Abstract
Objective
In this study, we performed a candidate genetic risk score (GRS) analysis of early-onset bipolar disorder.
Method
Treatment of Early Age Mania (TEAM) study enrollment and sample collection took place from 2003–2008. Mayo Clinic Bipolar Biobank samples were collected from 2009–2013. Genotyping and analyses for the present study took place from 2013–2014. The diagnosis of bipolar disorder was based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision criteria. Eight single-nucleotide polymorphisms (SNPs), previously reported in genome-wide association studies to be associated with bipolar disorder, were chosen for GRS analysis in early-onset bipolar disease. These SNPs map to 3 genes: CACNA1C (calcium channel, voltage-dependent, L type, alpha 1C subunit), ANK3 (ankyrin-3, node of Ranvier [ankyrin G]), and ODZ4 (teneurin transmembrane protein 4 [formerly “odz, odd Oz/ten-m homolog 4 {Drosophila}, ODZ4”]). The 8 candidate SNPs were genotyped in patients from the TEAM study (n=69), adult patients with bipolar disorder (n=732) including a subset with early-onset illness [n=192]), and healthy controls (n=776). GRS analyses were performed comparing early-onset cases with controls. In addition, associations of early-onset BD with individual SNPs and haplotypes were explored.
Results
GRS analysis revealed associations of the risk score with early-onset bipolar disorder (P=.01). Gene-level haplotype analysis comparing TEAM patients with controls suggested association of early-onset bipolar disorder with a CACNA1C haplotype (global test, P=.01). At the level of individual SNPs, comparison of TEAM cases with healthy controls provided nominally significant evidence for association of SNP rs10848632 in CACNA1C with early-onset bipolar disorder (P=.017), which did not remain significant after correction for multiple comparisons.
Conclusion
These preliminary analyses suggest that previously identified bipolar disorder risk loci, especially CACNA1C, have a role in early-onset bipolar disorder, possibly with stronger effects than for late-onset bipolar disorder.
Keywords: ANK3, CACNA1C, early onset bipolar disorder, genetics, ODZ4
Introduction
Early-onset bipolar disorder (BD) is a devastating illness that confers significant morbidity and demonstrates continuity with adult mood disorders (1–4). Lifetime prevalence rates of bipolar disorder in children are as high as 2.1% (5). Early-onset BD may be associated with greater long-term morbidity and increased familial risk (6–9). However, the molecular underpinnings of this risk are poorly understood (10). Prior genome-wide association studies (GWAS) of adults have implicated several genes in BD, including calcium channel, voltage-dependent, L type, alpha 1C subunit (CACNA1C) (11–13), ankyrin-3, node of Ranvier (ankyrin G) (ANK3) (12,14), and teneurin transmembrane protein 4 (formerly “odz, odd Oz/ten-m homolog 4 [Drosophila], ODZ4”) (ODZ4) (15,16). However, it is not known whether single-nucleotide polymorphisms (SNPs) in these genes have stronger associations with early-onset BD than with adult-onset BD.
Early-onset BD may represent a distinct phenotype with greater severity and genetic loading. Hence, samples of early-onset cases (EOC) present an invaluable opportunity for study of this heterogenous disease. Prior work supports an increased familial risk for BD in relatives of probands with early-onset BD (5), and neuroimaging studies suggest that children and adolescents with early-onset BD may have unique neurobiologic features (17,18). Experts have also argued that this population has a differential response to pharmacologic agents, which bolsters the contention of a discrete phenotype (1).
Herein, we report on a study that utilized samples from the Treatment of Early Age Mania (TEAM) study (19), from adults with BD, and from healthy control adults to perform a candidate gene study of early-onset BD. The primary aim was to perform a genetic risk score (GRS) analysis of early-onset BD using SNPs previously implicated as risk factors for BD. Eight SNPs in 3 genes were selected: 4 in CACNA1C (rs1006737 [12], rs1024582 [12], rs4765913 [15], and rs10848632 [20]); 3 in ANK3 (rs1938526 [12], rs9804190 [15], and rs10994336 [12]); and 1 in ODZ4 (rs12576775 [15]). We hypothesized that these 8 SNPs would be associated with a risk for BD and that the associations might be greater for patients with early-onset BD.
Methods
Patients
The study protocol and procedures were approved by the Mayo Clinic Institutional Review Board. Research clinicians obtained informed consent from adult participants, assent from child participants, and informed consent from primary caretakers. The protocol and procedures related to the execution of the TEAM study (19) and sample collections were approved through each local institutional review board (Washington University School of Medicine, St. Louis, Missouri; Children’s National Medical Center, Washington, DC; The University of Texas Medical Branch, Galveston, Texas; and the Johns Hopkins Medical Institutions, Baltimore, Maryland).
The present study included samples from patients with early-onset BD from the TEAM study (n=82), samples from adult BD patients (n=855), and samples from healthy controls (n=857) (21). TEAM study enrollment and sample collection took place from 2003–2008. Mayo Clinic Bipolar Biobank samples were collected from 2009–2013. Genotyping and analyses for the present study took place from 2013–2014. After quality control and removal of non-Caucasian samples (to avoid confounding by population structure), we analyzed a total of 69 TEAM samples, 732 samples from adults with BD (192 with early-onset BD, 256 with late-onset BD, and 284 with an undetermined age of onset), and 776 control samples. Demographics and comorbidities of the cohorts are described in Table 1.
Table 1.
