Abstract
Current corneal assessment technologies make the process of corneal evaluation extremely fast and simple, and several devices and technologies show signs that help in identification of different diseases thereby, helping in diagnosis, management, and follow-up of patients. The purpose of this review is to present and update readers on the evaluation of cornea and ocular surface. This first part reviews a description of slit lamp biomicroscopy (SLB), endothelial specular microscopy, confocal microscopy, and ultrasound biomicroscopy examination techniques and the second part describes the corneal topography and tomography, providing up-to-date information on the clinical recommendations of these techniques in eye care practice. Although the SLB is a traditional technique, it is of paramount importance in clinical diagnosis and compulsory when an eye test is conducted in primary or specialist eye care practice. Different techniques allow the early diagnosis of many diseases, especially when clinical signs have not yet become apparent and visible with SLB. These techniques also allow for patient follow-up in several clinical conditions or diseases, facilitating clinical decisions and improving knowledge regarding the corneal anatomy.
Keywords: Confocal microscopy, slit lamp biomicroscopy, specular microscopy, ultrasound biomicroscopy
Current corneal assessment technologies make the process of corneal evaluation extremely fast and simple.[1] Corneal assessment requires the use of several devices and technologies to identify signs of disease, plan treatment, and assist in follow-up visits to monitor changes
The most common device used in eye examinations to explore the cornea is slit lamp biomicroscopy (SLB), which allows for detailed anterior and posterior segment assessment. However, sometimes, it is necessary to conduct additional examinations involving endothelial specular microscopy (SM), confocal microscopy (CM), ultrasound biomicroscopy (UBM), corneal topography or tomography (Placido disc, slit scanning, and/or Scheimpflug imaging technologies) to conduct the final diagnosis or complete the patients' follow-up.
The purpose of this review is to present an update on the evaluation of the cornea and ocular surface. This first part reviews a description of SLB, endothelial SM, CM, and UBM examination techniques, and in the second part, the corneal topography and tomography will be described, providing updated information on the clinical recommendations of these techniques in eye care practice.
Slit Lamp Biomicroscopy
SLB has been an essential device in eye care practice since the beginning of the 20th century.[2] This instrument allows us to identify different clinical situations, diagnose different corneal or eye pathologies, complete the pre- and post-operative assessment, and help in contact lens fitting and follow-up and other such efforts.
SLB involves two major arms, which are able to rotate independently about a common axis. The first is the observational arm or microscope that allows the direct visualization of the cornea with different magnifications (usually from ×6 to ×40, with various steps or continuously variable in the case of zoom optics). The microscope includes two individually adjustable eyepieces (objective typically with ×3 to ×3.5 magnification) to compensate for the examiner's uncorrected spherical ametropia. Nowadays, it is common for the observation arm to include a digital camera, which is connected to a computer to save patients' pictures or video. Second, SLB has an independent illumination arm centered on the focal plane of the microscope, which allows one to modify the illumination angle with the observation arm, tilt of the light beam, thickness of the beam, the level of illumination, and also to introduce different filters, usually red-free (green), useful to increase contrast in blood vessel or hemorrhages or to view Fleischer ring in keratoconus- and cobalt blue and others (neutral density – useful to decrease brightness, for example, in photosensitive patients, polarizing – reduce patient discomfort).[3,4]
Knowledge of the SLB components and controls is fundamental to linking it with the anatomical structures to be observed and choosing the best configurations of illumination (direct, indirect or proximal, retro, and sclerotic scatter) and illumination features [Table 1], thickness of the beam (wide beam, narrow beam of approximately 1 mm parallelepiped or until almost closed [0.1–0.4 mm] - optic section), shape of the beam (rectangular, conical, or circular beam), angle between illumination and magnification arms, and magnification.
Table 1.
