Abstract
Background & objectives:
Pioglitazone was suspended for manufacture and sale by the Indian drug regulator in June 2013 due to its association with urinary bladder carcinoma, which was revoked within a short period (July 2013). The present questionnaire-based nationwide study was conducted to assess its impact on prescribing behaviour of physicians in India.
Methods:
Between December 2013 and March 2014, a validated questionnaire was administered to physicians practicing diabetes across 25 centres in India. Seven hundred and forty questionnaires fulfilling the minimum quality criteria were included in the final analysis.
Results:
Four hundred and sixteen (56.2%) physicians prescribed pioglitazone. Of these, 281 used it in less than the recommended dose of 15 mg/day. Most physicians (94.3%) were aware of recent regulatory events. However, only 333 (44.8%) changed their prescribing pattern. Seventeen of the 416 (4.1%) physicians who prescribed pioglitazone admitted having come across at least one type 2 diabetes mellitus patient (T2DM) who had urinary bladder carcinoma, and of these 13 said that it was in patients who took pioglitazone for a duration of more than two years. Only 7.8 per cent of physicians (n=58) categorically advocated banning pioglitazone, and the rest opined for its continuation or generating more evidence before decision could be taken regarding its use in T2DM.
Interpretation & conclusions:
Majority of the physicians though were aware of the regulatory changes with regard to pioglitazone, but their prescribing patterns were not changed for this drug. However, it was being used at lower than the recommended dose. There is a need for generating more evidence through improved pharmacovigilance activities and large-scale population-based prospective studies regarding the safety issues of pioglitazone, so as to make effectual risk-benefit analysis for its continual use in T2DM.
Keywords: Compliance, drug safety, peroxisome proliferator-activated receptor-gamma agonist, pioglitazone, type 2 diabetes mellitus, urinary bladder carcinoma
India, with 65.1 million people aged 20-79 yr having diabetes, with prevalence rate of 8.56 per cent, is home to the second largest population suffering from diabetes in the world1. Further, Indian patients with diabetes are at substantial higher risk of cardiovascular complications due to ‘Asian Indian genotype’2. There are two categories of physicians engaged in the management of diabetes in the country; first, specialized physicians having done residency in Internal Medicine (MD Medicine) with or without fellowship in Endocrinology (DM Endocrinology), and second, primary care physicians having done MBBS with or without Diploma/Certificate Courses in Diabetology. However, there might be variations in prescribing habits of the physicians due to their professional training level, personal preferences, past experiences and institutional affiliations.
Currently, 10 different classes of drugs are available for the management of type 2 diabetes mellitus (T2DM)3. Due to the safety concerns, many of these drugs have witnessed variations in their sale patterns. Pioglitazone, a thiazolidinedione group of anti-diabetic drug (ADD) and classical example which displayed this type of sale pattern, was approved for marketing in the USA in 1999 and in India in 20004,5. However, its use has been associated with congestive heart failure exacerbation and increased incidence of fractures in women6. There were reports of incidence of urinary bladder carcinoma with pioglitazone both globally as well as nationally7,8. This alerted Indian drug regulators and its manufacture and sale were suspended due to this safety concern in June 20139. The suspension was revoked in July 2013, with some caveats regarding cautionary use10. This quick change in regulatory action was widely covered by both professional and social media all over the world. This regulatory spin has created apprehension among physicians treating diabetes regarding prescription of pioglitazone. To address this, this pan-India questionnaire-based cross-sectional study was conducted with the primary objective to evaluate the impact of recent regulatory events with regard to pioglitazone prescribing behaviour of physicians in the country.