Demographic Characteristics
| Characteristic | TEAM (n=69) | Mayo, All Cases (n=732) | Mayo, Early-Onset Cases (n=192) | Mayo, Late-Onset Cases (n=256) | Controls (n=776) |
|---|---|---|---|---|---|
| Age at enrollment, mean (SD), y | 10.5 (2.9) | 42.0 (15.1) | 35.9 (12.8) | 47.3 (15.2) | 52.5 (21.9) |
| Body mass index at enrollment, mean (SD), kg/m2 | 19.4 (4.2) | 30.1 (7.0) | 30.2 (7.3) | 29.6 (6.7) | 26.0 (8.5) |
| Sex, male, No. (%) | 30 (43.5) | 303 (41.4) | 65 (33.9) | 122 (47.7) | 318 (41.0) |
| Race, Caucasian, No. (%) | 69 (100) | 732 (100) | 192 (100) | 256 (100) | 776 (100) |
| Age of onset, mean (SD) | 4.9 (2.5) | 24.8 (13.9) | 13.7 (3.7) | 33.2 (12.8) | ... |
| Attention-deficit/hyperactivity disorder, No. (%) | 67 (97.1) | 183/691 (26.5) | 55/184 (29.9) | 54/244 (22.1) | ... |
| Psychosis, No. (%) | 57 (82.6) | 323/716 (45.1) | 109/187 (58.3) | 140/254 (55.1) | ... |
| Mixed episodes, No. (%) | 66 (95.7) | 92/575 (16.0) | 27/186 (14.5) | 38/244 (15.6) | ... |
| Separation anxiety disorder, No. (%) | 25 (36.2) | ... | ... | ... | ... |
| Panic disorder, No. (%) | 0 (0) | 224/700 (32.0) | 74/185 (40.0) | 66/249 (26.5) | ... |
| Obsessive-compulsive disorder, No. (%) | 14 (20.3) | 109/703 (15.5) | 38/184 (20.7) | 34/246 (13.8) | ... |
| Social phobia, No. (%) | 20 (29.0) | ... | ... | ... | ... |
| Social anxiety disorder, No. (%) | … | 179/696 (25.7) | 50/182 (27.5) | 68/247 (27.5) | ... |
| Generalized anxiety disorder, No. (%) | 23 (33.3) | 353/706 (50.0) | 87/183 (47.5) | 124/249 (49.8) | ... |
| Any substance dependence or abuse, No. (%) | 1 (1.4) | 411/722 (56.9) | 112/189 (59.3) | 138/255 (54.1) | ... |
Abbreviation: TEAM, Treatment of Early Age Mania study.
TEAM Cohort
The ascertainment and assessment procedures for the TEAM study are described elsewhere (19). Briefly, TEAM participants were child and adolescent outpatients aged 6 to 15 years with a diagnosis of BD and currently in a manic or mixed episode for a minimum of 4 weeks preceding enrollment. Diagnosis of BD was based on a semistructured interview using the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (22) to establish BD criteria as described in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (23). A diagnosis of mania required a threshold number of symptoms of at least moderate severity for a concurrent period. All participants had a Children’s Global Assessment Scale score of 60 or less, good health, and an intelligence quotient of 70 or greater. Those with pervasive developmental disorders, schizophrenia, and major medical comorbidities were excluded (19).
Adult Bipolar Disorder Cohorts
Adult samples were from 855 patients with a confirmed diagnosis of BD based on criteria from DSM-IV-TR (23). The diagnosis was confirmed by a senior psychiatrist upon review of a Structured Clinical Interview for DSM-IV-TR (SCID) (24). Data were also collected using a patient questionnaire, a clinical questionnaire, and, if available, the electronic health record (EHR). The ages of first manic and first depressive episodes were determined on the basis of a review of the SCID, a clinical questionnaire, and the EHR by a research coordinator. After this review, a subset of adult patients (n=192) was classified as having early-onset BD. Patients were classified as having early-onset BD if their first manic or depressive episode occurred at age 19 years or younger (3,25,26). This early-onset characterization was independently confirmed for each patient by 2 child and adolescent psychiatrists (P.E.C. and M.V.), who also reviewed the SCID, the clinical questionnaire, and the EHR. The overall demographics of the adult bipolar biobank study, from which samples in the present study were obtained, have been reported previously (27).
Adult Controls
The control group comprised 857 participants from a biobank (21). Patients were excluded from the control group if they reported having a first-degree relative with BD or a prior diagnosis of a psychiatric condition such as BD, schizophrenia, major depression, Down syndrome, autism, or attention-deficit/hyperactivity disorder. Eligible control patients were matched to adult cases by age, sex, and race/ethnicity.
Genotyping and Quality Control
Because of the small number of available early-onset cases from the TEAM study, this study focused on candidate loci previously reported to be associated with bipolar disorder. On the basis of the results of prior GWAS, we selected 8 candidate SNPs for study: 4 in CACNAIC (rs1006737 [12], rs1024582 [12], rs4765913 [15], and rs10848632 [20]); 3 in ANK3 (rs1938526 [12], rs9804190 [15], and rs10994336 [12]); and 1 in ODZ4 (rs12576775 [15]). TEAM samples were genotyped with the TaqMan genotyping assay (Life Technologies) at Washington University. Adult samples were genotyped at Mayo Clinic genotyping core facility using the Illumina GoldenGate Genotyping Assay (Illumina Inc) as part of a larger candidate gene study (28). For quality control, 10 Mayo Clinic samples were also genotyped on the Illumina platform, obtaining 100% concordant genotype calls between Mayo Clinic and Washington University. To reduce the likelihood of confounding by population structure, we restricted analyses to patients of self-reported Caucasian ancestry. SNP genotype distributions were tested for departure from Hardy-Weinberg equilibrium. No departures from Hardy-Weinberg equilibrium were detected in the full set of Caucasian subjects (all P>.05). Although 1 SNP (rs1938526) in the subset of controls showed marginal evidence for departure from Hardy-Weinberg equilibrium (P=.047), this evidence of disequilibrium is not significant, given the number of tests performed. After quality control, 69 TEAM early-onset BD cases, 732 adult BD cases (192 with early-onset BD), and 776 controls—all of European ancestry—were included in the analyses.
