Abstract
Background & objectives:
Lactobacilli species that are better adapted to vaginal environment of women may colonize better and offer protection against vaginal pathogenic bacteria. In this study, the distribution of common Lactobacillus species was investigated in pregnant women.
Methods:
Sixty seven pregnant women were included in the study and vaginal samples were collected for Gram staining. Women were classified as normal vaginal flora, intermediate flora and bacterial vaginosis (BV) based on Nugent's score. Vaginal samples were also collected for the identification of Lactobacillus spp. by multiplex polymerase chain reaction (PCR) profiling of 16S rDNA amplification method.
Results:
Lactobacillus crispatus (100%) was the most predominant Lactobacillus spp. present in pregnant women with normal flora, followed by L. iners (77%), L. jensenii (74%) and L. helveticus (60%). While, L. iners was commonly present across groups in women with normal, intermediate or BV flora, L. crispatus, L. jensenii and L. helveticus decreased significantly as the vaginal flora changed to intermediate and BV. In women with BV, except L. iners other species of lactobacilli was less frequently prevalent. Species such as L. rhamnosus, L. fermentum, L. paracasei and L. casei were not detected in any vaginal sample.
Interpretation & conclusions:
L. crispatus, L. jensinii and L. helveticus were predominant species in women with normal flora. L. crispatus alone or in combination with L. jensinii and L. helveticus may be evaluated for probiotic properties for the prevention and treatment of BV.
Keywords: Bacterial vaginosis, Lactobacillus crispatus, multiplex polymerase chain reaction, Nugent score, pregnant women, vaginal Lactobacillus
The beneficial microbiota such as Lactobacillus spp. in vaginal ecosystem is thought to have been adapted and coevolved by mutualistic association with its human host1. It is essential to explore the Lactobacillus diversity of vagina in health and disease and to understand whether changes in the individual vaginal Lactobacillus spp. can be correlated with changes in vaginal infections2. Bacterial vaginosis (BV) is a common vaginal infection caused by imbalance of indigenous microbiota3,4. Disturbance in the vaginal flora with overgrowth of bacteria that are present in the vagina in small numbers such as Gardnerella vaginalis, Prevotella, Mobiluncus, Peptostreptococcus and decrease in the number of Lactobacillus spp. often associated with high pH and clue cells is generally described as BV5,6. BV is known to increase predisposition to sexually transmitted diseases, including gonorrhoea, chlamydia, syphilis, trichomoniasis, human immunodeficiency virus and human papilloma virus7,8. In pregnancy, BV increases the risk of post-abortal sepsis, early miscarriage, recurrent abortion, late miscarriage, preterm premature rupture of membranes, spontaneous preterm labour and histologic chorioamnionitis9,10,11.
Therapy of BV involves oral or local administration of metronidazole or intravaginal clindamycin and varies in efficacy3. The long-term cure rate is low, and BV recurs in up to 40 per cent of women within three months after initiation of antibiotic therapy and in up to 50 per cent of women after three months12. There are several side-effects and disadvantages associated with these therapies, including superinfections by pathogenic microorganisms and disturbance of gut flora when treated by oral supplementation13. Moreover, vaginal opportunistic pathogens, particularly G. vaginalis and anaerobic bacteria show increasing drug resistance. In this context, Lactobacillus spp. administered orally or locally may be an effective alternative therapy which would re-establish the indigenous Lactobacillus and prevent BV as well as associated complications2.
In humans, about 120 Lactobacillus species have been identified and more than 20 species have been found in the vagina14. Based on the previous molecular-based vaginal microbiome studies, three or four species (mainly Lactobacillus crispatus, Lactobacillus iners, Lactobacillus jensenii and Lactobacillus gasseri) normally predominate14,15,16. Colonization by lactobacilli ensures low pH in the genital tract (pH 4.5), which protects against colonization by other microbes7. Lactobacillus species also protect vaginal health by producing antimicrobial compounds such as hydrogen peroxide and bacteriocins17. This study was undertaken to identify and study the vaginal lactobacilli profile of pregnant women with normal, disturbed (intermediate flora) and BV flora.