Material & Methods
This cross-sectional, multicentre, questionnaire-based study was conducted between November 2013 and March 2014. The department of Pharmacology, All India Institute of Medical Sciences (AIIMS), New Delhi, was the nodal centre of the study. A total of 111 prospective centres were identified for participation on the basis of their location (north, west, south, east and centre) and affiliation (government and private) from all over the country. The majority of Adverse Drug Reaction (ADR) Monitoring Centres (AMCs) under the Pharmacovigilance Programme of India were contacted to be part of this study. Besides this, the centres from some other institutes/hospital were contacted to be part of this study to cover up for States/Regions which were not fully covered by these AMCs. Of all, 36 centres initially consented to participate in the study. But later on, eight centres withdrew their consent citing their inability to undertake the study and two centres withdrew due to their inability to complete the study in the required period. One centre was removed for not conducting the study in accordance with the protocol. Hence, a total of 25 centres from all over the country participated in the study (Fig. 1).
Fig. 1.
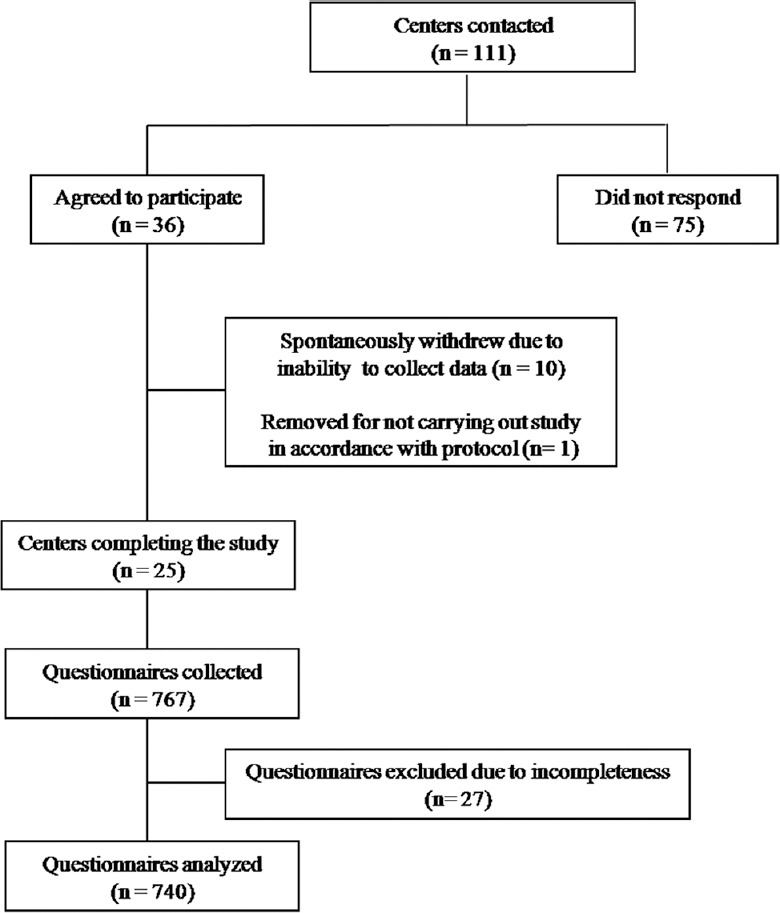
Flow chart showing the study design.
Study population: Each investigator was assigned to collect a minimum of 20 responses from the study population in and around their centre. The study population consisted of physicians managing T2DM using allopathic system of medicine and having one of the following professional degrees: (i) DM Endocrinology (Fellowship in Endocrinology), (ii) MD or DNB Internal Medicine (Residency in Internal Medicine), (iii) MBBS with Diploma/Certificate Course in diabetology, and lastly (iv) MBBS only, were included in the study. To ensure quality, in the last group, i.e. MBBS without additional degree/diploma/certificate course, physicians having minimum of five years of experience in clinical practice after getting their professional degree, were included in the study. Further, each centre was told to strive for inclusion of equal number of participants having private and government affiliations and also from all the four qualification categories, as far as possible.