Summary of illumination and observation techniques for slit lamp biomicroscopy of the anterior eye. Illumination and observation techniques may been combining
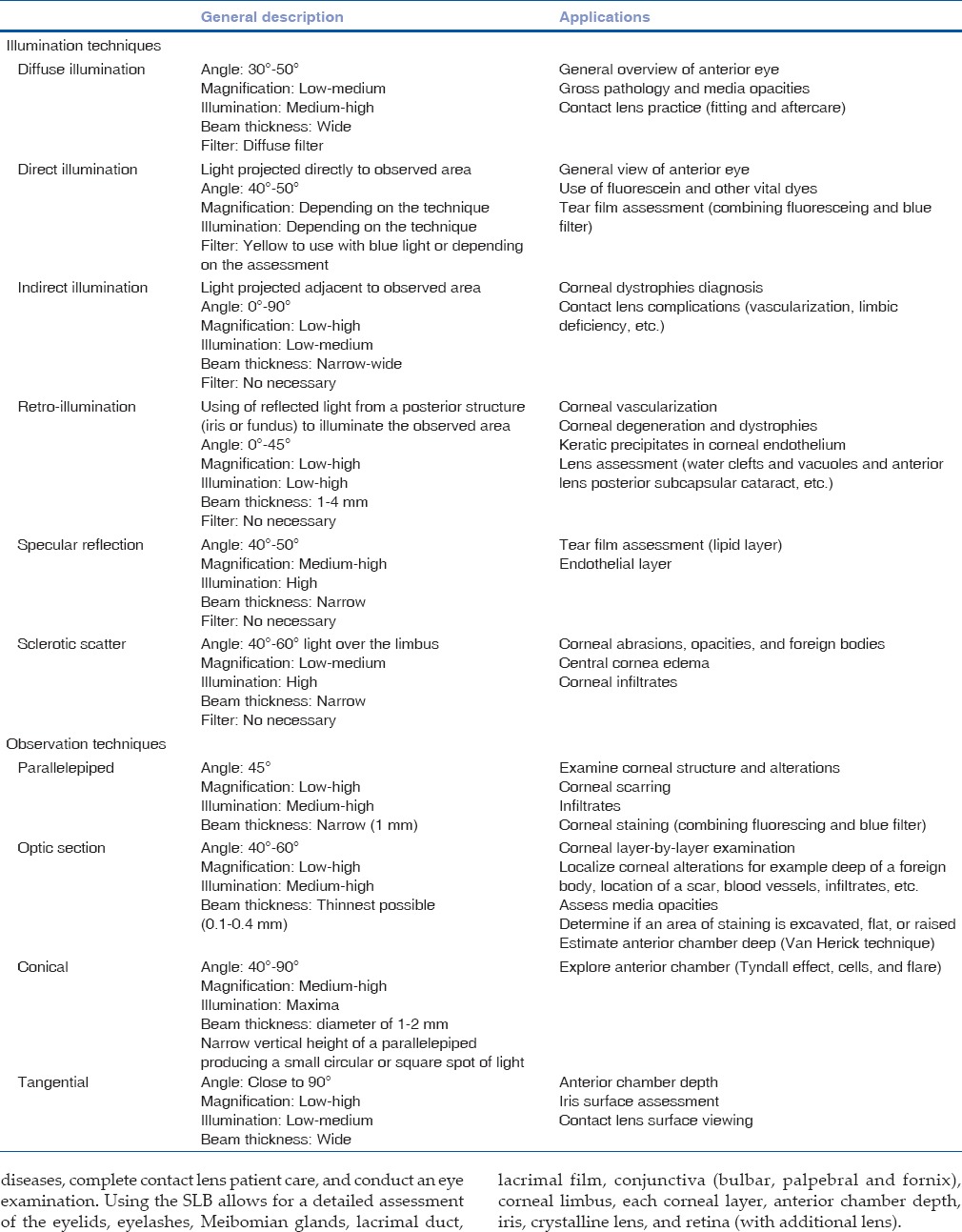
Direct illumination (when the light is projected directly on the point of interest) allows for different corneal assessment. For example, the complete opening of the slit lamp beam with low intensity to avoid dazzling the patient is recommended when conducting a general overview with low magnification at the beginning of the examination. This illumination is commonly used in contact lens practice, with fluorescein and other vital dyes (for example, in dry eye patient assessment, keratitis diagnosis, and gas permeable contact lens fitting). However, with narrow beam (parallelepiped or optical section), a more detailed assessment of the cornea, lens, and media can be completed [Fig. 1]. To complete a layer-by-layer viewing of the cornea, it is recommended that one modify the angle between illumination and observation arms.
Figure 1.
Slit section in normal cornea showing the tear film/epithelium, anterior and posterior stroma, endothelium, anterior chamber with aqueous humor, iris, pupil, tear meniscus, eyelid, and eyelashes
Indirect illumination (when light is projected just adjacent to the point of interest, which is illuminated by scattered light) is usually conducted with narrow beam to complete corneal dystrophies diagnosis, contact lens complication (vascularization), limbic deficiency, and other procedures. Slight side-to-side movements of the joystick to compare the appearance of an area in direct and indirect illumination are recommended for the detection and study of structural details that may be “washed out” under direct illumination.[3]
Retroillumination (when light is reflected from the retina or iris to illuminate the point of interest from behind) allows for the detailed assessment of some corneal dystrophies (corneal epithelial basement membrane dystrophies [also called map-dot-fingerprint or Cogan dystrophy], Meesmann dystrophy, Lattice corneal dystrophy), corneal edema, or blood vessels (corneal pannus or limbal stem cell deficiency). This technique allows opacity media assessment as well. A particular type of retroillumination called marginal retroillumination is useful for the observation of refractive abnormalities in the cornea, such as epithelial microcysts or vacuoles.[4,5]
Specular reflection (specific parallelepiped when the angle of the beam is equal to the microscope typically 40°–50° and light is moved until a very bright reflex is obtained) is generally used to visualize tear film (lipid layer) or endothelium.
