Abstract
A careful examination of cornea and ocular surface eliciting the basic signs will help a clinician toward an accurate diagnosis. Flipping the upper lid or pulling the lower lid to look at the inferior fornix may help to pick up any subtle clinical sign. Meticulous documentation by diffuse and slit view will help in following up the disease. Eyelids and ocular surface are evaluated externally and by slit lamp. Slit-lamp examination with the use of the stains such as fluorescein, rose bengal, or lissamine green provides extensive knowledge about the ocular surface. Tests of tear production are also detailed herein. This review is intended to help the eye practitioners in eliciting common clinical signs seen in cornea and ocular surface diseases.
Keywords: Clinical signs, cornea, corneal diagram, follicles, papillae, tear film
Conjunctival Signs of Inflammation
Conjunctival signs of inflammation can be nonspecific and specific. Nonspecific signs include hyperemia and papillae <1 mm. Specific signs of conjunctival inflammation include giant papillae, follicles, and membranes.
Papillae are elevations of hyperplastic epithelium with a central vascular core [Figs. 1a and 2a]. It is pink or red in color with white borders. Causes of papillae include allergic conjunctivitis, vernal keratoconjunctivitis, atopic keratoconjunctivitis, and giant papillary conjunctivitis caused by sutures, prosthesis, foreign body, etc.
Figure 1.
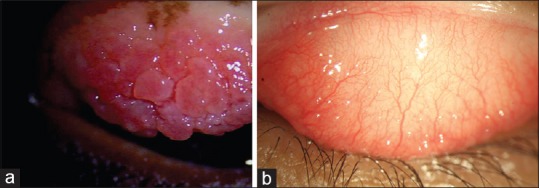
(a) Diffuse slit-lamp biomicroscope view of upper tarsus showing giant papillae and (b) follicles
Figure 2.
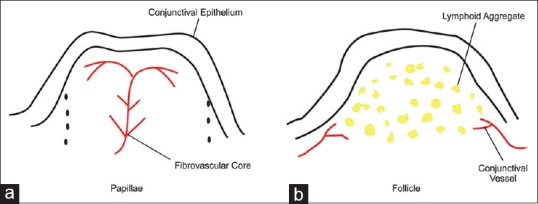
(a) Cross-sectional diagram of conjunctival papilla with a central vascular tuft surrounded by acute and chronic leukocytes. (b) Cross-sectional diagram of mononuclear cells obscuring conjunctival blood vessels
Follicles present as smooth, sago grain-like elevation in the conjunctiva representing lymphoid tissue aggregation [Figs. 1b and 2b]. Causes include adenoviral conjunctivitis and herpes simplex infection, toxic reactions to medications, chlamydial infections, Molluscum, Trachoma, and Parinaud's ocular glandular syndrome.
Conditions mimicking follicles include benign lymphoid hyperplasia, lymphoma, leukemia, and amyloidosis.
Membranes are made of fibrin, protein, and polymorphs. True membranes peel with bleeding and pseudomembranes peel without bleeding. Causes of membrane include severe conjunctivitis (adenoviral, streptococcus, gonococcus, and diphtheria), severe chemical burns, Stevens–Johnson's syndrome, and cicatricial pemphigoid.
Phlyctenules are localized acute nodular infiltrates located on the conjunctiva, limbus, or peripheral cornea. In the cornea, it is a triangular infiltrate with the base at the limbus.
Corneal Signs of Inflammation
Filaments are formed because of epithelium growing over a central core of mucin [Fig. 3]. Patients experience pain on blinking. Causes of filament are aqueous tear deficiency (ATD), superior limbic keratoconjunctivitis (SLK), postcataract surgery, patching, and herpes simplex keratitis.
