manipulate the biological activity of peptide macrocycles. Toward this end, we recently published a report detailing the binding and pharmacokinetic differences that result from cross-linker manipulation utilizing bis-palladium reagents for macrocycle diversification.17 It was found that a solution of 50% acetonitrile was required in order to dissolve the palladium reagents and obtain high conversion efficiencies.
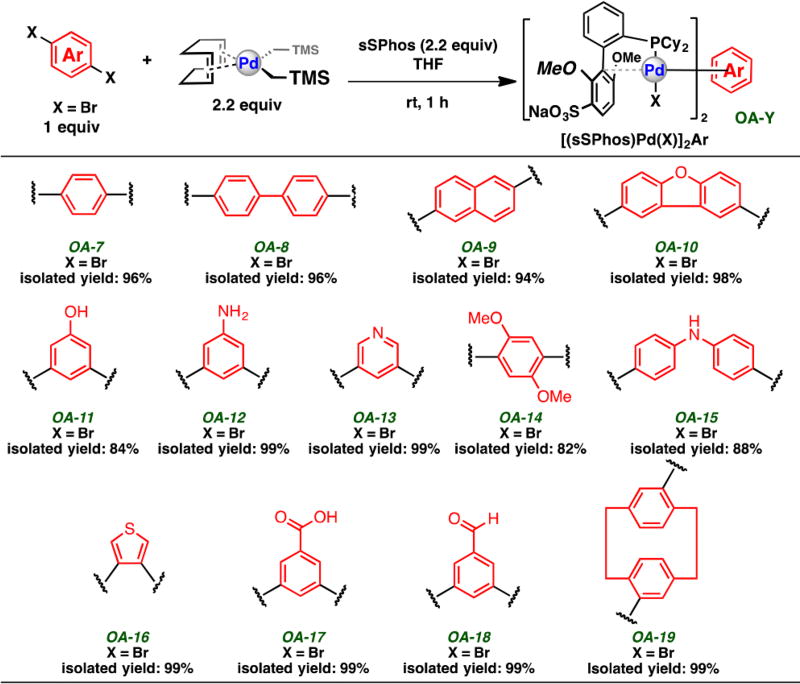
We set out to determine whether our previously described method could be carried out without the aid of an organic cosolvent using sSPhos-derived oxidative addition complexes. Thus, we prepared 13 bis-palladium macrocyclization reagents ([(sSPhos)Pd(X)]2Ar) of varying structure, functional group presence, substitution pattern, and conformation (Scheme 1) from commercially available aryl dihalides. We chose these, in most instances, to be structurally different than the examples we previously published. We note that, regardless of these structural features, each of the bis-palladium macrocyclization reagents could be isolated in good to excellent yield (82–99% yield).
Scheme 1.
Preparation of bis-Palladium Macrocyclization Reagentsa
aReported isolated yields are an average of two runs after purification.
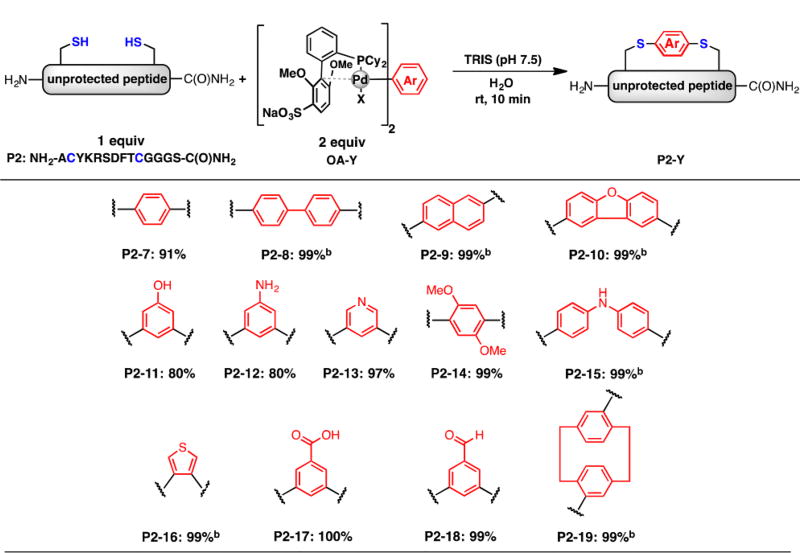
The high functional group tolerance of the cysteine arylation method may provide a general method for the tuning of macrocyclic peptides. To test the efficiency of peptide macrocyclization using sSPhos supported reagents, a model peptide (P2) was prepared with two cysteines separated in an i, i+7 position. After the peptide was dissolved in water and tris(hydroxymethyl)aminomethane (TRIS, pH 7.5), the palladium reagents were introduced in an aqueous solution, and the mixture was gently vortexed for 5 s. The reactions were allowed to proceed for 10 min at room temperature, followed by addition of 3-thiopropionic acid to quench the reaction, and LC-MS analysis indicated that most of the macrocyclization reactions were high yielding under these standard conditions (Scheme 2).
Scheme 2.
Macrocyclization of Peptides Containing Two Unprotected Cysteinesa
aYield determined by LC-MS analysis. bRequired 15% acetonitrile solution to fully dissolve bis-palladium reagents.