Sclerotic scatter (when the beam is focused on the corneal limbus and produced by a fiber-optic effect of the cornea) is generally used to visualize central corneal edema, haze, scarring, infiltrates, and foreign bodies.[3]
SLB allows for the exploration of the lens, anterior vitreous, and retina. In this case, pupil dilatation is recommended. The retina or optic nerve may be explored in great detail with additional condensing lenses (e.g., the 60 D, 78 D, and 90 D lenses) that enable the examiner to achieve the desired magnification, which provides a magnified stereoscopic view of the retina, thus helping in optic disk and macular assessment.[6]
The eye care practitioner should conduct a standard examination routine with an anterior-to-posterior approach which should be completed and repeated on each patient, to guarantee that nothing is overlooked and all structures examined for abnormality.[3,7]
With this approach, the lashes, eyelids, and their margins are examined first (direct and diffuse illumination with low [less than, ×10] or medium magnification [between ×10 to ×25] is recommended in this step), followed by the assessment of the tear film, conjunctiva (bulbar and fornix), and cornea (parallelepiped and/or optic section with high illumination and medium-to-high magnification is recommended).[3,4] Two or three scans across the cornea, with the beam directed from both the nasal and temporal sides, superior, inferior, and center, are recommended to guarantee a complete examination. High magnification (more than ×25) is recommended for the detailed assessment of corneal findings. Anterior chamber, angle, iris, and lens scans may be conducted after corneal examination, looking to direct and indirect illuminated corneal areas.[3,4] A complete conjunctival assessment, involving palpebral conjunctiva (with direct and diffuse illumination and low-to-medium magnification is recommended) may be conducted at the end of the examination routine.[4] The use of fluorescein (and other vital dyes such as lissamine green or rose bengal) to explore under blue light and yellow filter (Kodak written #12 or similar) is recommended to complete the ocular surface assessment (epithelial integrity [corneal keratitis] and dry eye diagnosis).[4,7] Complete lens (capsule and nucleus), anterior vitreous, and retina assessment with SLB may be conducted, and in these cases, dilated pupil is recommended and compulsory if patients show symptoms of retinal detachment such as floaters or flashes.
In summary, SLB is a compulsory device in eye care consulting to diagnose and monitor corneal and anterior eye diseases, complete contact lens patient care, and conduct an eye examination. Using the SLB allows for a detailed assessment of the eyelids, eyelashes, Meibomian glands, lacrimal duct, lacrimal film, conjunctiva (bulbar, palpebral and fornix), corneal limbus, each corneal layer, anterior chamber depth, iris, crystalline lens, and retina (with additional lens).
In contact lens practice, the SLB plays a paramount role in contact lens fitting and aftercare follow-up;[8] it allows one to choose the adequate contact lens, assessing lens movement and centering, and in gas permeable contact lenses assess the tear film between lens and cornea (with fluorescein). In contact lens wearers with SLB, assessment, detection, and management of any possible complication or secondary effects related with contact lens wear are possible.[4]
Although eye assessment with SLB is a subjective affair that requires deep knowledge of eye anatomy and pathology, this instrument is indispensable in the consulting room. SLB is compulsory in eye examination to diagnose several eye diseases. Sometimes, especially in certain corneal diseases, diagnosis can only be completed with further objective examinations (endothelial SM, CM, UBM, corneal topography or tomography, and others) to reach deeper and more comprehensive evaluation of the cornea and facilitate eye care practitioners' decisions and patient management.
Specular Microscopy
SM is a technique for in vivo visualizing of the corneal endothelium, introduced at the beginning using the specular reflex light with the SLB.[9,10] Because the refractive index of the endothelial cells is greater than that of the aqueous humor,[11] the endothelial cells can be differentiated using a high-magnification image of the specular-reflected light from the corneal endothelium.[12]
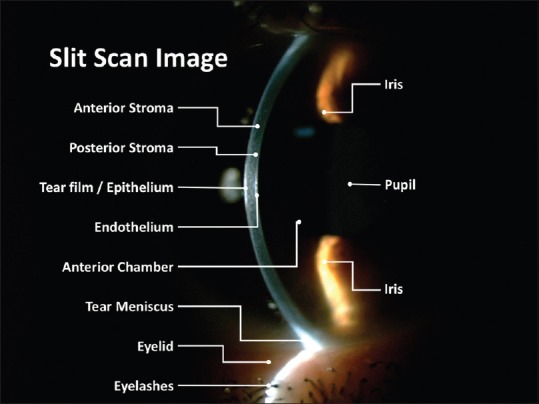
However, nowadays, specific instruments (specular microscopes) are noncontact optical instruments that include more sophisticated technology for image capture (automatic image focusing technology[12]) and analysis of the corneal endothelium (cell shape, number [density], and morphology),[12] including an interface with customized software and computers that allow better knowledge of corneal endothelium [Fig. 2], which is of paramount importance in some patient assessment (cataract surgery, penetrating or lamellar keratoplasty, etc.).[13] SM has been used in contact lens research and clinical contact lens practice to explore the increase in polymegethism[7] and the transient and chronic endothelial cell morphology changes, especially in long-term contact lens wearers.[14]
Figure 2.