Corneal vascularization can be superficial or deep or both. “Superficial” blood vessels cross the limbus. Causes include contact lens, blepharitis, herpes simplex infection, rosacea, phlyctenules, and atopic keratoconjunctivitis. “Deep corneal vascularization” does not cross the limbus. Causes are chronic inflammation and edema in interstitial keratitis due to congenital syphilis, herpes simplex, herpes zoster, Lyme disease, and measles.
-
Corneal stromal infiltration: Marginal infiltrates involve the peripheral cornea separated from the limbus by a clear zone. There is no minimal overlying staining. Sterile immune reactions occur to various antigens, especially staphylococcus.
Peripheral infiltrates in soft contact lens wearers occur due to hypoxia, reaction to contact lens solution preservatives, and reaction to bacteria antigens.
Infected infiltrates are associated with pain, anterior chamber reaction, corneal edema, and epithelial defect.
Corneal scarring: There is a loss of regular lamellar arrangement of collagen resulting in loss of clarity on slit view and thinning of cornea can be seen.
Keratic precipitates (KPs): KPs can be “fine,” or “mutton-fat” KPs are cells seen on the back of the cornea. Fine KPs are polymorphonuclear cells and lymphocytes. It is seen in nongranulomatous uveitis. Mutton fats are granulomatous KPs which are macrophages seen in graft rejection, herpes simplex, herpes zoster, and sarcoid.
Figure 3.
Diffuse slit-lamp biomicroscope view showing filament on inferior cornea
Corneal diagrams are important in day-to-day clinics to document the corneal finding [Table 1]. It is extremely important for following up cornea patients in busy practice involving more than one clinician.
Table 1.
Color codes used in corneal diagrams

Black color is used to denote corneal scars, degeneration, deposit, guttata, contact lens (dotted line), foreign body, tissue adhesive, corneal nerves, Vogt's striae, and intraocular lens [Fig. 4].
Figure 4.
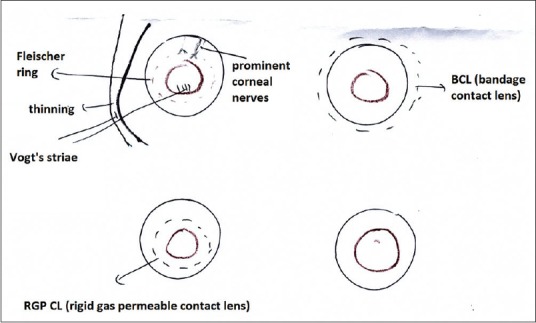
Corneal diagram showing keratoconus and contact lens represented by black color
Blue color is used for epithelial edema, epithelial bullae, and stromal edema and Descemet's folds [Fig. 5].
Figure 5.
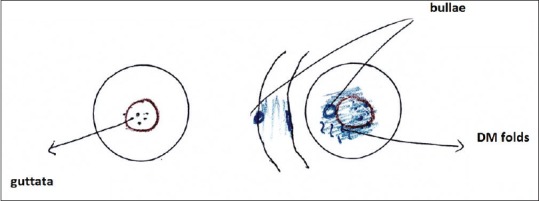
Corneal diagram showing central stromal edema and Descemet's folds
Yellow color is used to denote infiltrate, hypopyon, KPs, and lens (cataract) [Fig. 6].
Figure 6.
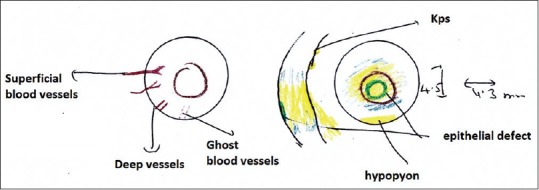
Corneal diagram showing infiltrate and epithelial defect
Green color is used for epithelial defect, fluorescein staining, superficial punctate keratopathies, filaments, and vitreous.
Brown color is used for pigment, iris, pupil, and peripheral anterior synechiae.
Signs of limbal stem cell deficiency are a loss of palisades of Vogt, dull or irregular corneal reflex, epithelium of variable thickness, and opacification and surface staining. Stroma may show blood vessels and opacification. In severe cases, fibrovascular pannus, and calcification on the surface of cornea may be seen.