Statistical Analysis
To determine whether previously identified BD risk associated SNPs have a role in early-onset illness that might be greater than their effect on general BD risk, we performed global tests of association of the genotyped SNPs with BD and early-onset BD using GRS analysis. For the GRS analyses, we excluded the CACNA1C SNP rs1024582 from the risk scores because rs1006737 and rs1024582 are in very high linkage disequilibrium (r2=.9; D′=1), and thus, including both SNPs would weigh the effect of these SNPs too heavily by double-counting the risk from that locus. We used 2 methods to calculate a GRS: 1) a simple count GRS (SC-GRS) obtained by summing the total number of risk alleles in the 7 remaining SNPs and 2) an odds ratio–weighted GRS (OR-GRS), with risk alleles weighted by their odds ratios (ORs) from prior studies (29). Thus, for each patient, the SC-GRS and OR-GRS were calculated as:
where Gi = genotype at SNP i coded as the number of risk alleles and ORi is the risk allele OR for SNP i. Although the SNPs were selected on the basis of several prior GWAS (12,15,16,20), for consistency, the risk alleles and ORs used in the GRS calculations were based on the large analysis performed by the Psychiatric GWAS Consortium (15). Logistic regression models were used to test for association of the risk scores with BD or early-onset BD. Results of the GRS analysis were considered statistically significant if P<.05 because all SNPs were included in a single analysis (ie, the risk score calculated from all SNPs formed a single predictor variable that was tested in the analysis).
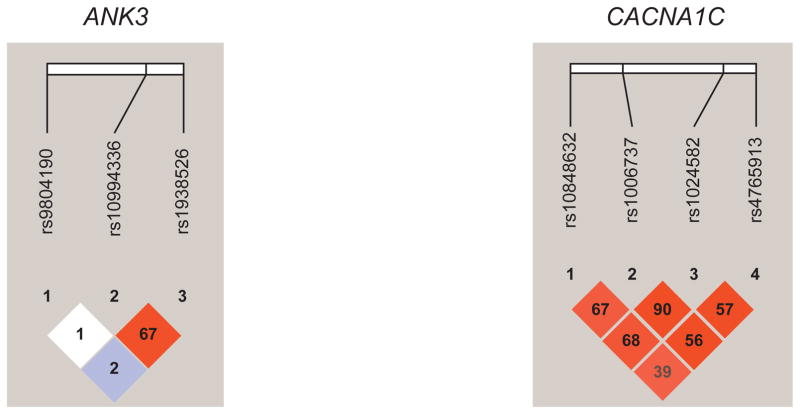
To determine whether particular SNPs may be driving the results of the GRS analysis, genetic data were also analyzed at the individual SNP and haplotype levels. We first used the adult patient (n=732) and control samples (n=776) to determine whether each SNP was associated with BD. We then tested whether the SNPs were associated with early-onset BD by comparing the TEAM early-onset samples (n=69) with the controls. We subsequently divided the adult BD sample into EOC and late-onset cases, and we performed analyses comparing the EOC (n=192) with controls and all available EOC (adult plus TEAM cases, n=261) with controls (n=776). Analyses were based on logistic regression models with SNP genotypes coded as 0, 1, or 2 copies of the minor allele. Haplotype analyses used all genotyped SNPs in each gene (for CACNA1C and ANK3 genes) and were performed using the score statistic implemented in the R package Haplo.stats (Mayo Foundation for Medical Education and Research) (30), comparing the same groups as in the individual SNP analysis. Linkage disequilibrium plots for ANK3 and CACNA1C were generated in Haploview (Broad Institute) (31) using 1,000-genome Caucasian ancestry data (32) (Figure).
Figure.
Linkage Disequilibrium Plots for ANK3 and CACNA1C Single-Nucleotide Polymorphisms. Colors are based on D′, whereas numbers represent r2 measures of linkage disequilibrium.
All data cleaning and statistical analyses were performed using R statistical computing software (The R Foundation for Statistical Computing) or SAS version 9.2 (SAS Institute Inc).
Results
The results of risk score analyses are presented in Table 2. Analysis of adult cases compared with controls provided no evidence of association of the SC-GRS or the OR-GRS with BD (SC-GRS, P=.71; OR-GRS, P=.56). Further, comparison of the adult late-onset group with controls did not demonstrate significant associations of risk scores and BD (SC-GRS, P=.96; OR-GRS, P=.85). The associations with GRS were also not significant in comparisons of TEAM EOC vs controls, although the OR point estimates for risk score analysis of TEAM EOC compared with controls were larger than in the adult case–control comparison. Analyses comparing the adult EOC with controls did not show significant differences with a simple count approach, but when using an OR-weighted approach, significant evidence of association of risk scores with early-onset BD was observed (SC-GRS, P=.06; OR-GRS, P=.02). Furthermore, analysis of the combined group of EOC (TEAM EOC plus adult EOC) vs controls also demonstrated association of risk scores with early-onset BD (SC-GRS, P=.02; OR-GRS, P=.01). The OR in the SC-GRS analysis (OR, 1.08) provides an estimate of the mean impact on the risk for early-onset BD of a risk allele at the 7 SNPs included in the risk score. Results were similar when the combined EOC group was compared with the late-onset cases, demonstrating that the loci investigated appear to primarily contribute to risk of early-onset, rather than late-onset, BD.
Table 2.
Genetic Risk Score Analysis of Patients With Early-onset Bipolar Disorder Compared With Controls
| Groups Compared | Risk Score | Mean Risk Score
|
OR | P Value | |
|---|---|---|---|---|---|
| Cases | Controls | ||||
| Adult cases vs controls | SC-GRS | 4.10 | 4.06 | 1.01 | .71 |
| OR-GRS | 0.27 | 0.26 | 1.10 | .56 | |
| TEAM vs controls | SC-GRS | 4.48 | 4.06 | 1.10 | .11 |
| OR-GRS | 0.30 | 0.26 | 1.58 | .24 | |
| Adult EOC vs controls | SC-GRS | 4.38 | 4.06 | 1.08 | .06 |
| OR-GRS | 0.32 | 0.26 | 1.77 | .02 | |
| TEAM+adult EOC vs controls | SC-GRS | 4.41 | 4.06 | 1.08 | .02 |
| OR-GRS | 0.31 | 0.26 | 1.74 | .01 | |
| TEAM+adult EOC vs adult LOC | SC-GRS | 4.41 | 4.05 | 1.09 | .047 |
| OR-GRS | 0.31 | 0.26 | 1.74 | .06 | |
| Adult LOC vs controls | SC-GRS | 4.05 | 4.06 | 0.99 | .96 |
| OR-GRS | 0.26 | 0.26 | 1.05 | .85 | |
Abbreviations: EOC, early-onset cases; LOC, late-onset cases; OR, odds ratio; OR-GRS, odds ratio–weighted genetic risk score; SC-GRS, simple count genetic risk score; TEAM, Treatment of Early Age Mania.