Specular microscopy in healthy eye (45 years old) showing central corneal thickness, endothelial cells density, polymegethism or coefficient of variation, pleomorphism (HEX), and others outcomes
Current specular microscopes provide different outcomes that include central corneal pachymetry with an endothelial cell analysis, which includes the cell density (CD) (cells/square millimeter – an average value for adults is 2400 cells/mm2 – ranged from 1500 to 3500 – but corneas with <1000 cells/mm2 might not tolerate intraocular surgery),[7] cell morphology providing the polymegathism or coefficient of variation (>0.40 might not tolerate intraocular surgery)[7] and/or the pleomorphism (the number of six-sided [hexagonal] cells, that may be <50% to tolerate intraocular surgery),[7] and the endothelial cell loss (e.g., in endothelial disease, trauma, or chemical toxicity) measured as the increase of the endothelial individual cell surface (square micrometers), decrease of the endothelial CD and the increase of the polymegathism and/or the pleomorphism.[4]
SM is an invaluable tool to diagnose different endothelial disorders such as Fuch's dystrophy, posterior polymorphous dystrophy (PPD), or iridocorneal endothelial (ICE) syndrome. In Fuch's dystrophy, the endothelium mosaic presents black holes (known as cornea guttata).[15] In the PPD, the endothelial cells have the characteristics of epithelial cells, showing clearly defined dark rings, and the involvement is normally bilateral.[16] In eyes with ICE syndrome (which is normally unilateral), the endothelium presents abnormal cells characterized by a bright margin, a dark paracentral area, and a central spot of light.[16]
With the appearance of SM, endothelial cell changes can be detected and monitored. Furthermore, its noncontact nature allows data to be collected from people from a wide age range, included young children and the elderly.[12]
Confocal Microscopy
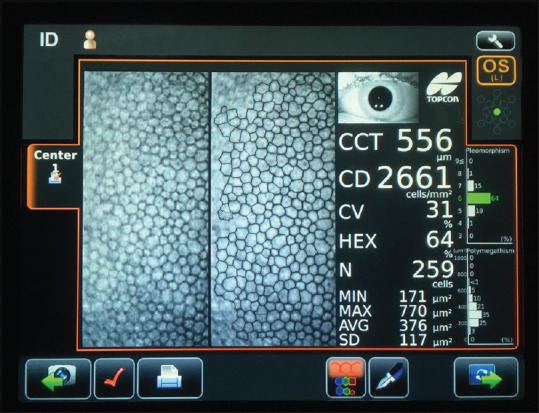
The in vivo human cornea was first examined with CM at the end of the 90s,[17] and nowadays, this technology allows us to assess the cornea at a cellular level, providing images which are comparable to in vitro histochemical techniques delineating each layer,[18,19] Including descemet's membrane, and the corneal endothelium [Fig. 3], which is important for recognizing minimal changes in the structures for early and reliable diagnoses.[5,20,21,22,23] This technique is based on the confocal principle; where the illumination and detection paths share the same focal plane (hence the term “confocal”), overcoming the problem of defocused light and avoiding the limitations in image quality achieved with a conventional light microscope.[1] Different technologies provide different depths of focus, slit scanning microscopes (26 μm), tandem scanning confocal microscopes (7-9 μm), and laser confocal microscopes (5-7 μm).
Figure 3.
Image of epithelium (a) and endothelium (b) in healthy eye. Epithelium image was captured with Heidelberg Retina Tomograph II (Heidelberg, Germany) and endothelium image with Confoscan4 (Nidek Technologies, Japan)
Some commercially available CMs are Confoscan P4 (Tomey Corporation, Cambridge, MA, USA), Confoscan 4 (Nidek Technologies, Japan), and the Heidelberg Retina Tomograph II Rostock Corneal Module (Heidelberg, Germany).[18,23,24]
The resolution and magnification of the CM images allow the imaging of the corneal cells and their nuclei; thus, any morphologic changes in cells or the presence of abnormal structures can be detected in vivo. This quality is very useful to investigate numerous corneal diseases, for example, corneal dystrophies (such as keratoconus,[23,25] Fuch's endothelial dystrophy,[26] PPD,[27] Bowman's layer dystrophies,[28,29] stromal dystrophies),[29,30] monitoring contact lens-induced corneal changes,[31] pre- and post-surgical evaluation (PRK, LASIK and LASEK, flap evaluations, and others),[18,23] diagnosis and assessment of limbal stem cell deficiency and cell grafts (aniridia, Stevens-Johnson syndrome),[32,33,34,35] monitoring penetrating keratoplasty, assessment of sub-basal nerve architecture,[36,37,38] and also of great interest in diabetic neuropathy diagnosis.[39,40,41] Other reported uses of the CM include the early diagnosis of infective keratitis (Acanthamoeba cysts).[42]
CM is a powerful diagnostic technique which allows noninvasive microscopic examination of the cornea. It is mainly used in research but has great potential in clinical practice.[18]
Ultrasound Biomicroscopy
UBM is a high-resolution technique which allows in vivo assessment of the structures of the anterior segment of the eye [Fig. 4].[43] Commercially available ultrasound biomicroscopes employ high-frequency ultrasound transducers (between 25 and 50 MHz) with an immersion technique, to obtain high-frequency B-scan ultrasonography images to a depth between 4 and 5 mm with lateral resolution of approximately 25 μm and 50 μm. Many devices include A-scan ultrasonography to measure the eye axial length (from the cornea to the retina) and explore posterior segment of the eye.