In approximately half of the examples, full conversion was not observed under our standard conditions (P2–8, 9, 10, 15, 16, and 19). In these cases, the addition of a small amount of acetonitrile (15% by volume) was found to be beneficial, allowing reactions previously capped at 60% yield to reach full conversion (see SI). We believe this is necessitated by the enhanced hydrophobicity of some of these macrocyclization reagents. The macrocyclic peptide products are readily purified through reversed-phase high performance liquid chromatography. ICP-MS analysis of the peptides showed that more than 99% of palladium was removed during the purification process. Moreover, no side reactions forming peptide oligomers through intermolecular cross-linking were observed (see SI).
In summary, we have developed a general method for the S-arylation of cysteine on biomolecules using bench-stable and water-soluble palladium reagents, prepared without the exclusion of air or ambient moisture. In most instances, no organic cosolvent was necessary. Key to the improvement of this method was the application of the commercially available sulfonated biaryl dialkyl phosphine ligand sSPhos to the synthesis of palladium arylation reagents. Given the importance of developing bioconjugation methods that adhere to physiological reaction conditions, we anticipate that the availability of a water-soluble Pd arylation reagent will facilitate the adoption of this methodology by chemical biologists and medicinal chemists for the synthesis of peptide macrocycles, antibody–drug conjugates, modified proteins, and other biochemical probes.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (GM-46059), MIT start-up funds (B.L.P.), a Damon Runyon Cancer Research Foundation Award (B.L.P.), and the Sontag Foundation Distinguished Scientist Award (B.L.P.). A.J.R. gratefully acknowledges support from the National Science Foundation Graduate Research Fellowship under Grant No. (1122374). The Varian 300 spectrometer used for portions of this work was purchased with funds from the NSF (Grant CHE-9808061). We thank Dr. Yuri Tulchinsky (MIT) and the Center for Environmental Health Sciences for assistance with ICP-MS, Chi Zhang (MIT) and Drs. Mycah Uehling, Yiming Wang, and Nicholas White (MIT) for assistance on the preparation of this manuscript.
Footnotes
ASSOCIATED CONTENT
- Experimental procedures and characterization data (PDF)
The authors declare the following competing financial interest(s): MIT has obtained or has filed patents on some of the ligands that are described in the paper from which S.L.B. and former coworkers receive royalty payments.
References
- 1.Schnolzer M, Kent SBH. Science. 1992;256:221–225. doi: 10.1126/science.1566069. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal P, Bertozzi CR. Bioconjugate Chem. 2015;26:176–192. doi: 10.1021/bc5004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwe K, Brechbiel MW. Cancer Biother.Radiopharm. 2009;24:289–302. doi: 10.1089/cbr.2008.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witus LS, Francis MB. Acc. Chem. Res. 2011;44:774–783. doi: 10.1021/ar2001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephanopoulos N, Francis MB. Nat. Chem. Biol. 2011;7:876–884. doi: 10.1038/nchembio.720. [DOI] [PubMed] [Google Scholar]
- 6.Boutureira O, Bernardes JL. Chem. Rev. 2015;115:2174–2195. doi: 10.1021/cr500399p. [DOI] [PubMed] [Google Scholar]
- 7.Pauly H. Hoppe-Seyler's Z. Physiol. Chem. 1904;42:508–518. [Google Scholar]
- 8.Vinogradova EV, Zhang C, Spokoyny AM, Pentelute BL, Buchwald SL. Nature. 2015;526:687–691. doi: 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KW, Buchwald SL. Angew. Chem., Int. Ed. 2005;44:6173–6177. doi: 10.1002/anie.200502017. [DOI] [PubMed] [Google Scholar]
- 10.White CJ, Yudin AK. Nat. Chem. 2011;3:509–524. doi: 10.1038/nchem.1062. [DOI] [PubMed] [Google Scholar]
- 11.Mallinson J, Collins I. Future Med. Chem. 2012;4:1409–1438. doi: 10.4155/fmc.12.93. [DOI] [PubMed] [Google Scholar]
- 12.Hewitt WM, Leung SSF, Pye CR, Ponkey AR, Bednarek M, Jacobson MP, Lokey RS. J. Am. Chem. Soc. 2015;137:715–721. doi: 10.1021/ja508766b. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Graves B, Guerlavais V, Tovar C, Packman K, To K-H, Olson KA, Kesavan K, Gangurde P, Mukherjee A, Baker T, Darlak K, Elkin C, Filipovic Z, Qureshi FZ, Cai H, Berry P, Feyfant E, Shi XE, Horstick J, Annis DA, Manning AM, Fotouhi N, Nash H, Vassilev LT, Sawyer TK. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E3445–54. doi: 10.1073/pnas.1303002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driggers EM, Hale SP, Lee J, Terrett NK. Nat. Rev. Drug Discovery. 2008;7:608–624. doi: 10.1038/nrd2590. [DOI] [PubMed] [Google Scholar]
- 15.Lau YH, de Andrade P, Wu Y, Spring DR. Chem. Soc. Rev. 2015;44:91–102. doi: 10.1039/c4cs00246f. [DOI] [PubMed] [Google Scholar]
- 16.Lau YH, de Andrade P, Quah S-T, Rossmann M, Laraia L, Sköld N, Sum TJ, Rowling PJE, Joseph TL, Verma C, Hyvönen M, Itzhaki LS, Venkitaraman AR, Brown CJ, Lane DP, Spring DR. Chem. Sci. 2014;5:1804–1809. [Google Scholar]
- 17.Rojas AJ, Zhang C, Vinogradova EV, Buchwald NH, Reilly J, Pentelute BL, Buchwald SL. Chem. Sci. 2017;8:4257–4263. doi: 10.1039/c6sc05454d. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.