Material & Methods
Pregnant women were selected from Government Maternity Hospital, Hyderabad, India; from January 2014 to March 2015. Sixty seven women were selected for the study after obtaining written informed consent. The study was approved by the Institutional Ethical Committee (IEC), ICMR-National Institute of Nutrition, Hyderabad. The sample size was calculated with preliminary data on vaginal lactobacilli in normal women; based on which, 18 per group were found to be sufficient to detect significance at 5 per cent between groups with 80 per cent power.
At the first study visit, weight, age, height and blood sample (0.2 ml) for haemoglobin levels were collected. Gestational age was calculated based on the last menstrual period, and birth weight was recorded. Vaginal samples (vaginal exudates of lateral wall) were collected for the identification of Lactobacillus spp. from all the 67 pregnant women. The women were classified into BV, intermediate and normal according to the Nugent score (NS) criteria based on vaginal smear Gram staining scores18 using Microscope (Olympus B202, Japan). NS of 1-3 is considered normal vaginal flora or normal microbiota (BV negative), NS of 4-6 is considered as intermediate vaginal flora or intermediate microbiota, and 7-10 is considered as BV positive18. All women diagnosed with BV were treated with local antibiotic (Clindamycin 2% vaginal cream) for one week as per the WHO guidelines19. The first swab was used to prepare a smear on a glass slide for the purpose of grading18. The second swab was transferred to a sterile phosphate buffer saline (PBS) tube for DNA extraction.
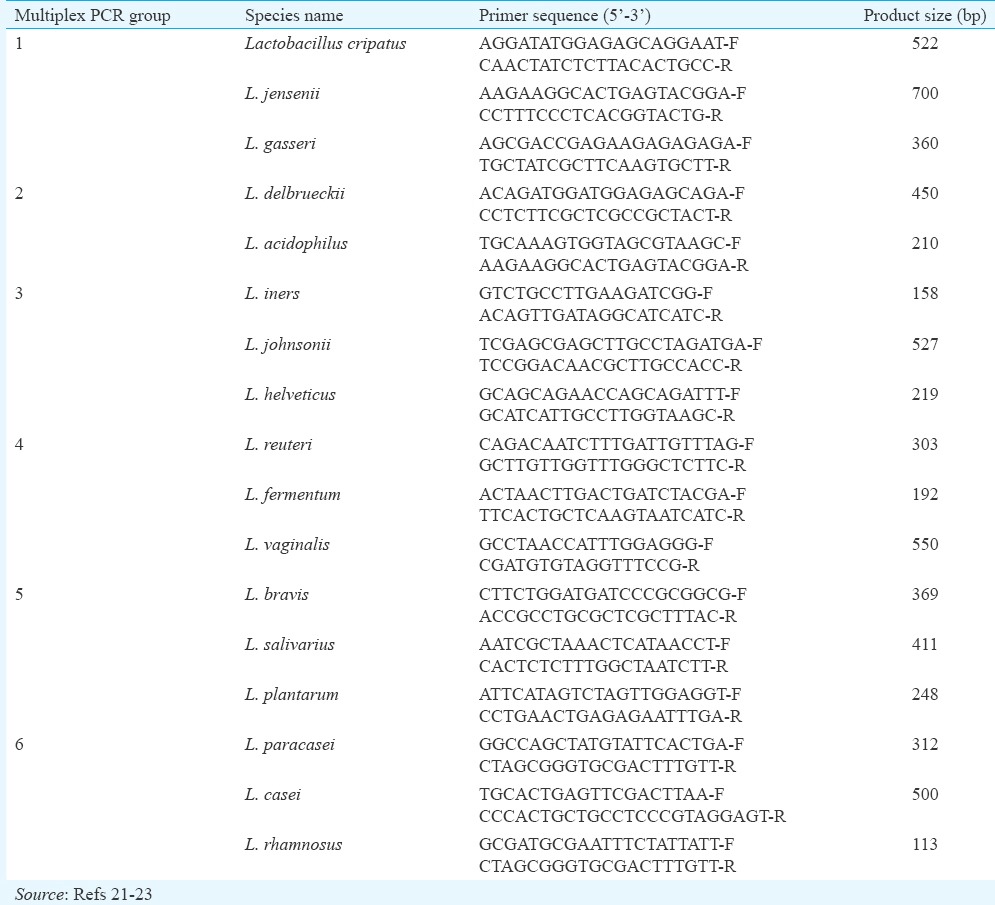
DNA Extraction from vaginal swabs and Lactobacilli identification by Multiplex PCR: DNA extraction from vaginal swab samples was carried out as described by Kumar et al20. The polymerase chain reaction (AESTAC, Japan) was carried out for isolated DNA samples. Each sample was initially identified to the genus level by amplification with genus-specific primers, [forward primer (F) CTCAAAACTAAACAAAGTTTC-F and reverse primer (R), CTTGTACACACCGCCCGTTCA-R] [250 base pairs (bp) product size]. PCR programme included initial denaturation at 94°C for five minutes, followed