Abstract
Purpose:
The aim of this study is to analyze the yield of retinal images obtained in a rural diabetes eye care model.
Methods:
An analysis of a sample of nonmydriatic fundus photography (NMFP) of posterior segment ophthalmic images, obtained by an indigenous equipment (3 nethra-Forus Royal), was done in a district-wide rural diabetic retinopathy (DR) screening program; a trained optometrist did the initial image grading. DR and diabetic macular edema (DME) were classified based on international DR and DME severity scale. The agreement between the optometrist and retina specialist was very good (κ = 0.932; standard error = 0.030; 95% confidence interval = 0.874–0.991).
Results:
Posterior segment images of 2000 eyes of 1000 people with diabetes mellitus (DM) were graded. The mean age of the participants was 55.7 ± 11.5 standard deviation years. Nearly 42% of the screened participants (n = 420/1000) needed referral. The most common referable posterior segment abnormality was DR (8.2%). The proportion of people with any form of DR was seen in 110/1225 eyes, and sight-threatening DR was seen in 35/1225 eyes. About 62% of posterior segment images were gradable. The reasons for ungradable posterior segment images (34%) were small pupil, unfocused/partially available field of images, and cataract.
Conclusion:
A NMFP model was able to detect referable posterior segment abnormalities in a rural diabetes eye care program. Reasons found for ungradability of images in the present study can be addressed while designing future DR screening programs in the rural areas.
Keywords: Diabetes, eye care model, retinal imaging, rural, yield
India and other Asian countries are facing an epidemic of diabetes mellitus (DM), with China and India having the highest number of people with DM in the world.[1,2] Diabetes is a major public health problem in India and is growing to epidemic proportions with approximately 79 million people predicted to have diabetes by 2030.[3] It is important to find effective ways of screening for visual complications in people with diabetes. Recent epidemiological studies suggest that there is an increase in the prevalence of DM in rural India[4] Nearly 1 of 10 individuals in rural South India, above the age of 40 years, showed evidence of type 2 DM. Likewise, among participants with diabetes, the prevalence of diabetic retinopathy was around 10%; the strongest predictor being the duration of diabetes.[4] In a Pan India study of the prevalence of diabetic retinopathy done by All India ophthalmological society in 2014, the prevalence of DR was 21.7% across the nation.[5]
In India, 70% of the population lives in rural areas whereas 70% of health-care personnel and doctors live in urban areas. To bridge this rural and urban divide, we need effective rural diabetes eye care models, which can screen for visual complications of DM.
Objectivity, accuracy, sustainability, and cost-effectiveness are essential in building an eye complication screening model for rural population with DM. The first step in this direction is using an ophthalmic image screening model with indigenous cost-effective equipment and an inbuilt standard protocol for operating systems.
Ophthalmic imaging has been established as an effective screening modality for DR in various studies.[6,7,8,9,10] A single-field nonmydriatic fundus image showed good sensitivity and specificity in detection of any DR/referable DR.[11] Similarly, in a large, widely distributed DR ocular telehealth program, as compared with nonmydriatic fundus photography (NMFP), nonmydriatic ultra-widefield imaging reduced the number of ungradable eyes by 81%, increased the identification of DR nearly two-fold, and identified peripheral lesions suggesting more severe DR in almost 10% of patients, thus demonstrating significant benefits of this imaging method for large DR teleophthalmology programs.[10] Teleophthalmology has shown benefit in improving access to DR care in the rural areas.[12]
The present study was done with the objective of analyzing the yield of retinal imaging in a rural diabetes eye care model. The study results will help in understanding the efficacy and gaps in using a retinal imaging screening model for rural population with DM.