Haplotype analyses demonstrated no significant associations between ANK3 and CACNA1C haplotypes when adult BD cases were compared with healthy controls. However, the comparison of TEAM EOC with controls revealed significant associations of early-onset BD with CACNA1C haplotypes composed of the SNPs rs10848632, rs1006737, rs1024582, and rs4765913 (global test, P=.01 in analysis of haplotypes with frequencies ≥5%) (Table 3); the result of the CACNA1C global haplotype test is marginally significant after correction for the number of genes (3) that were examined at the gene level. The CACNA1C haplotype analysis results in Table 3 show that the TGGT haplotype was associated with an increased risk for early-onset BD (maximum statistic simulation, P=.009) in the comparison of TEAM cases vs controls. A similar CACNA1C haplotype analysis found no significant difference between adult EOC and controls or between the TEAM EOC and the adult EOC compared with controls (global statistic, P>.10) (Table 3). ANK3 haplotypes were not significantly associated with early-onset BD.
Table 3. CACNA1C.
Haplotype Analysis for TEAM and Adult Early-onset Cases of Bipolar Disorder Compared With Controls
| Analysis | Haplotypea | Case Freq | Control Freq | P Value | Max-stat Sim P Value | Global-stat P Value |
|---|---|---|---|---|---|---|
| TEAM vs controls | CGGT | 0.48 | 0.59 | .01 | .01 | |
| TAAT | 0.14 | 0.13 | .63 | |||
| TAAA | 0.22 | 0.20 | .46 | |||
| TGGT | 0.11 | 0.05 | .002 | .009 | ||
| Adult EOC vs controls | CGGT | 0.56 | 0.59 | .33 | .76 | .79 |
| TGGT | 0.05 | 0.05 | .66 | |||
| TAAT | 0.13 | 0.13 | .63 | |||
| TAAA | 0.22 | 0.20 | .37 | |||
| TEAM+adult EOC vs controls | CGGT | 0.54 | 0.59 | .048 | .15 | .21 |
| TAAT | 0.14 | 0.13 | .54 | |||
| TAAA | 0.22 | 0.20 | .28 | |||
| TGGT | 0.07 | 0.05 | .07 |
Abbreviations: EOC, early-onset cases; Freq, frequency; Global-stat, global statistic; Max-stat Sim, maximum statistic simulation; TEAM, Treatment of Early Age Mania.
Haplotype consists of rs10848632, rs1006737, rs1024582, and rs4765913.
No statistically significant associations with the 8 candidate SNPs were observed when adult cases were compared with controls (Table 4). The comparison of TEAM EOC with controls (Table 5) showed nominally significant evidence of association of the minor allele at rs10848632 in CACNA1C with early-onset BD (OR, 1.54; P=.017), which is not significant after correction for multiple comparisons. Comparison of adult EOC with controls provided nominally significant evidence of association of rs10994336 in ANK3 with early-onset BD (OR, 1.49; P=.049) (Table 5), which was also not significant after correction for multiple testing.
Table 4.
Association of Single-Nucleotide Polymorphisms With Bipolar Disorder Based on an Analysis of Adult Cases Compared With Controls
| SNP | Gene | MAF Cases | MAF Controls | OR | P Value |
|---|---|---|---|---|---|
| rs10848632 | CACNA1C | 0.39 | 0.39 | 1.02 | .77 |
| rs1006737 | CACNA1C | 0.34 | 0.35 | 0.99 | .92 |
| rs1024582 | CACNA1C | 0.35 | 0.35 | 0.99 | .94 |
| rs4765913 | CACNA1C | 0.22 | 0.23 | 0.98 | .79 |
| rs1938526 | ANK3 | 0.07 | 0.07 | 1.04 | .76 |
| rs9804190 | ANK3 | 0.22 | 0.23 | 0.98 | .77 |
| rs10994336 | ANK3 | 0.07 | 0.06 | 1.10 | .53 |
| rs12576775 | ODZ4 | 0.17 | 0.16 | 1.07 | .48 |
Abbreviations: MAF, minor allele frequency; OR, odds ratio; SNP, single-nucleotide polymorphism.
Table 5.
Association of Candidate Single-Nucleotide Polymorphisms With Early-onset Bipolar Disorder
| TEAM Cases vs Controls
|
Adult EOC vs Controls s
|
TEAM+Adult EOC vs Controls s
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Gene | Control MAF | MAF TEAM | OR | P Value | MAF Mayo EOC | OR | P Value | OR | P Value |
| rs10848632 | CACNA1C | 0.39 | 0.49 | 1.54 | .017 | 0.42 | 1.12 | .32 | 1.22 | .056 |
| rs1006737 | CACNA1C | 0.35 | 0.39 | 1.20 | .34 | 0.36 | 1.08 | .52 | 1.11 | .33 |
| rs1024582 | CACNA1C | 0.35 | 0.40 | 1.23 | .26 | 0.38 | 1.12 | .34 | 1.15 | .19 |
| rs4765913 | CACNA1C | 0.23 | 0.27 | 1.28 | .24 | 0.24 | 1.08 | .59 | 1.13 | .32 |
| rs1938526 | ANK3 | 0.07 | 0.07 | 1.02 | .95 | 0.10 | 1.37 | .10 | 1.28 | .16 |
| rs9804190 | ANK3 | 0.23 | 0.24 | 1.07 | .76 | 0.20 | 0.85 | .26 | 0.91 | .43 |
| rs10994336 | ANK3 | 0.06 | 0.07 | 1.06 | .88 | 0.09 | 1.49 | .049 | 1.37 | .086 |
| rs12576775 | ODZ4 | 0.16 | 0.20 | 1.25 | .34 | 0.18 | 1.14 | .38 | 1.17 | .24 |
Abbreviations: EOC, early-onset cases; MAF, minor allele frequency; OR, odds ratio; SNP, single-nucleotide polymorphism; TEAM, Treatment of Early Age Mania.