by 30 cycles of denaturation at 94°C for 30 sec, annealing at 56°C and extension at 72°C for five minutes. Amplified product was identified on Low EEO agarose gel using Geldoc (Syngene, UK). Samples positive for Lactobacillus genus were subjected to species identification using species-specific primers21,22,23 (Table I). Multiplex PCR was used for the identification of 17 Lactobacillus spp. as given in Table I. Species was identified on Low EEO agarose gel using Geldoc based on product size and 1 kb ladder DNA. For each sample, PCR reaction was carried out independently in duplicates.
Table I.
Sequences of polymerase chain reaction primers used for the identification of lactobacilli by multiplex polymerase chain reaction
Cultivation of lactobacilli bacteria from vaginal swabs in MRS broth: The vaginal swabs were vortexed in 1 ml sterile PBS (pH 7.4) to prepare bacterial suspensions and 100 μl of sample was inoculated in freshly prepared sterile MRS broth (BD Difco™, USA). After incubation for 48 h under anaerobic condition (anaerobic workstation, N2 80%, CO2 10%, H2 10%) at 37°C, samples positive for growth were used for DNA extraction. DNA extraction and PCR procedures followed were similar to those mentioned above.
Statistical analysis: ANOVA was performed to compare means of NS and pH in women with normal, intermediate and BV flora and Chi-square was used to compare proportions between groups using SPSS version 19.0 software (SPSS, Chicago, IL, USA). Heatmap was created using R-programme software package (G-PLOT HEATMAP 2) to depict the frequency of the lactobacilli species.
Results & Discussion
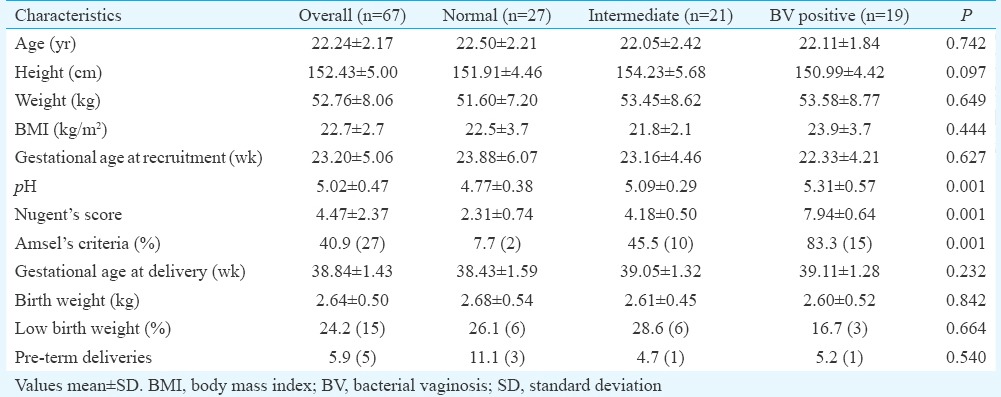
Mean age, weight, height, body mass index and haemoglobin concentration were similar in women with normal, intermediate and BV flora. Of the 67 pregnant women, 15 had low birth weight babies (birth weight <2.5 kg) and five had preterm deliveries (gestational age at delivery <37 wk) (Table II). The mean birth weight and gestational age at delivery were comparable between groups. Of the 67 pregnant women, 27 had normal vaginal flora, 21 had intermediate flora and 19 had BV. The vaginal pH and NS means were significantly (P<0.001) higher in women with BV compared to normal.