Figure 4.
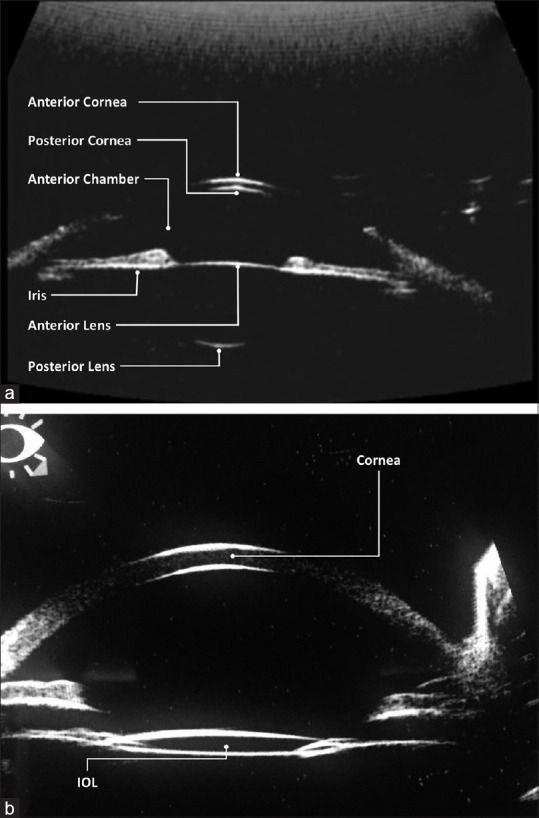
Example of ultrasound biometry (a) in healthy eye showing anterior and posterior surfaces of the cornea and lens and (b) in a pseudophakic 61-year-old male patient
UBM allows in vivo observation (qualitative and quantitative evaluation) of the anatomy and pathology of the eye from the conjunctiva, cornea, anterior chamber, iridocorneal angle, iris, zonules, ciliary body, lens, basal vitreous body, and retina, which provides significant information on several clinical situations, for example, retinal detachment, vitreous bleeding, eye tumors, inflammation, eye orbit lesions, or trauma and foreign bodies in the eye.
Moreover, UBM has been used in evaluating corneal dystrophies, corneal scars, cysts, excimer laser photokeratectomy and LASIK procedures, to explore intraocular lens (IOL) implants, glaucoma diagnosis (open-angle glaucoma, primary angle closure glaucoma included plateau iris syndrome, pigmentary glaucoma, and pupillary block glaucoma, pseudoexfoliation syndrome), explore anterior and posterior segment tumors (providing valuable information on the extension of pigmented lesions or malignant melanoma from the iris to the ciliary body), and other rare eye diseases (such as mesodermal dysgenesis of the neural crest, ICE syndrome, phakomatoses, and metabolic disorders).[43] UBM has been used in IOLs patient assessment (nonsutured and sutured fixation of IOL) measuring anterior chamber depth, sulcus-to-sulcus diameter, capsular bag thickness, capsular bag diameter, ciliary ring diameter, ciliary process-capsular bag distance, ciliary apex-capsular bag plane, IOL tilting, haptic location, iris-IOL contact, vitreous incarceration, and conducting a scleral fixation assessment.[44,45]
High-frequency digital UBM or very high-frequency digital UBM (Artemis [ArcScan Inc, Morrison, Colorado]) is a recent technology that allows in vivo corneal epithelium measurement,[46,47,48] stromal measurement,[49] flap and residual stromal bed measurement in LASIK procedures,[50] and in anterior or posterior lamellar keratoplasty,[51,52,53,54] for the whole cornea; providing valuable information in keratoconus screening.[55,56,57] Anterior and posterior chamber dimensions[58] or the assessment of different glaucomatous diseases (including pupillary block or plateau iris syndrome) are, also, possible with this technology.