Discussion
This study of a relatively small sample of early-onset BD cases is, to our knowledge, the first investigation of CACNA1C, ANK3, and ODZ4 genetic variations in early-onset BD. On the basis of prior GWAS studies, we selected 8 SNPs, including rs10848632, rs1006737, rs1024582, and rs4765913 in CACNA1C; rs1938526, rs9804190, and rs10994336 in ANK3; and rs12576775 in ODZ4 (12,15,16). Our GRS analyses suggested an association between a risk score composed of the genotyped candidate SNPs and early-onset BD, but not with BD in general. Furthermore, our results suggest a CACNA1C haplotype may be associated with early-onset BD. Contrary to our expectations, the individual SNPs of interest were not significantly associated with risk of adult-onset BD (with the index episode of depression or mania occurring after age 19 years).
The haplotype analysis of TEAM EOCs vs controls provided marginally significant evidence of association of early-onset BD with CACNA1C genetic variation. However, the T-G-G-T haplotype that was associated with increased risk of early-onset BD had a relatively low frequency, and thus the results should be interpreted cautiously, given the small sample size. Nevertheless, the preliminary results, which suggest that CACNA1C may have a more prominent role in early-onset BD, are interesting and warrant further investigation. Genes that regulate calcium channel functioning, such as CACNA1C, may have widespread effects on the development and manifestations of psychiatric illness (33–36). Analyses of genome-wide SNP data, from autism spectrum disorder, attention-deficit/hyperactivity disorder, BD, unipolar depression, and schizophrenia, have implicated risk loci in the CACNA1C and CACNB2 L-type voltage-gated calcium channel subunits across a range of psychiatric illnesses. Hence, genotypic variations in calcium channel genes may have broad implications for psychiatric phenotypes. These findings also underscore the potential limitations of traditional, descriptive approaches to psychiatric diagnosis (37). The National Institute of Mental Health Research Domain Criteria (RDoC) program is shifting how research hypotheses are structured (38). For example, recent studies of biomarker panels have characterized neurobiologically distinct categories among patients with bipolar disorder, psychosis, and schizoaffective disorder that are not congruent with clinical diagnostic categories (39). Further research using the RDoC framework could inform studies of children and adolescents with bipolar disorder.
The pleomorphic nature of BD and challenges in its phenotypic classification have likely hindered the progress of genetic risk studies of this lifelong disease, which produces considerable individual and societal burden (38). Clinical characteristics (eg, psychotic symptoms, panic attacks, and alcohol misuse) have shown promise as refined phenotypic characterizations with familial aggregation in BD (40,41). Early-onset BD, in particular, is an important subgroup for study, because this characteristic confers greater familial risk and likely presents a more homogenous population to study (42,43). The diagnostic stability and adult continuity of childhood bipolar disorder is poorly understood and understudied (44). A recent systematic review describing the dearth of positive findings in genetic research of early-onset bipolar disorder concluded that the genetic heterogeneity of childhood bipolar disorder is a substantial barrier (45). Emotional dysregulation and irritability in childhood are important targets of contemporary research efforts (46). The SNPs of interest in the present study may have broader relationships with dimensional symptoms, as opposed to a DSM-IV-TR (23) diagnosis of bipolar disorder.
It is widely recognized that genetic risk for complex psychiatric diseases such as BD is conferred by many alleles both within and across genes. For example, an 8-locus CACNA1C haplotype has been shown to be associated with risk for BD (47). Polygenic risk scores have been successfully utilized in recent studies, for instance to show genetic overlap between BD and the clinical dimensions of mania in schizophrenia (48) and to identify new associations in schizophrenia (49). Although the small number of SNPs examined in the present study is not suitable for developing GRS for the purpose of clinical risk prediction, we applied the GRS methodology to facilitate investigation of the overall impact of well-established BD loci in the risk of a subphenotype (early-onset illness). Our results support the notion that polygenic methodologies may be important for future work examining genetic risk for early-onset BD.
Strengths of our study include its focus on a narrow and decidedly heritable phenotype in well-characterized samples. Patients in the TEAM EOC with DSM-IV-TR BD I were rigorously characterized by child and adolescent psychiatrists with a high level of expertise in early-age mania (19). Patients in the adult EOC were also rigorously characterized on the basis of a review of SCID and EHR data, with independent confirmation by 2 child and adolescent psychiatrists. These factors are critical, given the heterogeneity of BD and of early-onset mood disorders in general.
However, it is important to note the limitations of this exploratory work. First, the sample size was small and the TEAM sample was particularly small, limiting the power of the study. Although no statistically significant associations were observed for individual SNPs (after correction for multiple testing), the OR estimates, particularly in the comparisons of early-onset BD cases with controls, indicated the same direction of effect as in the original reports of association with BD (12,15). Thus, low study power likely had a role in the failure to identify significant associations at the SNP level. Second, the analyses were restricted to patients of European ancestry, which limits the generalizability of our results; however, this approach has the important advantage of reducing the likelihood of confounding by population structure. Reliance on self-reported race/ethnicity is also a limitation, in that we cannot exclude the possibility of residual population stratification. Because genome-wide SNP data were not available for these samples, more robust methods such as principal components could not be applied to control for population stratification. Nevertheless, prior research has shown that self-reported ancestry is usually a reliable approach for accounting for population structure in candidate gene studies (50). Third, in most cases, the classification of adult samples as early-onset vs late-onset BD was based on a retrospective review of records and patient report, which may have been biased or inexact. Also, it was not possible to characterize numerous, potentially important factors such as co-occurring disorders, treatment history, and functional impairment. Finally, this study was limited to a small number of SNPs. It was not meant to fully characterize the genetics of early-onset BD; rather, we aimed to explore whether previously identified risk loci might have a more prominent role in early-onset illness. Other important limitations include the potential differences in comorbidity among cohorts. We were unable to fully characterize and control for comorbidities in the present study because of differences between the case groups and data collection methods. This may explain the lack of specificity among some of our findings. Finally, as we discussed above, the poor longitudinal diagnostic stability of bipolar disorder in childhood presents inherent limitations in the interpretation of our findings.