Table II.
Demographic and clinical characteristics of pregnant women and neonates
Only 13 of the 17 lactobacilli species were detected by multiplex PCR. Heatmap (Fig. 1) shows distribution of lactobacilli species in pregnant women L. jensenii, L. vaginalis and L. helveticus. Species such as L. rhamnosus, L. fermentum, L. paracasei and L. casei were not detected by multiplex PCR.
Fig. 1.
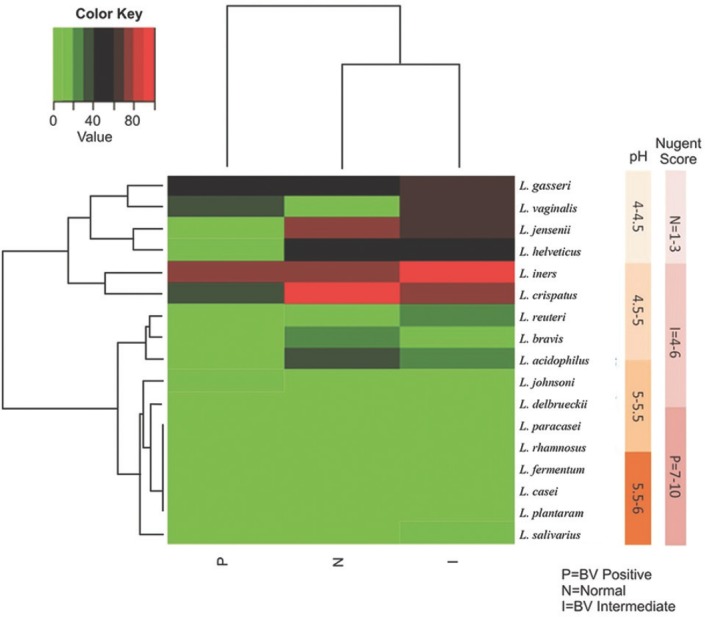
Heatmap shows proportions of microbial species found in the vaginal bacterial communities of 67 pregnant women. As the colour key indicates, light green colour represents the absence of the organisms, and the darker coloured tiles indicate the presence of percentage of that particular organism. Lactobacillus crispatus was the dominant flora in the normal and intermediate group and L. iners was the more frequent organism in the positive group. Nugent's score and pH bars are shown on the right side of the Figure. The Nugent's score increased with increasing pH.
The proportion of pregnant women with Lactobacillus spp. in normal, intermediate and BV flora are shown in Fig. 2. L. crispatus (100%) was the most predominant Lactobacillus spp. present in pregnant women with normal flora, followed by L. iners (77%), L. jensenii (74%) and L. helveticus (60%) (Fig. 2). Significantly (P<0.05) higher proportion of women with normal flora had L. crispatus compared to women with intermediate flora and BV. Similarly, L. jensenii and L. helveticus were significantly (P<0.05) higher in women with normal flora compared to women with BV (Fig. 2). L. iners was commonly present across groups in women with normal, intermediate or BV flora. Except L. iners, other species of lactobacilli were less frequently prevalent in women with BV.
Fig. 2.