All participants were approached individually by the investigator at each centre and were explained the purpose of the study. The questionnaire was administered to the physicians who were willing to participate and were given sufficient time to answer the questions by striking out the most appropriate option(s) against each item. They were given the alternative to leave any question unanswered, if desired. The collected responses in form of filled questionnaire from each centre were sent to nodal centre for the final analysis. Completeness check and quality analysis of all the questionnaires received from various centres was carried out at the nodal centre. The questionnaires received from other centres and not having demographic data and official seal of the participating physician were removed from the final analysis. Also from each centre, 10 per cent of the questionnaires were randomly selected and a telephone call was made to the participants to check their authenticity of having been participated in the study.
Questionnaire: A 25-item questionnaire was used to evaluate the diabetes practicing pattern in the study. The questionnaire was validated by administering it to five physicians from each category mentioned above and also to five physicians not involved in the management of T2DM. In the final questionnaire, 16 items were used for soliciting information regarding knowledge and the impact of recent regulatory developments regarding pioglitazone practicing pattern in the country. The copyright of the final version of questionnaire was applied with the Registrar of Copyright, Government of India. The study protocol was reviewed and approved by the Institute Ethics Committee, AIIMS, New Delhi (Ref No. IEC/NP-610/2014 RP).
Statistical analysis: All the questionnaires fulfilling the inclusion criteria were taken for the final analysis. The items not attempted by the respondents were removed from the final count. However, other marked items of that questionnaire were taken up in the final analysis. For the items, where multiple options were marked by the respondents against only one applicable option, all the chosen options were considered towards the final analysis. The data were analyzed using the Statistical Package for the Social Sciences (SPSS, Chicago, USA) version 18 for Windows. Data were presented as frequency (percentage) or median (range). For analyzing item responses of the questionnaire, descriptive statistics was applied. The association between two categorical variables was analyzed using Chi-square/Fisher's exact test, whichever was appropriate.
Results
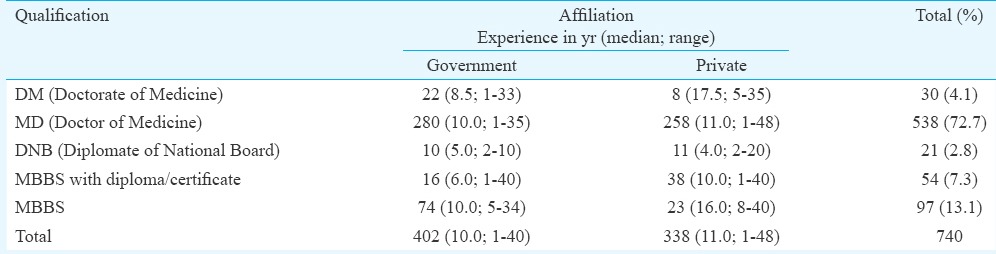
A total of 767 questionnaires were collected from 25 centres enrolled in the study. Twenty seven questionnaires were excluded from the study for not meeting the inclusion criteria. Hence, a total of 740 completed questionnaires were taken up for the final analysis (Fig. 1). The profile of participants who were taken up for the final analysis is shown in the Table. Participants having government affiliation were more than those having private affiliation (54.32 vs 45.68%), with majority having MD Internal Medicine (72.7%) qualification.
Table.
Profile of physicians participated in the study
Metformin was used as first-line ADD by 705 (95.27%) physicians, of whom 301 (40.68%) used it as monotherapy, and the remaining used in combination with other drugs such as sulphonylureas, pioglitazone, dipeptidyl peptidase-4 (DPP-4) inhibitors and insulin. Sulphonylureas were used by 306 (41.35%) physicians as the first-line drug, but only 17 reported to be using this as monotherapy with rest of them using in combination with other drugs. Pioglitazone was used by 41 (5.54%), DPP-4 inhibitors by 103 (13.92%) and insulin by 102 (13.78%) as first-line ADD but only in combination with other drugs.