In conclusion, this study used GRS analysis to demonstrate that the selected candidate loci contribute primarily to the risk of early-onset BD rather than to the risk of late-onset BD. Our results also suggested an association of early-onset BD with a CACNA1C haplotype. Future studies with larger, ethnically diverse samples and more detailed phenotypic characterizations will refine the understanding of genetic risk profiles in early-onset BD.
Clinical Points.
Early-onset bipolar disorder may represent a distinct phenotype with greater severity and genetic loading.
Despite considerable prior work, the genetics of early-onset bipolar disorder are poorly understood.
A candidate genetic risk score with 8 single-nucleotide polymorphisms showed an association with early-onset bipolar disorder.
Acknowledgments
Funding: This study was funded by the Marriott Foundation and Mayo Clinic Center for Individualized Medicine. The original TEAM study described in this manuscript received funding from NIMH cooperative grants U01 MH064846, U01 MH064850, U01 MH064851, U01 MH064868, U01 MH064869, U01 MH064887, U01 MH064911, and R01 MH051481. The funding organizations (Marriott Foundation, Mayo Clinic Center for Individualized Medicine, and NIMH) had no role in the study design, data collection, analysis, interpretation of results, writing of the report, and the submission of the manuscript.
The authors gratefully acknowledge the pivotal contributions of Barbara Geller, MD, Professor Emerita of Child Psychiatry, Department of Psychiatry, Washington University School of Medicine, St Louis, Missouri, to designing and overseeing this project from its inception through the main publication.
The authors wish to acknowledge the essential contributions of the TEAM investigators for the design, oversight, and execution of the original TEAM study. Dr Croarkin is supported by NIMH under Award Number K23MH100266.
Abbreviations
- ANK3
ankyrin-3, node of Ranvier (ankyrin G)
- BD
bipolar disorder
- CACNA1C
calcium channel, voltage-dependent, L type, alpha 1C subunit
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision
- EHR
electronic health record
- EOC
early-onset cases
- GRS
genetic risk score
- GWAS
genome-wide association studies
- ODZ4
teneurin transmembrane protein 4 (formerly “odz, odd Oz/ten-m homolog 4 [Drosophila], ODZ4”)
- OR
odds ratio
- OR-GRS
odds ratio–weighted genetic risk score
- RDoC
National Institute of Mental Health Research Domain Criteria
- SC-GRS
simple count genetic risk score
- SCID
Structured Clinical Interview for DSM-IV-TR
- SNPs
single-nucleotide polymorphisms
- TEAM
Treatment of Early Age Mania
Footnotes
Disclaimers
The content of this report is solely the responsibility of the authors and does not necessarily represent the official views of the US Department of Health and Human Services, the National Institutes of Health, or the NIMH.
Conflict of Interest and Financial Disclosures
Dr Croarkin has received grant support from Pfizer Inc, the National Institute of Mental Health (NIMH) (K23 MH100266), the Brain and Behavior Research Foundation, and the Mayo Foundation. He has received in-kind support for equipment and supplies from Neuronetics Inc and AssureRx Health Inc.
Dr Luby has received grant or research support from NIMH, the Communities Healing Adolescent Depression and Suicide (CHADS) Coalition, and the Sydney R. Baer Jr Foundation. Dr Luby has received royalties from Guilford Press.
Dr Simonson is currently employed by Sqrrl Data Inc.
Dr Wagner has received honoraria from UBM Medica, American Psychiatric Association, Las Vegas Psychiatric Society, American Academy of Child and Adolescent Psychiatry, Partners Healthcare, Doctors Hospital at Renaissance, Oxford University Press, University of California San Diego, Baylor College of Medicine, Weill Cornell Medical School, and Nevada Psychiatric Association.
Dr Walkup has received free medication/placebo from the following pharmaceutical companies for NIMH-funded studies: Eli Lilly and Co (2003), Abbott (2005), and Pfizer Inc (2007). Dr Walkup also was paid for a one-time consultation with Shire PLC (2011). Dr Walkup is a paid speaker for the Tourette Syndrome National Education and Outreach Program of the National Center on Birth Defects and Developmental Disabilities at the US Centers for Disease Control and Prevention. Dr Walkup also receives grant funding from The Hartwell Foundation and the Tourette Syndrome Association. Dr Walkup receives royalties for books on Tourette syndrome from Guilford Press and Oxford University Press, the American Academy of Child and Adolescent Psychiatry, and the American Psychiatric Association. He is an unpaid adviser to the Anxiety and Depression Association of America, Consumer Reports, and the Trichotillomania Learning Center.
Dr Casuto is employed by the University of Cincinnati College of Medicine and the University of Cincinnati Health Physicians/UC Health at the Lindner Center of HOPE. Dr Casuto is presently, or has been in the past year, a coinvestigator on research studies sponsored by Cephalon Inc, Forest Laboratories Inc, the Marriott Foundation, Naurex Inc, Shire PLC, and Takeda Pharmaceutical Co Ltd.
Dr McElroy is employed by the University of Cincinnati College of Medicine, University of Cincinnati Health Physicians/UC Health, and the Lindner Center of HOPE. She is a consultant to, or a member of the scientific advisory boards of, in the past year: Bracket, F. Hoffmann-La Roche Ltd, MedAvante Inc, Naurex Inc, Novo Nordisk A/S, Shire PLC, and Sunovion Pharmaceuticals Inc. Dr McElroy is presently, or has been in the past year, a principal investigator or a coinvestigator on research studies sponsored by the Agency for Healthcare Research and Quality in the US Department of Health and Human Services, Alkermes PLC, Cephalon Inc, Forest Laboratories Inc, the Marriott Foundation, NIMH, Naurex Inc, Orexigen Therapeutics Inc, Shire PLC, and Takeda Pharmaceutical Co Ltd. Dr McElroy is also an inventor on US Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and, along with the patent’s assignee, University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson Pharmaceutical Research & Development LLC, which has exclusive rights under the patent.