Examination of the Eyelids and Ocular Surface
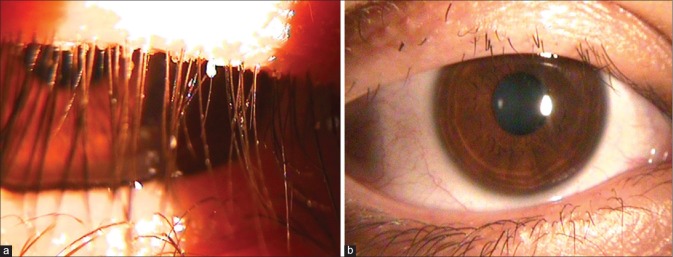
The eyelids should be examined externally and with the slit lamp. The external examination reveals blinking rate, lagophthalmus, or gross anatomical problems. The slit-lamp examination provides detailed knowledge about Meibomian gland orifices, eyelash problems, and ocular surface. Demodex infestation may lead to cylindrical dandruff-like scaling (collarettes) around the base of the eyelashes though this is not always present [Fig. 7a]. Loss (madarosis) [Fig. 7b] and whitening (poliosis) of lashes, eyelid crusting, and eyelid ulceration are associated with blepharitis. During slit-lamp examination, the eyelids should be everted to check for papilla, scars, or hyperemia. Eyelid keratinization such as in Stevens–Johnson syndrome causes blink-related microtrauma and results in chronic ocular surface inflammation that is difficult to manage. Furthermore, the inferior fornix should be examined for follicles or symblepharon by pulling down the lower eyelid.[1,2]
Figure 7.
(a) Collarettes and (b) madarosis
The slit-lamp examination begins with direct illumination of the eyelids (margin, Meibomian glands, and eyelashes), conjunctiva, and sclera. The cornea and the overlying tear film are examined with a broad beam and then a narrow beam. The height of the tear meniscus, mucin cells, and other debris in the tear film are evaluated. Discrete lesions are measured with a slit-beam micrometer. Multiple illumination methods should be used to bring out details.[1]
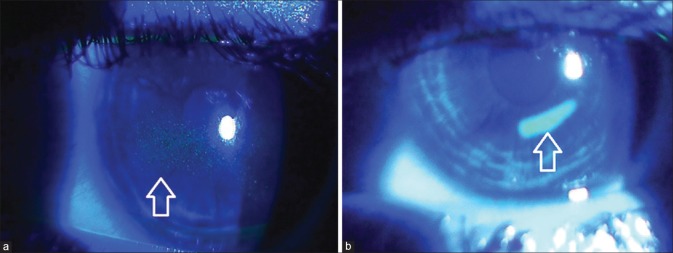
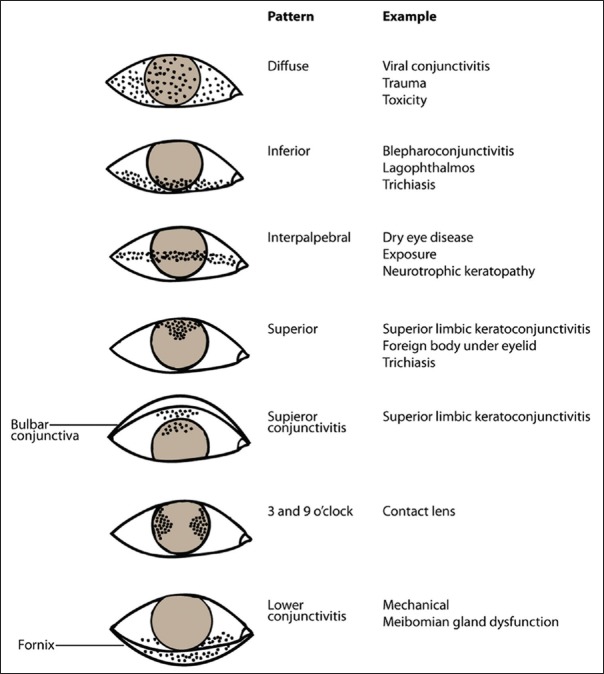
Conjunctivochalasis is poor adherence of bulbar conjunctiva. It occurs commonly with chronic inflammation or aging. SLK is a chronic, recurrent condition of ocular irritation and redness. Stains such as fluorescein, rose bengal, and lissamine green provides useful information about the ocular surface. Topical fluorescein staining is detected with a cobalt blue filter [Fig. 8]. Fluorescein detects disruption of intercellular junctions and will stain punctate and macroulcerative epithelial defects (positive staining) such as