Most of the participating physicians (n=535, 72.3%) preferred prescribing fixed dose combinations (FDCs) rather than individual ADDs, if available in the market. Increased patient compliance was found to be the most important factor for prescribing FDC by the physicians (n=543, 73.4%), others being reduction in cost (n=272, 36.7%), ease of prescription (n=127, 17.2%) and increased efficacy of the drugs (n=72, 9.7%). The most commonly prescribed FDCs by the participating physicians included metformin plus sulphonylurea (n=493, 66.6%) followed by metformin plus DPP-4 inhibitors (n=103, 13.9%), metformin plus sulphonylurea plus pioglitazone as triple combination (n=70, 9.5%) and metformin plus pioglitazone (n=56, 7.6%).
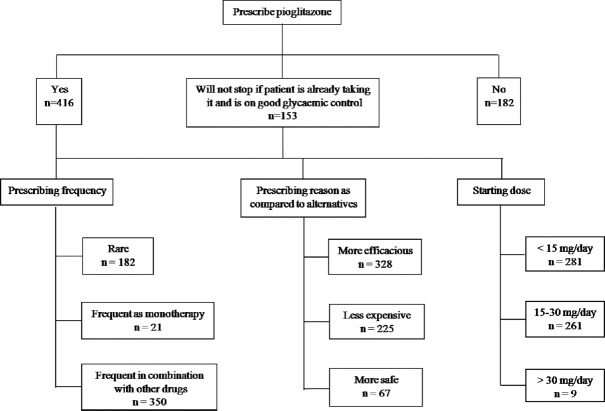
Pioglitazone was prescribed by 416 (56.22%) physicians for the management of T2DM (Fig. 2). Further, 153 (20.7%) physicians did not prescribe pioglitazone to a newly diagnosed T2DM patient but did not discontinue if patient was already taking it and was having good glycaemic control. There was no significant difference in pioglitazone prescription, either according to affiliation or according to qualification of the prescribing physician. Further, majority of the physicians preferred to prescribe it in combination with other ADDs, monotherapy being less preferred way of prescribing this medication. Almost all of the physicians prescribed pioglitazone at dose of up to 30 mg/day as a starting dose, with almost equal division between <15 mg/day (n=281, 37.9%) and between 15 and 30 mg/day (n=261, 35.27%) (Fig. 2).
Fig. 2.
Prescribing pattern of pioglitazone.
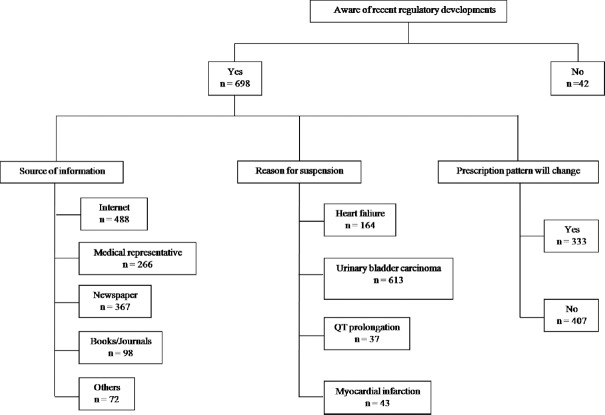
Six hundred and ninety eight (94.3%) respondents were aware of the recent regulatory development regarding pioglitazone in India (Fig. 3). More number of physicians having government affiliations were unaware than their private counterparts regarding these recent regulatory developments (29 vs 13, P<0.05). Internet was the most common source of information regarding these recent developments, followed by newspapers, interaction with medical representatives of pharmaceutical companies and medical journals. Only 82.8 per cent (n=613) were aware of the correct reason for the suspension of the drug as increased risk of urinary bladder carcinoma. Despite the recent events, majority of the physicians (n=401, 55.0%) were not in favour of change in their current prescribing pattern regarding pioglitazone in T2DM. There was no significant difference for change in prescribing behaviour among physicians having different affiliation or qualification. DPP-4 inhibitors were mentioned as the alternative drug to pioglitazone by majority of the physicians (n=550, 74.3%), other being acarbose (n=375, 50.7%), insulin (n=305, 41.2%) and glucagon like peptide-1 (GLP-1) agonists (n=113, 15.27%). According to 49.9 per cent (n=369) of respondents, these alternatives were equally efficacious as compared to pioglitazone while 13.5 per cent (n=100) mentioned these to be less efficacious and rest as more efficacious.