Conclusion
Improvement in technology for corneal assessment has made the process of corneal evaluation extremely fast and simple. The SLB is a traditional technique of paramount importance in clinical diagnosis and compulsory when an eye test is conducted in primary or specialist eye care practice. However, different techniques allow for an improvement in early diagnosis of many diseases, especially when clinical signs have not yet become apparent and visible when using SLB. These techniques also allow for a careful patient follow-up in several clinical conditions or diseases, facilitating clinical decisions and improving knowledge regarding the corneal anatomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rio-Cristobal A, Martin R. Corneal assessment technologies: Current status. Surv Ophthalmol. 2014;59:599–614. doi: 10.1016/j.survophthal.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Brody J, Waller S, Wagoner M. Corneal topography: History, technique, and clinical uses. Int Ophthalmol Clin. 1994;34:197–207. doi: 10.1097/00004397-199403430-00018. [DOI] [PubMed] [Google Scholar]
- 3.Fleming JB, Semes LP. Benjamin WJ. Borish's Clinical Refraction. 2nd ed. St Louis, Missouri (USA): Butterworth-Heinemann-Elsevier; 2006. Anterior segment evaluation. [Google Scholar]
- 4.Efron N. Contact Lens Complications. 3rd ed. New York (USA): Elsevier Saunders; 2012. [Google Scholar]
- 5.Efron N. Contact lens-induced changes in the anterior eye as observed in vivo with the confocal microscope. Prog Retin Eye Res. 2007;26:398–436. doi: 10.1016/j.preteyeres.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Flanagan JG, Prokopich CL. Indirect fundus biomicroscopy. Ophthalmic Physiol Opt. 1995;15(Suppl 2):S38–41. [PubMed] [Google Scholar]
- 7.Liesegang TJ, Skuta GL, Cantor LB. External Disease and Cornea. San Francisco (USA): American Academy Ophthalmology; 2005. [Google Scholar]
- 8.American Optometric Association. Optometric Clinical Practice Guideline: Care of the Contact Lens Patient. 2006. [Last accessed on 2017 Jul 25]. Available from: http://www.aoa.org/documents/optometrists/CPG-19.pdf .
- 9.Bourne WM. Corneal endothelium – Past, present, and future. Eye Contact Lens. 2010;36:310–4. doi: 10.1097/ICL.0b013e3181ee1450. [DOI] [PubMed] [Google Scholar]
- 10.Brooks AM, Grant G, Gillies WE. Specular microscopy in the identification of deep corneal opacities. Surv Ophthalmol. 1992;36:351–6. doi: 10.1016/0039-6257(92)90112-7. [DOI] [PubMed] [Google Scholar]
- 11.Maurice DM. Cellular membrane activity in the corneal endothelium of the intact eye. Experientia. 1968;24:1094–5. doi: 10.1007/BF02147776. [DOI] [PubMed] [Google Scholar]
- 12.McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27:1–6. doi: 10.1097/ICO.0b013e31815892da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acar BT, Buttanri IB, Sevim MS, Acar S. Corneal endothelial cell loss in post-penetrating keratoplasty patients after cataract surgery: Phacoemulsification versus planned extracapsular cataract extraction. J Cataract Refract Surg. 2011;37:1512–6. doi: 10.1016/j.jcrs.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Pflugfelder SC. The effects of long-term contact lens wear on corneal thickness, curvature, and surface regularity. Ophthalmology. 2000;107:105–11. doi: 10.1016/s0161-6420(99)00027-5. [DOI] [PubMed] [Google Scholar]
- 15.Bourne WM, McLaren JW. Clinical responses of the corneal endothelium. Exp Eye Res. 2004;78:561–72. doi: 10.1016/j.exer.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Laganowski HC, Sherrard ES, Muir MG, Buckley RJ. Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: Value of endothelial specular microscopy. Br J Ophthalmol. 1991;75:212–6. doi: 10.1136/bjo.75.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cavanagh HD, El-Agha MS, Petroll WM, Jester JV. Specular microscopy, confocal microscopy, and ultrasound biomicroscopy: Diagnostic tools of the past quarter century. Cornea. 2000;19:712–22. doi: 10.1097/00003226-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Tavakoli M, Hossain P, Malik RA. Clinical applications of corneal confocal microscopy. Clin Ophthalmol. 2008;2:435–45. doi: 10.2147/opth.s1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruzat A, Pavan-Langston D, Hamrah P. In vivo confocal microscopy of corneal nerves: Analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–7. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guthoff RF, Zhivov A, Stachs O. In vivo confocal microscopy, an inner vision of the cornea-A major review. Clin Exp Ophthalmol. 2009;37:100–17. doi: 10.1111/j.1442-9071.2009.02016.x. [DOI] [PubMed] [Google Scholar]