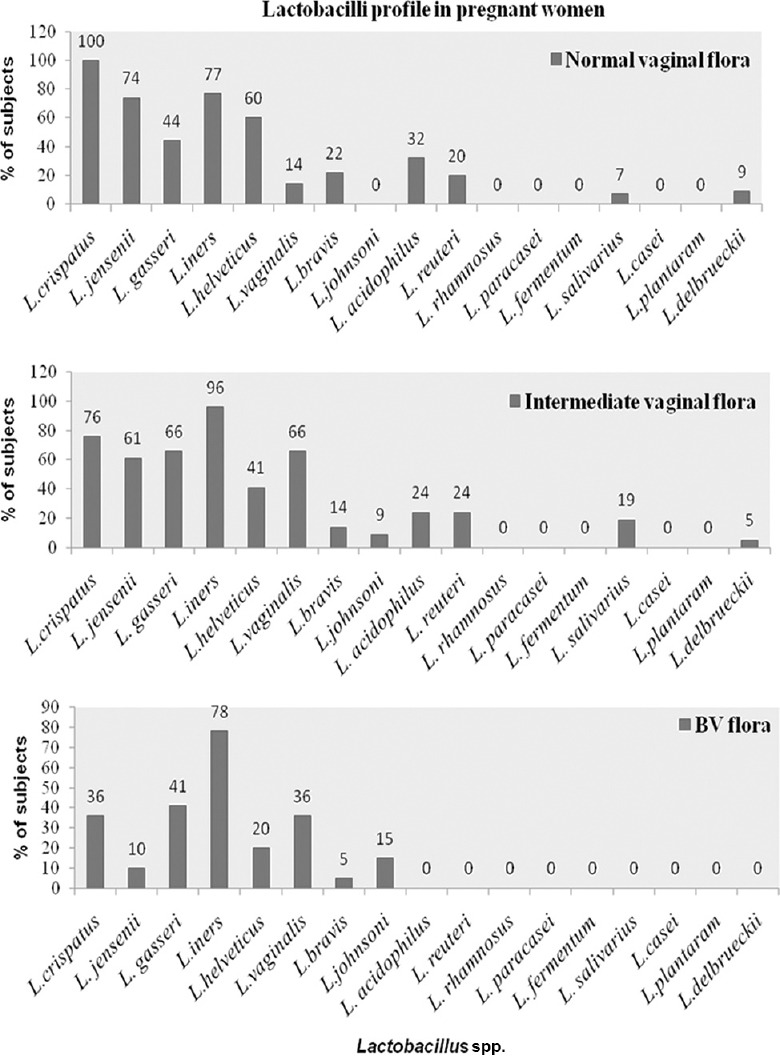
Percentage of the most frequent vaginal Lactobacillus spp. in women with normal, intermediate and bacterial vaginosis flora as determined by multiplex polymerase chain reaction profile.
Four of 27 pregnant women with normal flora had a combination of L. crispatus, L. jensenii, L. helveticus and L. acidophilus and this combination was not found in women with intermediate or BV flora. In contrast, L. iners, L. gasseri, L. vaginalis and L. salivarius combination were found in three women with intermediate and two with BV flora; interestingly, this combination was not found in normal group. Combination of L. iners, L. gasseri and L. vaginalis was found in both intermediate and BV groups, but not in normal flora. However, a combination of L. iners along with L. crispatus, L. jensenii, L. helveticus and L. reuteri was found in three of 27 women with normal flora, a similar combination was observed less frequently in women with intermediate and BV microbiota.
By culture-dependant method, 12 lactobacilli spp. (L. crispatus, L. jensenii L. gasseri, L. iners, L. helveticus, L. vaginalis, L. bravis, L. johnsoni, L. acidophilus, L. reuteri, L. paracasei and L. salivarius) could be identified from women with normal flora and intermediate flora while, none of the lactobacilli spp. could be isolated from vaginal samples of women with BV flora. Lactobacillus delbrueckii, which could be detected by multiplex PCR, could not be isolated by culture-dependent method from any vaginal samples. In culture-dependent method L. iners, L. jensenii and L. crispatus were detected only in 33, 39 and 61 per cent compared to 78.3, 70.2 and 91.8 per cent by multiplex PCR method. L. gasseri (50%) and L. reuteri (28%) isolation rates in MRS broth, however, were similar to multiplex PCR (L. gasseri, 51%; L. reuteri, 22%). L. paracasei on the other hand, which was not detected by multiplex PCR was isolated from eight per cent of pregnant women.
Our findings showed that L. crispatus, L. iners, L. gasseri, L. jensenii and L. vaginalis dominated the vaginal microbiota of Indian women, which was similar to those found in European and Brazilian women2,22. A similar Lactobacillus spp. profile in vagina has been reported from South Africa24. In our study, L. helveticus was identified more commonly in Indian women with normal flora which was less frequent in several other studies25,26.