Fig. 3.
Knowledge of regulatory issues about pioglitazone and its impact on the prescription pattern.
Four hundred and eighty one physicians (65.0%) informed patients about the potential risks involved with the pioglitazone use while 181 (24.5%) discussed the risk only if the patients themselves enquired about the adverse effects. Three hundred and forty three (46.3%) doctors responded that the patient refused to pioglitazone, if informed about the potential adverse effects associated with it. Importantly, 17 physicians responded to have encountered at least one case of urinary bladder carcinoma (associated or not associated) in T2DM patients, who were taking pioglitazone. Of these, 13 physicians responded that those patients had been taking pioglitazone for more than two years before disease occurrence. Significantly, 350 (47.3%) respondents were in favour of continuation of pioglitazone sale in India, with only 58 (7.8%) physicians in favour of ban of the drug. Rest of the respondents (n=338, 45.6%) were of the opinion that more data need to be generated to conclusively decide about the association of urinary bladder carcinoma with pioglitazone. There was no significant difference among the opinion of physicians having different affiliations or qualifications with regard to continuation of pioglitazone sale in the country.
Discussion
In the current study, 56.2 per cent of the physicians were observed prescribing pioglitazone for the management of T2DM. There could be a decrease in pioglitazone prescription as discussed in a report from a tertiary care private hospital from north India11. A drug utilization study conducted in a tertiary care hospital among T2DM patients found that thiazolidinediones were prescribed to 1.83 per cent of T2DM patients12. At global level, minor effects were observed on its utilization patterns in Australia, the sales of pioglitazone were substantially reduced in the USA after Food and Drug Administration (FDA) warnings13,14. Majority of the physicians (94.3%) were aware of the recent regulatory developments regarding this drug in the country, but only 82.8 per cent of physicians were aware of the correct reason for the suspension of pioglitazone as increased risk of urinary bladder carcinoma.
Diabetes mellitus itself is associated with increased incidence of urinary bladder carcinoma15. Among ADDs, contrary to previous reports, metformin has not been found to be associated with decreased incidence of bladder carcinoma as compared to sulphonylureas16. Among sodium-glucose cotransporter type 2 inhibitors, increased incidence of bladder carcinoma has been observed with dapagliflozin in preclinical studies, however, subsequent studies did not find any increased carcinoma risk with dapagliflozin or other SGLT-2 inhibitors17. A population-based cohort study in France concluded that pioglitazone exposure increases the risk of bladder cancer, which was related to the cumulative dose (≥28,000 mg) and duration of exposure (≥24 months) of the drug18. The report on safety study commissioned by the US FDA concluded that the significant increased risk of bladder cancer could not be linked with pioglitazone use in T2DM patients although previously observed increased risk could not be completely ruled out19. A repeat analysis of PROactive study also demonstrated the increased risk of bladder carcinoma with pioglitazone20. A meta-analysis reported that pioglitazone use was associated with clinically significant increased risk of bladder cancer which was related to total duration and cumulative dose of the therapy21.