herpetic dendritic lesions or dysplastic epithelium. It can also highlight nonstaining lesions that project through the tear film (negative staining) such as basement membrane dystrophy or Thygeson superficial keratitis. Furthermore, in recurrent cornea erosions, areas of early negative staining are noted. Different disease states can produce various punctuate staining patterns as shown in Fig. 9. Pooling of the dye due to an indentation or thinning of the cornea must be distinguished from actual staining. Rose bengal [Fig. 10a] has affinity for dead or devitalized epithelial cells that have a lost or altered mucous layer. The dye may cause intense stinging. Lissamine green [Fig. 10b] stains in a similar fashion to rose bengal but causes less irritation. This is why, in clinical practice lissamine green is preferred over rose bengal.[1,2,3,4]
Figure 8.
(a) Superficial punctate keratopathy with fluorescein dye and (b) corneal epithelial defect with fluorescein dye
Figure 9.
Punctate staining patterns of the ocular surface
Figure 10.
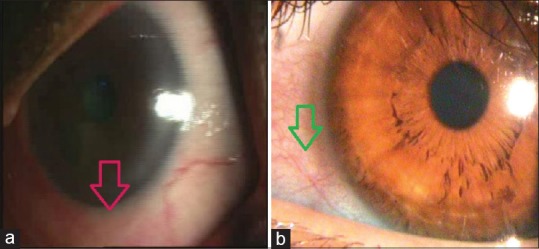
(a) Rose Bengal stain. (b) Lissamine green stain
Evaluation of the Tear Film
Tear meniscus height is evaluated by slit lamp. A tear meniscus height of <0.2 mm is considered abnormal and is a clue for ATD.[5] A foamy tear film is indicative for Meibomian gland dysfunction. The following tests are performed to evaluate tear production and tear quality.
Tear break-up time
It is measured by instilling fluorescein, asking the patient to hold the eyelids open after 1 or 2 blinks, and counting the seconds until a dry spot appears with cobalt blue filter [Fig. 11]. The appearance of dry spots in <10 s is considered abnormal. The normal tear break-up time is 20–30 s.[1,6]
Figure 11.
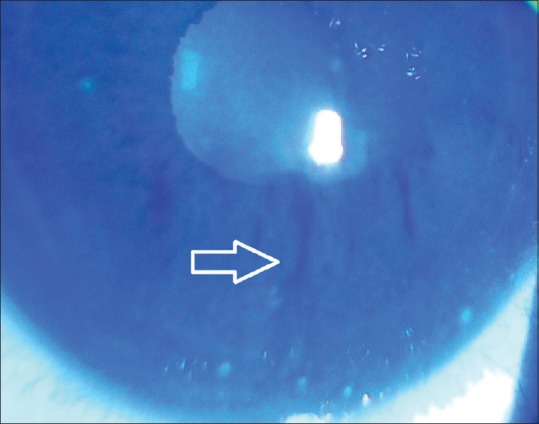
Tear break-up time. Note the white arrow showing the dry spots
Basic secretion test
After instillation of a topical anesthetic, a thin filter paper strip is placed at the junction of the middle and lateral thirds of the lower eyelid [Fig. 12]. After 5 min, <3 mm wetting of the paper is highly suggestive of ATD, whereas 3–10 mm is equivocal.[1]
Figure 12.
