Abstract
Purpose:
The aim of this study was to evaluate an innovative approach for closing retinal tears using DuraSeal™ (DS) hydrogel sealant in a rabbit model.
Methods:
Retinal detachment with a small tear was performed on 20 New Zealand rabbits. Thereafter, rabbits were divided into two groups; the experimental group received a transscleral injection of 0.1 ml DS into the subretinal space whereas the control group received sham injection of saline. Eyes were clinically evaluated using indirect ophthalmoscopy, retinal function was recorded in ten rabbits by electroretinography and the sealant's toxicity was evaluated histopathologically.
Results:
We found that the DS hydrogel was easily injected transsclerally into the subretinal space of the detached retinas with no major complications. Retinal reattachment was seen in both groups within 2 weeks with no toxicity to the sensory retina. There were no significant differences in retinal function between groups.
Conclusion:
Subretinal injection of hydrogel through a transscleral route is easy to perform and may open a new avenue in the treatment of retinal detachment. However, the efficacy of the DS as a tamponade for sealing retinal tear is yet to be definite. Long-term clinical, functional, and toxicological studies are needed to evaluate its full potential for clinical applications.
Keywords: DuraSeal hydrogel sealant, retinal detachment, retinal tear, transcleral approach
Rhegmatogenous retinal detachment (RRD) is a serious condition in which the retina is detached from the underlying retinal pigment epithelium (RPE), and if left untreated it can lead to vision loss and blindness. In the course of the disease, vitreous traction, trauma, or injury to the eye may cause a small tear or a break in the retina allowing vitreous fluids to accumulate in the subretinal space and thus lead to its detachment.[1,2] Therefore, closure of the retinal hole and reattachment of the retina are the primary aims of treatment. Several gold-standard approaches are available to treat retinal holes, including laser, cryotherapy, sclera buckle, gas, and silicon oil; however, they all have disadvantages due to their high invasiveness, potential cellular toxicity, and complications leading to cataract formation and glaucoma.[3,4] In view of the limitations associated with these surgical techniques, development of a safe and efficacious treatment with minimal toxicity and invasiveness which takes into consideration the anatomical and physiological constraints of the eye remains challenging.
In recent years, the need to improve the treatment of retinal detachment envisioned a logical alternative in the form of a biocompatible and biodegradable sealant to close the retinal breaks. Some intraocular adhesive glues, including cyanoacrylate, fibrin glue, and others have been used to patch retinal breaks in experimental retinal detachment models and in clinical cases yet, each glue has limitations and is not an ideal treatment for closing retinal breaks. Recently, adhesive and liquid hydrogels are potential tissue sealants since they are hydrophilic, flexible, and biodegradable, and therefore, they have been evaluated for their efficacy in closing retinal breaks with minimal toxicity.[5,6,7,8] Campbell et al. reported DuraSeal™ (DS) sealant to have the fastest reaction time, highest strength, and moderate water uptake among the tested sealants.[9] This hydrogel was later evaluated by Sueda et al.[7] as treatment for closing retinal breaks. In spite of the promising advantages of the hydrogel, the authors encountered a low success rate, mainly due to technical problems in the mixing of the liquid glue and side effects associated with its intraocular application. This, in turn, led us to develop a different strategy for closing retinal holes using this DS hydrogel delivered through a transscleral route as discussed by Gupta et al. and Patel et al.[10,11]
Developing a new delivery approach which takes into consideration the anatomical and physiological constraints of the eye, as well as the chemical properties of the hydrogel, should be further explored and we believe that transscleral delivery may offer a potential new route using DS hydrogel for tissue repair.
In this study, we evaluated the efficacy of DS hydrogel sealant in closing retinal holes and explored a transcleral approach as a new paradigm in retinal reattachment surgeries.
Methods
Surgery to induce rhegmatogenous retinal detachment
Twenty New Zealand albino rabbits (Harlan Biotech Israel Ltd., Jerusalem, Israel) weighing 1.5–1.7 kg were used in this study. Animals were handled according to the recommendations of the ARVO statement for the Use of Animals in Ophthalmic and Visual Research and the hospital's Institutional Animal Care and Use Committee.
All procedures were carried out in the right eyes with sterile techniques under a surgical microscope. Animals were anesthetized by intramuscular (IM) ketamine hydrochloride (35 mg/kg), and xylazine (5 mg/kg). Pupils were dilated with topical 0.5% phenylephrine hydrochloride, 0.5% tropicamide, and 1% atropine. Conjunctival peritomy and sclerotomy of 180° (from 0800 to 1400 h) were carried out at the corneoscleral limbus, and a two-port vitrectomy was performed. An infusion port was made 1 mm posterior to the sclerocorneal limbus in the inferotemporal quadrant using a small gauge stiletto (Accurus Surgical System 25-gauge; Alcon, TX, USA), and a 25Ginfusion cannula that delivered a balanced salt solution (BSS; Alcon Japan) was then inserted into the trocar cannula.
The second port, created in the same manner, was used for inserting a vitreous cutter into the superotemporal quadrant. Subsequently, we performed a core vitrectomy, and retinal detachment was made approximately 2–3 DD from the optic nerve by injecting 0.1 ml BSS under the retina using a 25G-soft-tip needle (Alcon, TX, USA). The same 25G-soft-tip needle was used to create a small tear in the retina.