Evidence is also available that pioglitazone use does not increase the risk for bladder carcinoma. A retrospective study conducted on database from 34,970 Taiwanese T2DM patients concluded that there was no association of pioglitazone (either cumulative dose consumed or duration of the therapy) with the development of urinary bladder carcinoma. However, it was associated with an increased risk of newly developed chronic kidney disease22. Another retrospective study on patient database having undergone radical cystectomy for bladder cancer concluded that pioglitazone use among T2DM patients did not have any negative effect of pathological tumour staging23. In India, an open-label clinical study found no increased risk of bladder-related abnormalities even after two years of treatment with pioglitazone24. A retrospective study involving 2222 patients from India concluded that pioglitazone use was not associated with occurrence of bladder carcinoma25. Similarly, no correlation could be established between the pioglitazone and urinary bladder carcinoma in diabetes mellitus patients in a retrospective study conducted in a cancer hospital in Chennai26.
The US FDA, although not banned the drug, had issued statement regarding its cautious use following interim analysis of an ongoing epidemiological study27. The recent guidelines for management of T2DM issued by the National Institute for Health and Care Excellence (NICE) have mentioned pioglitazone as one of the first-line drugs for T2DM, if metformin is contraindicated28. However, un-investigated macroscopic haematuria has been added as one of the contraindications for this drug28. In India, the drug's manufacturing, sales and distribution were suspended in June 20139, and within a span of six weeks, the ban was revoked along with some conditions for its cautionary use10. Despite this controversy, in this study, majority of the physicians (n=401, 55.0%) were not in favour of change of their current prescribing pattern of pioglitazone. This was further corroborated by the fact that only a few respondents (7.8%) were in favour of discontinuation of the drug from the market. As evident from this study, pioglitazone was being prescribed very frequently at low starting dose ranging from 7.5 to 15 mg/day, with very few prescriptions above 30 mg/day. This indicated the awareness among physicians about the cumulative dose of pioglitazone and bladder carcinoma relationship. Although 7.5 mg is not the recommended dose of pioglitazone18,20 but giving low dose will theoretically decrease the risk of development of urinary bladder carcinoma.
In this study, 17 of the 416 (4.1%) physicians who prescribed pioglitazone responded to encounter minimum one case of urinary bladder carcinoma (associated or not associated) in T2DM patients who were taking pioglitazone. Of them, 13 confirmed that these patients were taking pioglitazone for more than two years. This confirms the earlier reports from France, Germany and the USA that when patients take a cumulative dose of 28,000 mg or more (i.e., 30 mg per day × 365 days × 2.5 yr) of pioglitazone, the risk of pioglitazone-associated bladder cancer increases significantly20. This needs to be investigated further for proper causality assessment for its association with urinary bladder carcinoma.
In our study, most of the participating physicians (72.3%) preferred prescribing FDC rather than individual ADDs, if available in the market. Further, increased patient compliance was found to be the most important factor for prescribing FDC by the physicians (73.4%), others being reduction in cost (36.7%), ease of prescription (17.2%) and increased efficacy of the drugs (9.7%). These factors were in agreement with a study in which patient compliance, reduction in cost because of decreased expenditure of packaging, etc., ease of prescription and sometimes increased efficacy of drugs when combined were listed as most common factors for prescription of FDCs29. Reduction in cost is an important consideration for prescribing FDCs, and an analysis from India demonstrated substantial reduction in price by combining two or more drugs in diabetes30.
Our study had certain limitations. First, the responding physicians were practicing in urban settings and the physicians in rural setting could not be included in the study. Second, only a limited number of participants having fellowship in endocrinology participated in this study. Third, there could be recall bias regarding the urinary bladder carcinoma cases among T2DM patients being observed by the physicians. However, in India, due to under-reporting of ADRs by the physicians and lack of other reliable data source, this study could provide the initial trends which can be used further to carry out large-scale epidemiological studies. Fourth, participants from all the regions were not equally represented in the study. For this reason, all the participants were considered as one unit and subgroup analysis was not done for any regional variations in prescription habits. Finally, the information was not collected from the participating physicians whether they were following precautions (namely, total cumulative dose and duration of the therapy, history of bladder disease, un-investigated haematuria, etc.) while prescribing pioglitazone in T2DM patients.