Methods
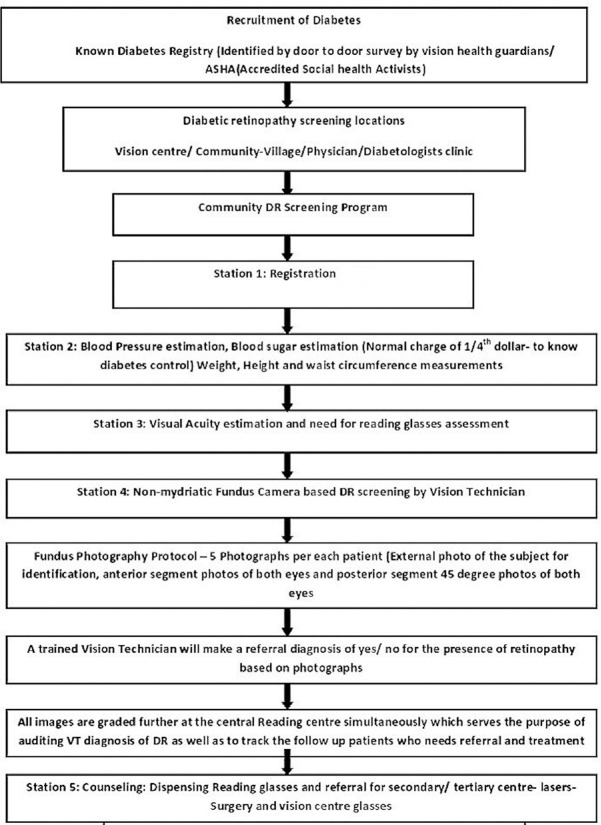
The present study is analysis of a sample of ophthalmic images obtained by an indigenous equipment (3 nethra-Forus Royal) in a district-wide rural DR screening program implemented in Prakasam District, Andhra Pradesh, India. Detailed demographic data of each patient were recorded which were followed by their medical history including blood sugar level of patients. Detailed Demographic data of each patients was recorded which was followed by their medical history including Blood sugar level of patients, Visual acuity, Anterior Segment Photography and un-dilated Posterior segment Imaging. The patients were recruited even when their blood sugars are normal when they are taking medication for diabetes and/or under care of a physician who deals with diabetes. Fig. 1 shows the study flowchart. All images collected in the study were stored at a central server located at the base hospital. We analyzed anterior and posterior segment images obtained from a sample of 2000 eyes of 1000 people with known DM who were screened in DR screening programs in rural areas. All images were assessed for quality in terms of gradability, percentage of posterior segment abnormalities, and percentage of referable posterior segment abnormalities to an ophthalmologist [Table 1] for further management.
Figure 1.
Study flowchart
Table 1.
Referral criteria of an ophthalmic imaging screening model for people with diabetes mellitus
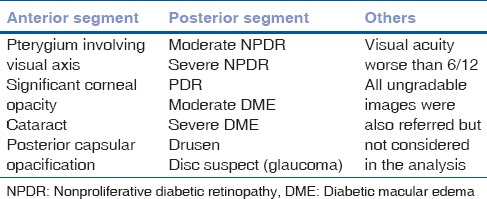
An optometrist who was trained for ophthalmic image grading and diabetic retinopathy classification by a retina specialist did the initial image grading and analysis. DR and diabetic macular edema (DME) was classified based on international DR and DME severity scale.[13] Sight-threatening or referral retinopathy was considered as moderate and severe nonproliferative DR and proliferative DR, and sight-threatening or referable DME was considered as moderate and severe DME.
A sample of 5% of normal, 5% of nongradable, and all referable posterior segment abnormalities grading done by the optometrist were sorted out in a different folder and were regraded by the retina specialist. The image acquisition was done by a vision technician. Separate folder was made for anterior segment images and posterior segment images. The agreement between optometrist and retina specialist was very good (κ = 0.932 standard error of κ = 0.030 and 95% confidence interval: From 0.874 to 0.991). As the agreement was very good for all categories of normal and abnormal images between the study optometrist and the retina specialist, separate results of sensitivity and specificity were not presented.