Dr Jensen is employed by the REACH Institute, New York, New York, and in the past 12 months has received charitable gift support from Shire PLC, and grant support from the Marriott Foundation and the Klingenstein Third Generation Foundation. He also is a part owner of the psychiatric consulting organization CATCH Services Inc, New York City, New York, and he receives book royalties from American Psychiatric Publishing, Civic Research Institute Inc, Ballantine Books, and Guilford Press.
Dr Frye has received grant support from AssureRx Health Inc, Myriad, Pfizer Inc, NIMH (R01 MH079261), the National Institute on Alcohol Abuse and Alcoholism (P20AA017830) in the National Institutes of Health at the US Department of Health and Human Services, and the Mayo Foundation. He has been a consultant to Janssen Global Services LLC, Mitsubishi Tanabe Pharma Corp, Myriad Genetics Inc, Sunovion Pharmaceuticals Inc, and Teva Pharmaceutical Industries Ltd. He has received continuing medical education/travel/presentation support from CME Outfitters LLC and Sunovion Pharmaceuticals Inc.
Dr Biernacka has received research funding as a principal investigator from the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism in the National Institutes of Health at the US Department of Health and Human Services; as a coinvestigator from the National Institute of Mental Health and the National Institute of General Medical Sciences in the National Institutes of Health at the US Department of Health and Human Services; and as a co-principal investigator from the Marriott Foundation for the Mayo Clinic Bipolar Disorder Biobank.
Drs Veldic, Joshi, and Cuellar-Barboza, and Mss Cercy and Geske report no financial or other relationship relevant to the subject of this article.
References
- 1.Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005 Apr;7(2):111–8. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 2.Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008 Oct;65(10):1125–33. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etain B, Dumaine A, Mathieu F, Chevalier F, Henry C, Kahn JP, et al. A SNAP25 promoter variant is associated with early-onset bipolar disorder and a high expression level in brain. Mol Psychiatry. 2010 Jul;15(7):748–55. doi: 10.1038/mp.2008.148. Epub 2009 Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraone SV, Biederman J, Wozniak J. Examining the comorbidity between attention deficit hyperactivity disorder and bipolar I disorder: a meta-analysis of family genetic studies. Am J Psychiatry. 2012 Dec 1;169(12):1256–66. doi: 10.1176/appi.ajp.2012.12010087. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas KR, Nakamura EF, Kessler RC. Epidemiology of mental disorders in children and adolescents. Dialogues Clin Neurosci. 2009;11(1):7–20. doi: 10.31887/DCNS.2009.11.1/krmerikangas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pauls DL, Morton LA, Egeland JA. Risks of affective illness among first-degree relatives of bipolar I old-order Amish probands. Arch Gen Psychiatry. 1992 Sep;49(9):703–8. doi: 10.1001/archpsyc.1992.01820090031005. [DOI] [PubMed] [Google Scholar]
- 7.Grigoroiu-Serbanescu M, Martinez M, Nothen MM, Grinberg M, Sima D, Propping P, et al. Different familial transmission patterns in bipolar I disorder with onset before and after age 25. Am J Med Genet. 2001 Dec 8;105(8):765–73. doi: 10.1002/ajmg.10047. [DOI] [PubMed] [Google Scholar]
- 8.Bellivier F, Golmard JL, Rietschel M, Schulze TG, Malafosse A, Preisig M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003 May;160(5):999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 9.Post RM, Leverich GS, Kupka RW, Keck PE, Jr, McElroy SL, Altshuler LL, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010 Jul;71(7):864–72. doi: 10.4088/JCP.08m04994yel. [DOI] [PubMed] [Google Scholar]
- 10.Nurnberger JI, Jr, Koller DL, Jung J, Edenberg HJ, Foroud T, Guella I, et al. Psychiatric Genomics Consortium Bipolar Group. Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry. 2014 Jun;71(6):657–64. doi: 10.1001/jamapsychiatry.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008 Jun;13(6):558–69. doi: 10.1038/sj.mp.4002151. Epub 2008 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Wellcome Trust Case Control Consortium. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008 Sep;40(9):1056–8. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M, et al. Bipolar Genome Study (BiGS) Consortium. Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatry. 2010 Nov 1;68(9):801–10. doi: 10.1016/j.biopsych.2010.06.019. Epub 2010 Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durak O, de Anda FC, Singh KK, Leussis MP, Petryshen TL, Sklar P, et al. Ankyrin-G regulates neurogenesis and Wnt signaling by altering the subcellular localization of β-catenin. Mol Psychiatry. 2015 Mar;20(3):388–97. doi: 10.1038/mp.2014.42. Epub 2014 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Psychiatric GWAS Consortium Bipolar Disorder Working Group. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat Genet. 2011 Sep 18;43(10):977–83. doi: 10.1038/ng.943. Erratum in: Nat Genet. 2012 Sep;44(9):1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014 Mar 11;5:3339. doi: 10.1038/ncomms4339. [DOI] [PubMed] [Google Scholar]
- 17.Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004 Aug;61(8):781–92. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 18.Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry. 2014 Oct 1;71(10):1148–56. doi: 10.1001/jamapsychiatry.2014.1031. [DOI] [PubMed] [Google Scholar]
- 19.Geller B, Luby JL, Joshi P, Wagner KD, Emslie G, Walkup JT, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry. 2012 May;69(5):515–28. doi: 10.1001/archgenpsychiatry.2011.1508. Epub 2012 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser C, Russell E, Jones IR, Craddock N, et al. WTCCC. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2013 Dec;18(12):1302–7. doi: 10.1038/mp.2012.142. Epub 2012 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson JE, Ryu E, Johnson KJ, Koenig BA, Maschke KJ, Morrisette JA, et al. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clin Proc. 2013 Sep;88(9):952–62. doi: 10.1016/j.mayocp.2013.06.006. Erratum in: Mayo Clin Proc 2014 Feb;89(2):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geller B, Williams M, Zimerman B, Frazier J. Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) St Louis (MO): Washington University; c1996. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Arlington (VA): American Psychiatric Publishing; c2000. Text Revision. [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, William JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York (NY): Biometrics Research, New York State Psychiatric Institute; c2002. [Google Scholar]