In conclusion, majority of the physicians were aware of the regulatory changes related to pioglitazone in the country and it had not impacted their prescription patterns. The economic consideration was also one of the reasons for prescription of this drug in developing country like India, and majority of the physicians were in favour of marketing of this drug. A large number of physicians were prescribing this drug at less than recommended dose of 15 mg/day, which might limit the cumulative dose-dependent toxicity of pioglitazone. Pharmacovigilance of ADDs in general and pioglitazone in particular, needs to be strengthened. Along with this, more evidence needs to be generated by large-scale population-based prospective studies to conclude about the association between pioglitazone use and urinary bladder carcinoma.
Acknowledgment
Authors thank Dr Mangala Lahkar for help in collecting data from Government Medical College, Guwahati, Assam.
Footnotes
Conflicts of Interest: None.
References
- 1.International Diabetes Federation. IDF diabetes atlas. 6th ed. Basel, Switzerland: IDF; 2013. [accessed on August 10, 2017]. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/19-atlas-6th-edition.html . [Google Scholar]
- 2.Radha V, Mohan V. Genetic predisposition to type 2 diabetes among Asian Indians. Indian J Med Res. 2007;125:259–74. [PubMed] [Google Scholar]
- 3.Salvo MC, Brooks AD, Thacker SM. Patient considerations in the management of type 2 diabetes - Critical appraisal of dapagliflozin. Patient Prefer Adherence. 2014;8:493–502. doi: 10.2147/PPA.S59169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Central Drugs Standard Control Organization. Minutes of the 64th meeting of drugs technical advisory board (DTAB) held on 19th July, 2013 in the Chamber of DGHS, Nirman Bhawan, New Delhi - 110002. [assessed on August 10, 2017]. Available from: http://www.cdsco.nic.in/writereaddata/Minutes%20of%2064th%20DTAB%20meeting.pdf .
- 5.Shukla R, Kalra S. Pioglitazone: Indian perspective. Indian J Endocrinol Metab. 2011;15:294–7. doi: 10.4103/2230-8210.85581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paul TV, Thomas N. Impact of oral antidiabetic agents on bone metabolism. Indian J Med Res. 2015;141:385–8. doi: 10.4103/0971-5916.159244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schernthaner G, Currie CJ, Schernthaner GH. Do we still need pioglitazone for the treatment of type 2 diabetes? A risk-benefit critique in 2013. Diabetes Care. 2013;36(Suppl 2):S155–61. doi: 10.2337/dcS13-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unnikrishnan R, Sundramoorthy C, Deshpande N, Sarvothaman R, Sahay RK, Mehtalia S, et al. Eight cases of bladder cancer in pioglitazone users from India. J Assoc Physicians India. 2012;60:66. [PubMed] [Google Scholar]
- 9.Hashmi A. Pioglitazone suspension and its aftermath: A wake up call for the Indian drug regulatory authorities. J Pharmacol Pharmacother. 2013;4:227–9. doi: 10.4103/0976-500X.119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministry of Health and Family Welfare India. Notification GSR 520(E) [assessed on August 10, 2017]. Available from: http://www.cdsco.nic.in/writereaddata/GRIjuly13.pdf .