- 21.Böhnke M, Masters BR. Long-term contact lens wear induces a corneal degeneration with microdot deposits in the corneal stroma. Ophthalmology. 1997;104:1887–96. doi: 10.1016/s0161-6420(97)30011-6. [DOI] [PubMed] [Google Scholar]
- 22.Niederer RL, McGhee CN. Clinical in vivo confocal microscopy of the human cornea in health and disease. Prog Retin Eye Res. 2010;29:30–58. doi: 10.1016/j.preteyeres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Patel DV, McGhee CN. Contemporary in vivo confocal microscopy of the living human cornea using white light and laser scanning techniques: A major review. Clin Exp Ophthalmol. 2007;35:71–88. doi: 10.1111/j.1442-9071.2007.01423.x. [DOI] [PubMed] [Google Scholar]
- 24.Erie JC, McLaren JW, Patel SV. Confocal microscopy in ophthalmology. Am J Ophthalmol. 2009;148:639–46. doi: 10.1016/j.ajo.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Efron N, Hollingsworth JG. New perspectives on keratoconus as revealed by corneal confocal microscopy. Clin Exp Optom. 2008;91:34–55. doi: 10.1111/j.1444-0938.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman SC, Beuerman RW, Kaufman HE. Diagnosis of advanced Fuchs' endothelial dystrophy with the confocal microscope. Am J Ophthalmol. 1993;116:652–3. doi: 10.1016/s0002-9394(14)73217-9. [DOI] [PubMed] [Google Scholar]
- 27.Grupcheva CN, Chew GS, Edwards M, Craig JP, McGhee CN. Imaging posterior polymorphous corneal dystrophy by in vivo confocal microscopy. Clin Exp Ophthalmol. 2001;29:256–9. doi: 10.1046/j.1442-9071.2001.00432.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi A, Sugiyama K. In vivo laser confocal microscopy findings for Bowman's layer dystrophies (Thiel-behnke and Reis-Bücklers corneal dystrophies) Ophthalmology. 2007;114:69–75. doi: 10.1016/j.ophtha.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 29.Werner LP, Werner L, Dighiero P, Legeais JM, Renard G. Confocal microscopy in Bowman and stromal corneal dystrophies. Ophthalmology. 1999;106:1697–704. doi: 10.1016/S0161-6420(99)90358-5. [DOI] [PubMed] [Google Scholar]
- 30.Roszkowska AM, Aragona P, Spinella R, Pisani A, Puzzolo D, Micali A, et al. Morphologic and confocal investigation on Salzmann nodular degeneration of the cornea. Invest Ophthalmol Vis Sci. 2011;52:5910–9. doi: 10.1167/iovs.11-7789. [DOI] [PubMed] [Google Scholar]
- 31.Martin R, De Juan V, Rodríguez G, Cuadrado R, Fernandez I. Measurement of corneal swelling variations without removal of the contact lens during extended wear. Invest Ophthalmol Vis Sci. 2007;48:3043–50. doi: 10.1167/iovs.06-1372. [DOI] [PubMed] [Google Scholar]
- 32.Pedrotti E, Passilongo M, Fasolo A, Nubile M, Parisi G, Mastropasqua R, et al. In vivo confocal microscopy 1 year after autologous cultured limbal stem cell grafts. Ophthalmology. 2015;122:1660–8. doi: 10.1016/j.ophtha.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Mastropasqua L, Calienno R, Lanzini M, Nubile M, Colabelli-Gisoldi RA, De Carlo L, et al. In vivo confocal microscopy of the sclerocorneal limbus after limbal stem cell transplantation: Looking for limbal architecture modifications and cytological phenotype correlations. Mol Vis. 2016;22:748–60. [PMC free article] [PubMed] [Google Scholar]
- 34.Shortt AJ, Bunce C, Levis HJ, Blows P, Doré CJ, Vernon A, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl Med. 2014;3:265–75. doi: 10.5966/sctm.2013-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nubile M, Lanzini M, Miri A, Pocobelli A, Calienno R, Curcio C, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol. 2013;155:220–32. doi: 10.1016/j.ajo.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 36.Patel DV, McGhee CN. Mapping the corneal sub-basal nerve plexus in keratoconus by in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2006;47:1348–51. doi: 10.1167/iovs.05-1217. [DOI] [PubMed] [Google Scholar]
- 37.Hollingsworth JG, Bonshek RE, Efron N. Correlation of the appearance of the keratoconic cornea in vivo by confocal microscopy and in vitro by light microscopy. Cornea. 2005;24:397–405. doi: 10.1097/01.ico.0000151548.46231.27. [DOI] [PubMed] [Google Scholar]
- 38.Somodi S, Hahnel C, Slowik C, Richter A, Weiss DG, Guthoff R, et al. Confocal in vivo microscopy and confocal laser-scanning fluorescence microscopy in keratoconus. Ger J Ophthalmol. 1996;5:518–25. [PubMed] [Google Scholar]