Results
A total of 32,959 diabetic patients underwent ophthalmic imaging in 1236 DR screening programs at 808 rural locations in the study area. In the present study, a sample of 1000 people with DM, whose data were complete, were sorted into a separate folder. Posterior segment images of 2000 eyes of 1000 people with DM were graded. The mean age of the participants was 55 years with 11.54 standard deviation. Men were 762 (76%) out of 1000 patients.
Utility of external photograph
An external photograph of every participant was taken before the start of ophthalmic imaging. This served as an audit measure to prevent the duplication of images.
Yield of retinal imaging in people with diabetes mellitus
Gradability of posterior segment images
Gradable posterior segment images were 62% (1225/2000 eyes), 682 (34%) were nongradable and 93 (4%) were not available. Images in the posterior segment were not gradable due to various reasons – major causes were miotic/undilated pupil resulting in unfocused images in 60%, and cataract in 37% and in 9% disc only was seen, but DR grading could not be done.
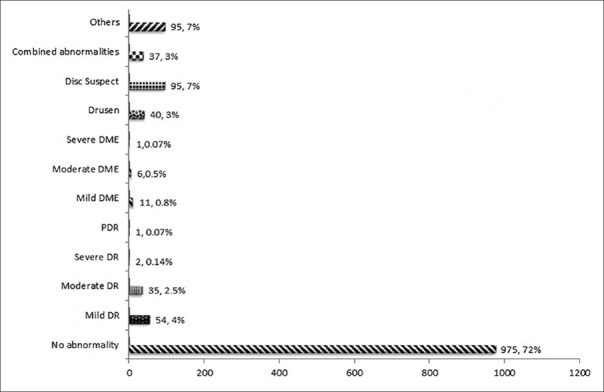
Among those 1225 gradable images, 848 (69%) eyes were normal and 377 (31%) eyes were abnormal. Referable abnormalities of posterior segment images were shown in [Fig. 2].
Figure 2.
Yield of posterior segment imaging in people with diabetes mellitus
Overall, 42% (420 participants/1000-840 eyes) of the screened participants needed referral for further management of their diabetic-related eye diseases. The most common referable posterior segment abnormality was DR seen in 8.2% of eyes (the proportion of people with any form of DR was seen in 110/1225 eyes and sight-threatening or referral DR was seen in 35/1225 eyes).
Discussion
Major findings of our rural diabetes eye care model are that 42% (420 participants/1000-840 eyes) of the screened participants needed referral for further management of their diabetic-related eye diseases. The most common referable posterior segment abnormality was DR seen in 8.2% of eyes (the proportion of people with any form of DR was seen in 110/1225 eyes and sight-threatening or referral DR was seen in 35/1225 eyes).
Image gradability
In our study, the ungradability was 34% and the major reasons for ungradability of posterior segment images were small pupil and an unfocused image was about 60% and due to the presence of significant cataract was about 37% and only part of image being available like optic disc only in 9% of eyes. In a study done by Gupta et al.,[14] 30% of posterior segment images were ungradable for screening. The major reason for ungradability in the Gupta et al. study was also the presence of cataract and small pupils. They have recommended pupillary dilatation for improving the gradability of photographs.[14]
In a study done by Raman et al.,[15] after pupillary dilatation, the nongradability of digital fundus images was reduced from 29.1% to 8.6%. With each line of improvement in Snellen visual acuity, the gradability improved by 12.1%; likewise, with each year of age, the gradability improved by 5.5% following mydriasis. They have recommended dilated retinal photography in people with age more than 50 years and visual acuity worse than 6/12 to improve the gradability.
The major limitation of our study was ungradable posterior segment images due to various anterior segment causes and technical issues resulting in a greater number of referrals to the next level.