- 11.Mithal A, Kaur P, Bansal B, Mishra SK, Wasir JS, Jevalikar G, et al. Usage of pioglitazone at Medanta, the Medicity. Indian J Endocrinol Metab. 2014;18:111–2. doi: 10.4103/2230-8210.126588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das S, Haroled Peter PL, Bhavani ML, Naresh P, Ramana MV. Age- and sex-related prevalence and drug utilization pattern in the management of type 2 diabetes mellitus and its comorbidity with cardiovascular diseases: A comparative study. Indian J Pharm Sci. 2015;77:478–84. doi: 10.4103/0250-474x.164776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu JC, Ross-Degnan D, Wagner AK, Zhang F, Lu CY. How did multiple FDA actions affect the utilization and reimbursed costs of thiazolidinediones in US medicaid? Clin Ther. 2015;37:1420–32.e1. doi: 10.1016/j.clinthera.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niyomnaitham S, Page A, La Caze A, Whitfield K, Smith AJ. Utilisation trends of rosiglitazone and pioglitazone in Australia before and after safety warnings. BMC Health Serv Res. 2014;14:151. doi: 10.1186/1472-6963-14-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Z, Wang X, Shen Z, Lu Y, Zhong S, Xu C. Risk of bladder cancer in patients with diabetes mellitus: An updated meta-analysis of 36 observational studies. BMC Cancer. 2013;13:310. doi: 10.1186/1471-2407-13-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamtani R, Pfanzelter N, Haynes K, Finkelman BS, Wang X, Keefe SM, et al. Incidence of bladder cancer in patients with type 2 diabetes treated with metformin or sulfonylureas. Diabetes Care. 2014;37:1910–7. doi: 10.2337/dc13-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HW, Tseng CH. A review on the relationship between SGLT2 inhibitors and cancer. Int J Endocrinol. 2014;2014:719578. doi: 10.1155/2014/719578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: A population-based cohort study. Diabetologia. 2012;55:1953–62. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265–77. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 20.Hillaire-Buys D, Faillie JL, Montastruc JL. Pioglitazone and bladder cancer. Lancet. 2011;378:1543–4. doi: 10.1016/S0140-6736(11)61662-0. [DOI] [PubMed] [Google Scholar]
- 21.Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: A systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258–73. doi: 10.1111/bcp.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MY, Hsiao PJ, Yang YH, Lin KD, Shin SJ. The association of pioglitazone and urinary tract disease in type 2 diabetic Taiwanese: Bladder cancer and chronic kidney disease. PLoS One. 2014;9:e85479. doi: 10.1371/journal.pone.0085479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero V, Peyton C, Gray I, Hemal A, Terlecki R. Pathology of bladder cancer among diabetic patients undergoing radical cystectomy with a history of pioglitazone (Actos) use. BMC Urol. 2014;14:10. doi: 10.1186/1471-2490-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balaji V. Efficacy and safety of pioglitazone in type 2 diabetes in the Indian patients: Results of an observational study. Indian J Endocrinol Metab. 2013;17:709–15. doi: 10.4103/2230-8210.113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S, Gupta K, Ravi R, Mehta V, Banerjee S, Joshi S, et al. Pioglitazone and the risk of bladder cancer: An Indian retrospective cohort study. Indian J Endocrinol Metab. 2015;19:639–43. doi: 10.4103/2230-8210.163187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balaji V, Seshiah V, Ashtalakshmi G, Ramanan SG, Janarthinakani M. A retrospective study on finding correlation of pioglitazone and incidences of bladder cancer in the Indian population. Indian J Endocrinol Metab. 2014;18:425–7. doi: 10.4103/2230-8210.131223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Food and Drug Administration. FDA Drug Safety Communication: Update to ongoing safety review of Actos (pioglitazone) and increased risk of bladder cancer. [accessed on August 10, 2017]. Available from: https://www.fda.gov/Drugs/DrugSafety/ucm259150.htm .
- 28.National Institute for Health and Care Excellence. Type 2 Diabetes in Adults: Management, NICE guideline 2015. [accessed on February 18, 2016]. Available from: http://www.nice.org.uk/guidance/ng28/chapter/1-Recommendations#drug-treatment-2 . [PubMed]
- 29.Guénette L, Moisan J, Breton MC, Sirois C, Grégoire JP. Difficulty adhering to antidiabetic treatment: Factors associated with persistence and compliance. Diabetes Metab. 2013;39:250–7. doi: 10.1016/j.diabet.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Hussain S. Cost variation analysis of oral hypoglycaemic agents available in Indian market: An economic perspective. Int J Pharm Sci Res. 2015;6:913–8. [Google Scholar]