- 39.Edwards K, Pritchard N, Dehghani C, Vagenas D, Russell A, Malik RA, et al. Corneal confocal microscopy best identifies the development and progression of neuropathy in patients with type 1 diabetes. J Diabetes Complications. 2017;31:1325–7. doi: 10.1016/j.jdiacomp.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 2015;10:e0123517. doi: 10.1371/journal.pone.0123517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Efron N. The Glenn A. Fry award lecture 2010: Ophthalmic markers of diabetic neuropathy. Optom Vis Sci. 2011;88:661–83. doi: 10.1097/OPX.0b013e3182171020. [DOI] [PubMed] [Google Scholar]
- 42.Parmar DN, Awwad ST, Petroll WM, Bowman RW, McCulley JP, Cavanagh HD, et al. Tandem scanning confocal corneal microscopy in the diagnosis of suspected Acanthamoeba keratitis. Ophthalmology. 2006;113:538–47. doi: 10.1016/j.ophtha.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 43.Mannino G, Abdolrahimzadeh B, Calafiore S, Anselmi G, Mannino C, Lambiase A, et al. A review of the role of ultrasound biomicroscopy in glaucoma associated with rare diseases of the anterior segment. Clin Ophthalmol. 2016;10:1453–9. doi: 10.2147/OPTH.S112166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horiguchi L, Garcia PN, Malavazzi GR, Allemann N, Gomes RL. Ultrasound biomicroscopy comparison of Ab interno and Ab externo intraocular lens scleral fixation. J Ophthalmol. 2016;2016:9375091. doi: 10.1155/2016/9375091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao S, Qin T, Wang S, Lu Y. Sulcus fixation of foldable intraocular lenses guided by ultrasound biomicroscopy. J Ophthalmol. 2015;2015:520418. doi: 10.1155/2015/520418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, et al. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16:414–30. doi: 10.3928/1081-597X-20000701-04. [DOI] [PubMed] [Google Scholar]
- 47.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Repeatability of layered corneal pachymetry with the artemis very high-frequency digital ultrasound arc-scanner. J Refract Surg. 2010;26:646–59. doi: 10.3928/1081597X-20091105-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogbuehi KC, Osuagwu UL. Repeatability and interobserver reproducibility of artemis-2 high-frequency ultrasound in determination of human corneal thickness. Clin Ophthalmol. 2012;6:761–9. doi: 10.2147/OPTH.S31690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reinstein DZ, Srivannaboon S, Archer TJ, Silverman RH, Sutton H, Coleman DJ, et al. Probability model of the inaccuracy of residual stromal thickness prediction to reduce the risk of ectasia after LASIK part I: Quantifying individual risk. J Refract Surg. 2006;22:851–60. doi: 10.3928/1081-597X-20061101-04. [DOI] [PubMed] [Google Scholar]
- 50.Reinstein DZ, Archer TJ, Gobbe M. LASIK flap thickness profile and reproducibility of the standard vs. zero compression Hansatome microkeratomes: Three-dimensional display with Artemis VHF digital ultrasound. J Refract Surg. 2011;27:417–26. doi: 10.3928/1081597X-20101110-01. [DOI] [PubMed] [Google Scholar]
- 51.Reinstein DZ, Gobbe M, Archer TJ. Anterior segment biometry: A study and review of resolution and repeatability data. J Refract Surg. 2012;28:509–20. doi: 10.3928/1081597X-20120620-02. [DOI] [PubMed] [Google Scholar]
- 52.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness in the normal cornea: Three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2008;24:571–81. doi: 10.3928/1081597X-20080601-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Stromal thickness in the normal cornea: Three-dimensional display with artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25:776–86. doi: 10.3928/1081597X-20090813-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dupps WJ, Jr, Qian Y, Meisler DM. Multivariate model of refractive shift in Descemet-stripping automated endothelial keratoplasty. J Cataract Refract Surg. 2008;34:578–84. doi: 10.1016/j.jcrs.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanellopoulos AJ, Aslanides IM, Asimellis G. Correlation between epithelial thickness in normal corneas, untreated ectatic corneas, and ectatic corneas previously treated with CXL; is overall epithelial thickness a very early ectasia prognostic factor? Clin Ophthalmol. 2012;6:789–800. doi: 10.2147/OPTH.S31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604–10. doi: 10.3928/1081597X-20090610-06. [DOI] [PubMed] [Google Scholar]
- 57.Konstantopoulos A, Hossain P, Anderson DF. Recent advances in ophthalmic anterior segment imaging: A new era for ophthalmic diagnosis? Br J Ophthalmol. 2007;91:551–7. doi: 10.1136/bjo.2006.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rondeau MJ, Barcsay G, Silverman RH, Reinstein DZ, Krishnamurthy R, Chabi A, et al. Very high frequency ultrasound biometry of the anterior and posterior chamber diameter. J Refract Surg. 2004;20:454–64. doi: 10.3928/1081-597X-20040901-08. [DOI] [PubMed] [Google Scholar]