To address the problem of ungradability and the risk of missing a patient with a disease, we incorporated strict referral criteria of referring all ungradable images as well as those patients with referable anterior and posterior segment abnormalities. We established a screening model having broad referral criteria so that we do not risk missing a patient with a referable problem either due to technology/training or an actual disease. We do agree that this might have resulted in more number of unnecessary referrals.
Gupta et al. attributed the ungradability of images also to the dark iridis seen in Indian eyes.[14] We also agree that this factor could have contributed to more ungradability of images, especially in undilated NMFP performed in the present study.
Our NMFP model has inbuilt elements of objectivity, sustainability, and audit and database management to tackle diabetes eye diseases. Pandit et al.[16] clearly given quality assurance standards for DR screening using retinal imaging by nonspecialist graders (included both diabetologists and trained retinal screeners). Quality assurance standards proposed by English DR screening programs are that at least 5% of all images should be audited periodically by in person dilated ophthalmoscopy by an ophthalmologist. A sensitivity of at least 80% and specificity of 90% should be aimed while comparing the nonspecialist graders with the reference standard ophthalmologist examination. We also followed similar quality assurance standards in our rural DR screening model. Periodic workshops and training programs at intervals of 6 months were conducted for technicians in the fundus photography, DR classification, referable criteria and photography data management.
In a pilot study by Srinivasan et al.,[17] on comparing the agreement and diagnostic accuracy of the algorithm among optometrists versus agold standardo (retinal specialist grading), they found that 99 optometrists correctly identified 91.5% images that required immediate referral (κ) = 0.696), 62.5% of images as requiring review after 6 months (κ = 0.462), and 51.2% of those requiring review after 1 year (κ = 0.532). Optometry students performed better than the working optometrists for all grades. Brady et al.[18] described an online tool to train nonexperts (Amazon mechanical Turk workforce) to diagnose the presence or absence of DR. By minimal training they could rapidly and correctly categorize normal versus abnormal. Gadkari et al.[19] used a portable mobile screening device in a telemedicine-based screening program with image acquisition done by technicians with reading of images done at a central reading center in real time. The agreement between the trained optometrist grader in the current study and retina specialist was very good for all categories of images. This suggests that a sustainable trained optometrist/vision technician led ophthalmic imaging model which is possible to detect the eye complications in a rural diabetes eye care program.
All participants screened in the present rural diabetes eye care model were linked to service delivery in an integrated model of eye care network. The pyramidal model of eye care network ensured comprehensive management of all diabetes-related eye complications from simple to complex (uncorrected refractive errors – cataract/glaucoma/DR) in the present study. Transport of referable patients by mobile van to the secondary center on the same day of screening program helped in improving the access of care to referral patients and prevented dropouts. We faced challenges in the running of cameras, especially in the summer seasons due to an erratic power supply. This problem was resolved through the use of portable solar chargers. This facilitated the running of the screening programs, providing an uninterrupted alternative power supply.
Conclusion
The present study results suggest that a NMFP model was able to detect referable posterior segment abnormalities in a rural diabetes eye care program. The lessons learned and the reasons found for ungradability of images in the present study can be addressed while designing future DR screening programs in the rural areas.
Financial support and sponsorship
This study was financially supported by the World Diabetes Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to acknowledge the World Diabetes Foundation.