An association between the presence of L. crispatus and absence of BV has been shown27. Association of L. crispatus has been observed with stability of the vaginal microbiota28. Several clinical trials have been performed to investigate the efficacy of specific strains of L. rhamnosus, L. fermentum and L. reuteri administered either orally or intravaginally in treating BV or urogenital infections27. L. fermentum and L. rhamnosus probiotic strains have been used with poor results in preventing BV26. Their uncommon presence in the vagina as observed in the current study and uncertain role in vaginal health may be the reason for the failure of efficacy with L. fermentum and L. rhamnosus.
The presence of L. gasseri, L. vaginalis and L. iners in women with intermediate and BV flora as observed in the current study could be due to their poorer colonization resistance to pathogens or inadequate production of antimicrobial substances, thereby allowing overgrowth of other pathogenic bacteria. Longitudinal studies in pregnant women have also shown that women harbouring Lactobacillus spp., particularly L. gasseri and L. iners, are more susceptible to BV compared to those colonized by L. crispatus26,28. Similar findings were observed in the present study, but L. vaginalis was also found to be commonly associated with intermediate and BV flora.
There are many commercially available probiotic strains for BV treatment. Most of the strains (L. acidophilus, L. casie, L. plantarum, L. lactis, L. jensenii and Bifidobacterium bifidum, Bifidobacterium infantis, etc.) that are available on the market are not frequently found in women with normal vaginal flora26. From our observations, it may be speculated that L. iners, L. gasseri and L. vaginalis may become a dominant part of the vaginal microbiota when the microbiota is in a transitional stage from normal to abnormal vaginal flora. Hence, L. crispatus individually or in combination with L. jensenii, L. helveticus and L. acidophilus, may be evaluated for probiotic potential to combat BV.
Acknowledgment
Authors acknowledge the Science and Engineering Research Board, Department of Science and Technology, Government of India, New Delhi, for providing the financial assistance (Project No: SB/FT/LS-272/2012). The authors thank Government Maternity Hospital, Hyderabad, for providing sample and Shrimati Nancharamma for assistance in sampling.
Footnotes
Conflicts of Interest: None.
References
- 1.Ma B, Forney LJ, Ravel J. Vaginal microbiome: Rethinking health and disease. Annu Rev Microbiol. 2012;66:371–89. doi: 10.1146/annurev-micro-092611-150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, et al. The vaginal microbiome: New information about genital tract flora using molecular based techniques. BJOG. 2011;118:533–49. doi: 10.1111/j.1471-0528.2010.02840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koumans EH, Markowitz LE, Hogan V, CDC BV Working Group Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: A synthesis of data. Clin Infect Dis. 2002;35:S152–72. doi: 10.1086/342103. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert NM, Lewis WG, Lewis AL. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 2013;8:e59539. doi: 10.1371/journal.pone.0059539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 6.Hemalatha R, Ramalaxmi BA, Swetha E, Balakrishna N, Mastromarino P. Evaluation of vaginal pH for detection of bacterial vaginosis. Indian J Med Res. 2013;138:354–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Lata I, Pradeep Y, Sujata, Jain A. Estimation of the incidence of bacterial vaginosis and other vaginal infections and its consequences on maternal/fetal outcome in pregnant women attending an antenatal clinic in a tertiary care hospital in North India. Indian J Community Med. 2010;35:285–9. doi: 10.4103/0970-0218.66855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witkin SS, Linhares IM, Giraldo P, Ledger WJ. An altered immunity hypothesis for the development of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44:554–7. doi: 10.1086/511045. [DOI] [PubMed] [Google Scholar]
- 9.Gunjan K, Anjali T. Should abnormal vaginal flora in 2nd trimester of pregnancy be treated to prevent preterm labor. MAMC J Med Sci. 2015;1:65–8. [Google Scholar]