- 25.Etain B, Mathieu F, Rietschel M, Maier W, Albus M, McKeon P, et al. Genome-wide scan for genes involved in bipolar affective disorder in 70 European families ascertained through a bipolar type I early-onset proband: supportive evidence for linkage at 3p14. Mol Psychiatry. 2006 Jul;11(7):685–94. doi: 10.1038/sj.mp.4001815. Epub 2006 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jamain S, Cichon S, Etain B, Muhleisen TW, Georgi A, Zidane N, et al. Common and rare variant analysis in early-onset bipolar disorder vulnerability. PLoS One. 2014 Aug 11;9(8):e104326. doi: 10.1371/journal.pone.0104326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frye MA, McElroy SL, Fuentes M, Sutor B, Schak KM, Galardy CW, et al. Development of a bipolar disorder biobank: differential phenotyping for subsequent biomarker analyses. Int J Bipolar Disord. 2015 Dec;3(1):30. doi: 10.1186/s40345-015-0030-4. Epub 2015 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuellar-Barboza AB, Winham SJ, McElroy SL, Geske JR, Jenkins GD, Colby CL, et al. Accumulating evidence for a role of TCF7L2 variants in bipolar disorder with elevated body mass index. Bipolar Disord. 2016 Mar;18(2):124–35. doi: 10.1111/bdi.12368. Epub 2016 Mar 2. [DOI] [PubMed] [Google Scholar]
- 29.Che R, Motsinger-Reif AA. Evaluation of genetic risk score models in the presence of interaction and linkage disequilibrium. Front Genet. 2013 Jul 23;4:138. doi: 10.3389/fgene.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet. 2002 Feb;70(2):425–34. doi: 10.1086/338688. Epub 2001 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005 Jan;21(2):263–5. doi: 10.1093/bioinformatics/bth457. Epub 2004 Aug 5. [DOI] [PubMed] [Google Scholar]
- 32.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012 Nov 1;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E Wellcome Trust Case Control Consortium. Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry. 2009 Mar;14(3):252–60. doi: 10.1038/mp.2008.133. Epub 2008 Dec 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, et al. Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry. 2010 Sep;67(9):939–45. doi: 10.1001/archgenpsychiatry.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014 Feb 12;7:33–42. doi: 10.2147/TACG.S39297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gershon ES, Grennan K, Busnello J, Badner JA, Ovsiew F, Memon S, et al. A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CACNA1C in brain. Mol Psychiatry. 2014 Aug;19(8):890–4. doi: 10.1038/mp.2013.107. Epub 2013 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013 Apr 20;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. Epub 2013 Feb 28. Erratum in: Lancet Apr 20, 2013, 381, (9875), 1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Insel TR, Cuthbert BN. Medicine: brain disorders? Precisely. Science. 2015 May 1;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- 39.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016 Apr 1;173(4):373–84. doi: 10.1176/appi.ajp.2015.14091200. Epub 2015 Dec 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013 May;381(9878):1654–62. doi: 10.1016/S0140-6736(13)60855-7. [DOI] [PubMed] [Google Scholar]
- 41.Craddock N, Sklar P. Genetics of bipolar disorder: successful start to a long journey. Trends Genet. 2009 Feb;25(2):99–105. doi: 10.1016/j.tig.2008.12.002. Epub 2009 Jan 12. [DOI] [PubMed] [Google Scholar]
- 42.Faraone SV, Glatt SJ, Tsuang MT. The genetics of pediatric-onset bipolar disorder. Biol Psychiatry. 2003 Jun 1;53(11):970–7. doi: 10.1016/s0006-3223(02)01893-0. [DOI] [PubMed] [Google Scholar]
- 43.Bellivier F, Etain B, Malafosse A, Henry C, Kahn JP, Elgrabli-Wajsbrot O, et al. Age at onset in bipolar I affective disorder in the USA and Europe. World J Biol Psychiatry. 2014 Jul;15(5):369–76. doi: 10.3109/15622975.2011.639801. Epub 2011 Dec 21. [DOI] [PubMed] [Google Scholar]
- 44.Carlson GA. Diagnostic stability and bipolar disorder in youth. J Am Acad Child Adolesc Psychiatry. 2011 Dec;50(12):1202–4. doi: 10.1016/j.jaac.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy KP, Cullen KR, DeYoung CG, Klimes-Dougan B. The genetics of early-onset bipolar disorder: a systematic review. J Affect Disord. 2015 Sep 15;184:1–12. doi: 10.1016/j.jad.2015.05.017. Epub 2015 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparks GM, Axelson DA, Yu H, Ha W, Ballester J, Diler RS, et al. Disruptive mood dysregulation disorder and chronic irritability in youth at familial risk for bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2014 Apr;53(4):408–16. doi: 10.1016/j.jaac.2013.12.026. Epub 2014 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez S, Xu C, Ramirez M, Zavala J, Armas R, Contreras SA, et al. Suggestive evidence for association between L-type voltage-gated calcium channel (CACNA1C) gene haplotypes and bipolar disorder in Latinos: a family-based association study. Bipolar Disord. 2013 Mar;15(2):206–14. doi: 10.1111/bdi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruderfer DM, Fanous AH, Ripke S, McQuillin A, Amdur RL Schizophrenia Working Group of the Psychiatric Genomics Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium; Cross-Disorder Working Group of the Psychiatric Genomics Consortium. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol Psychiatry. 2014 Sep;19(9):1017–24. doi: 10.1038/mp.2013.138. Epub 2013 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014 Jul 24;511(7510):421–7. doi: 10.1038/nature13595. Epub 2014 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Haiman CA, Kolonel LN, Henderson BE, Wilkens LR, Le Marchand L, et al. Self-reported ethnicity, genetic structure and the impact of population stratification in a multiethnic study. Hum Genet. 2010 Aug;128(2):165–77. doi: 10.1007/s00439-010-0841-4. Epub 2010 May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]