References
- 1.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Zhao W, Xiao J, Li R, Zhang P, Kissimova-Skarbek K, et al. Medical care and payment for diabetes in China: Enormous threat and great opportunity. PLoS One. 2012;7:e39513. doi: 10.1371/journal.pone.0039513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Raman R, Ganesan S, Pal SS, Kulothungan V, Sharma T. Prevalence and risk factors for diabetic retinopathy in rural India. Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study III (SN-DREAMS III), report no 2. BMJ Open Diabetes Res Care. 2014;2:e000005. doi: 10.1136/bmjdrc-2013-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadkari SS, Maskati QB, Nayak BK. Prevalence of diabetic retinopathy in India: The all India ophthalmological society diabetic retinopathy eye screening study 2014. Indian J Ophthalmol. 2016;64:38–44. doi: 10.4103/0301-4738.178144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chasan JE, Delaune B, Maa AY, Lynch MG. Effect of a teleretinal screening program on eye care use and resources. JAMA Ophthalmol. 2014;132:1045–51. doi: 10.1001/jamaophthalmol.2014.1051. [DOI] [PubMed] [Google Scholar]
- 7.DeBuc DC. The role of retinal imaging and portable screening devices in tele-ophthalmology applications for diabetic retinopathy management. Curr Diab Rep. 2016;16:132. doi: 10.1007/s11892-016-0827-2. [DOI] [PubMed] [Google Scholar]
- 8.Kanjee R, Dookeran RI, Mathen MK, Stockl FA, Leicht R. Six-year prevalence and incidence of diabetic retinopathy and cost-effectiveness of tele-ophthalmology in Manitoba. Can J Ophthalmol. 2016;51:467–70. doi: 10.1016/j.jcjo.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Pareja-Ríos A, Bonaque-González S, Serrano-García M, Cabrera-López F, Abreu-Reyes P, Marrero-Saavedra MD, et al. Tele-ophthalmology for diabetic retinopathy screening: 8 years of experience. Arch Soc Esp Oftalmol. 2017;92:63–70. doi: 10.1016/j.oftal.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Silva PS, Horton MB, Clary D, Lewis DG, Sun JK, Cavallerano JD, et al. Identification of diabetic retinopathy and ungradable image rate with ultrawide field imaging in a national teleophthalmology program. Ophthalmology. 2016;123:1360–7. doi: 10.1016/j.ophtha.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 11.Williams GA, Scott IU, Haller JA, Maguire AM, Marcus D, McDonald HR, et al. Single-field fundus photography for diabetic retinopathy screening: A report by the American academy of ophthalmology. Ophthalmology. 2004;111:1055–62. doi: 10.1016/j.ophtha.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Das T, Pappuru RR. Telemedicine in diabetic retinopathy: Access to rural India. Indian J Ophthalmol. 2016;64:84–6. doi: 10.4103/0301-4738.178151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–82. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V, Bansal R, Gupta A, Bhansali A. Sensitivity and specificity of nonmydriatic digital imaging in screening diabetic retinopathy in Indian eyes. Indian J Ophthalmol. 2014;62:851–6. doi: 10.4103/0301-4738.141039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raman R, Rani PK, Mahajan S, Paul P, Gnanamoorthy P, Krishna MS, et al. The tele-screening model for diabetic retinopathy: Evaluating the influence of mydriasis on the gradability of a single-field 45 degrees digital fundus image. Telemed J E Health. 2007;13:597–602. doi: 10.1089/tmj.2006.0084. [DOI] [PubMed] [Google Scholar]
- 16.Pandit RJ, Taylor R. Quality assurance in screening for sight-threatening diabetic retinopathy. Diabet Med. 2002;19:285–91. doi: 10.1046/j.1464-5491.2002.00722.x. [DOI] [PubMed] [Google Scholar]
- 17.Srinivasan S, Shetty S, Natarajan V, Sharma T, Raman R. Development and validation of a diabetic retinopathy referral algorithm based on single-field fundus photography. PLoS One. 2016;11:e0163108. doi: 10.1371/journal.pone.0163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brady CJ, Villanti AC, Pearson JL, Kirchner TR, Gupta OP, Shah CP, et al. Rapid grading of fundus photographs for diabetic retinopathy using crowdsourcing. J Med Internet Res. 2014;16:e233. doi: 10.2196/jmir.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadkari S. Innovative model for telemedicine-based screening for diabetic retinopathy in the developing world. Can J Ophthalmol. 2016;51:e109–11. doi: 10.1016/j.jcjo.2015.11.010. [DOI] [PubMed] [Google Scholar]