- 10.Martínez-Peña MD, Castro-Escarpulli G, Aguilera-Arreola MG. Lactobacillus species isolated from vaginal secretions of healthy and bacterial vaginosis-intermediate Mexican women: A prospective study. BMC Infect Dis. 2013;13:189. doi: 10.1186/1471-2334-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero R, Dey SK, Fisher SJ. Preterm labor: One syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemalatha R, Ramalaxmi BA, Krishnaswetha G, Kumar PU, Rao DM, Balakrishna N, et al. Cervicovaginal inflammatory cytokines and sphingomyelinase in women with and without bacterial vaginosis. Am J Med Sci. 2012;344:35–9. doi: 10.1097/MAJ.0b013e318235597b. [DOI] [PubMed] [Google Scholar]
- 13.Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, Krishna TP, et al. Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol. 2006;70:133–41. doi: 10.1016/j.jri.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Angeles-López M, García-Cano Ramos E, Aquino Santiago C. Hydrogen peroxide production and resistance to nonoxinol-9 in Lactobacillus spp.isolated from the vagina of reproductive-age women. Rev Latinoam Microbiol. 2001;43:171–6. [PubMed] [Google Scholar]
- 15.Dimitonova SP, Bakalov BV, Aleksandrova-Georgieva RN, Danova ST. Phenotypic and molecular identification of lactobacilli isolated from vaginal secretions. J Microbiol Immunol Infect. 2008;41:469–77. [PubMed] [Google Scholar]
- 16.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dover SE, Aroutcheva AA, Faro S, Chikindas ML. Natural antimicrobials and their role in vaginal health: A short review. Int J Probiotics Prebiotics. 2008;3:219–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation. Guidelines for the management of sexually transmitted infections. Geneva: WHO; 2003. [Google Scholar]
- 20.Kumar M, Rakesh S, Nagpal R, Hemalatha R, Ramakrishna A, Sudarshan V, et al. Probiotic Lactobacillus rhamnosus GG and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition. 2013;29:574–9. doi: 10.1016/j.nut.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Garg KB, Ganguli I, Das R, Talwar GP. Spectrum of Lactobacillus species present in healthy vagina of Indian women. Indian J Med Res. 2009;129:652–7. [PubMed] [Google Scholar]
- 22.Brolazo EM, Leite DS, Tiba MR, Villarroel M, Marconi C, Simoes JA, et al. Correlation between API 50 CH and multiplex polymerase chain reaction for the identification of vaginal lactobacilli in isolates. Braz J Microbiol. 2011;42:225–32. doi: 10.1590/S1517-83822011000100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Daroczy K, Xiao B, Yu L, Chen R, Liao Q, et al. Qualitative and semiquantitative analysis of Lactobacillus species in the vaginas of healthy fertile and postmenopausal Chinese women. J Med Microbiol. 2012;61:729–39. doi: 10.1099/jmm.0.038687-0. [DOI] [PubMed] [Google Scholar]
- 24.Pascual LM, Daniele MB, Pájaro C, Barberis L. Lactobacillus species isolated from the vagina: Identification, hydrogen peroxide production and nonoxynol-9 resistance. Contraception. 2006;73:78–81. doi: 10.1016/j.contraception.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 25.Damelin LH, Paximadis M, Mavri-Damelin D, Birkhead M, Lewis DA, Tiemessen CT, et al. Identification of predominant culturable vaginal Lactobacillus species and associated bacteriophages from women with and without vaginal discharge syndrome in South Africa. J Med Microbiol. 2011;60:180–3. doi: 10.1099/jmm.0.024463-0. [DOI] [PubMed] [Google Scholar]
- 26.Anukam K, Osazuwa E, Ahonkhai I, Ngwu M, Osemene G, Bruce AW, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: Randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450–4. doi: 10.1016/j.micinf.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Antonio MA, Meyn LA, Murray PJ, Busse B, Hillier SL. Vaginal colonization by probiotic Lactobacillus crispatus CTV-05 is decreased by sexual activity and endogenous Lactobacilli. J Infect Dis. 2009;199:1506–13. doi: 10.1086/598686. [DOI] [PubMed] [Google Scholar]
- 28.Wilks M, Wiggins R, Whiley A, Hennessy E, Warwick S, Porter H, et al. Identification and H2O2 production of vaginal lactobacilli from pregnant women at high risk of preterm birth and relation with outcome. J Clin Microbiol. 2004;42:713–7. doi: 10.1128/JCM.42.2.713-717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