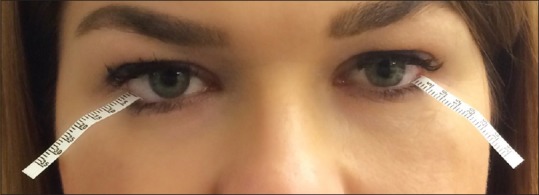
Filter strips located in the two-thirds of the inferior fornix for basic tear secretion and Schirmer tests
Schirmer I test
It is similar to basic secretion test but is done without local anesthetic. It measures basic and reflex tearing combined. Less than 5.5 mm of wetting after 5 min is diagnostic of ATD.[1]
Schirmer II test
It measures reflex secretion. It is performed with topical anesthetic. After the filter-paper strips have been inserted into inferior fornices, a cotton tip applicator is used to irritate the nasal mucosa. After 2 min, wetting of <15 mm is consistent with decreased reflex secretion.[1]
Tear Composition Assays
Tear film hyperosmolarity and reduced tear lysozyme or lactoferrin levels are highly suggestive of dry eye. Commercial assays to measure its various components are TearLab Osmolarity System (TearLab Corporation, USA), The Touch Tear Lactoferrin MicroAssay (Touch Scientific, Inc., USA), and InflammaDry Detector (Rapid Pathogen Screening, USA).[1,3]
Imaging Technologies
Videokeratoscopy can detect a break in the tear film. Wavefront sensors can evaluate sequential changes in visual performance related to tear film dynamics.[1] Anterior segment optical coherence tomography (OCT) measures inferior tear meniscus and the tear film and its components. The tear meniscus is 0.2 ± 0.09 mm in dry eyes and 0.5 ± 0.02 mm in healthy eyes by OCT.[7]
Impression Cytology
It is primarily a research tool. It provides precise assessment of the ocular surface epithelium. Sheets of epithelial conjunctival or corneal cells are harvested using a piece of filter paper. They then can be examined directly in morphological and histologic studies, or they may be processed as free cells for flow cytometry.[1]
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient have given her consent for her images and other clinical information to be reported in the journal. The patient understand that name and initial will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Weisenthal RW, Afshari NA, Bouchard CS, Colby KA, Rootman DS, Tu EY, et al. External Disease and Cornea, Basic and Clinical Science Course. Sec. 8. Singapore: American Academy of Ophthalmology; 2013. Examination techniques for the external eye and cornea; pp. 11–36. [Google Scholar]
- 2.Weisenthal RW, Afshari NA, Bouchard CS, Colby KA, Rootman DS, Tu EY, et al. External Disease and Cornea, Basic and Clinical Science Course. Sec. 8. Singapore: American Academy of Ophthalmology; 2013. Examination techniques for the external eye and cornea; pp. 37–82. [Google Scholar]
- 3.Zeev MS, Miller DD, Latkany R. Diagnosis of dry eye disease and emerging technologies. Clin Ophthalmol. 2014;8:581–90. doi: 10.2147/OPTH.S45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowling B. 8th ed. China: Elsevier Limited; 2016. Dry eye. Kanski's Clinical Ophthalmology: A systematic approach; pp. 119–29. [Google Scholar]
- 5.The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5:75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi C, Cursiefen C. Ophthalmological complications in Sjögren's syndrome. Z Rheumatol. 2010;69:32–40. doi: 10.1007/s00393-009-0517-4. [DOI] [PubMed] [Google Scholar]
- 7.Werkmeister RM, Alex A, Kaya S, Unterhuber A, Hofer B, Riedl J, et al. Measurement of tear film thickness using ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:5578–83. doi: 10.1167/iovs.13-11920. [DOI] [PubMed] [Google Scholar]