Hydrogel and its delivery
DS dura sealant system (Covidien Mansfield, MA, US) is a biodegradable and biocompatible hydrogel, Food and Drug Administration-approved for cranial and spinal dura surgeries.[12] It can adhere strongly to moist tissue. This hydrogel is composed of two synthetic liquids – a polyethylene glycol (PEG) solution with a gel-like texture (>90% water), and an amine component, which acts as a precursor. The PEG is a nontoxic, water-soluble chemical that increases biocompatibility. On mixing of the two components, polymerization occurs within 20 s without generating heat, leading to the formation of a solid, flexible, and adherent hydrogel layer. The hydrogel swells as it absorbs fluid from its surroundings. In reality in the eye, the polymerization process was instant.
Immediately after the detachment was performed, rabbits were divided into two groups, 12 in the experimental group and 8 in the control group. Since the hydrogel was used as a surrogate for subretinal tamponade, more animals were allocated to the experimental group. In this group, a total volume of 0.1 ml of the PEG and the amine component (0.05 ml each) were injected under direct visualization into the subretinal space through the sclera of the right eye of each treated rabbit. Since the detachment was induced in the peripheral retina closer to the limbus, there was no need to induce globe prolapse. The injection was conducted in two steps, with the PEG (containing a blue tracer) injected first to allow good visualization, immediately followed by the injection of the clear amine solution, thus enabling the mixing of the two liquids and formation of the gel. The tip of the needle was inserted into the globe tangentially (bevel-away-from-sclera so that when the needle was in the subretinal space of the previously induced retinal detachment, the bevel was away from the retina). In the control group, 0.1 ml of BSS was injected transsclerally into the subretinal space of the right eye of the rabbit. In both groups, the left eyes were left untouched as internal controls. All operated eyes were treated with 5% chloramphenicol ointment at the end of this procedure.
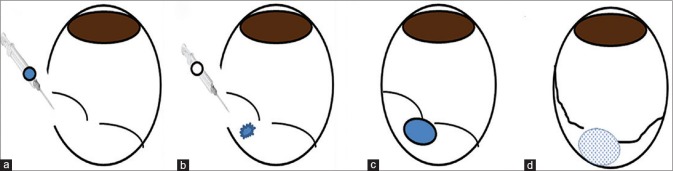
An illustration of the injection procedure is shown in Fig. 1.
Figure 1.
Illustration of transscleral delivery of DuraSeal™ hydrogel for closure of a previously induced retinal break and detachment. (a) Transscleral injection of DuraSeal™ polyethylene glycol solution to the subretinal space, (b) Transscleral injection of the amine solution, (c) The two components of the hydrogel mix and polymerize, (d) The hydrogel swells as it absorbs water from its surroundings. The hydrogel forms a subretinal tamponade and closes the retinal tear
Clinical examination of the fundus was conducted using an indirect ophthalmoscope (Vantage Plus Digital Indirect Ophthalmoscope©, Keeler Ltd., Windsor, UK) and performed on the 1st, 3rd, 7th, 14th, and 21st postoperative days. Operated eyes were monitored for the presence of a detachment and a retinal tear, as well as other potential ocular complications.
Electroretinography
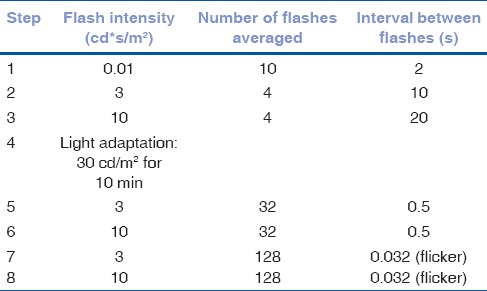
Electroretinographies (ERGs) were recorded on ten rabbits (5/group) a week before, and 7 and 20 days after surgery. Following overnight dark adaptation, the rabbits were anesthetized with an IM injection of ketamine (60 mg/kg) and xylazine (8 mg/kg) and pupils were dilated with 0.5% tropicamide at least 8 min before the beginning of the ERG session. Subcutaneous needles at the base of the left ear and at the lateral canthus of the recorded eye served as ground and reference electrodes, respectively. After application of topical anesthesia (oxybuprocaine 0.4%) and conductance medium (hydroxymethyl cellulose 1.4%), a jet corneal active electrode was placed. Impedance was kept below 2 kΩ. The operated eye was recorded first. Recordings were conducted using an integrated system (HMsERG, Ocuscience, NV, USA) with band-bass filter of 0.3–300 Hz. Flash stimuli and background adapting light were delivered unilaterally with a “miniganzfeld” dome placed about 3 cm from the recorded eye. The rabbit was positioned in lateral recumbency on a pillow, thus preventing light exposure of the unrecorded eye. Responses to the standard protocol of the International Society for Clinical Electrophysiology of Vision [Table 1] were recorded.
Table 1.
Electroretinography protocol sequence. The interval between consecutive steps was 2 s except for 30 s between steps 2 and 3
a-and b-wave amplitudes were measured from baseline to the first trough and from that trough to the next positive peak, respectively.
Electroretinography statistical analysis
Since the same individual rabbits were repeatedly recorded throughout the study, ANOVA with repeated measures was used. Within-Subjects ANOVA was used where the two different conditions were the day of recording and flash luminance. The group effect was measured both within subjects (i.e., as the interaction between recording day and group) and between subjects. Error bars and “±” indicate standard deviation.
Histology
At the end of a 3-week follow-up, animals were euthanized with an overdose of pentobarbital (Vetmarket, Shoham, Israel) and both eyes were enucleated for histological evaluation. Eyes were fixed in 4% Formalin for 24 h, dehydrated by increasing sucrose gradient and cryopreserved in OCT. Serial 10 μm cryosections were stained with H and E (American MasterTech Scientific®, Lodi, CA, USA) and examined by light microscopy (Olympus Optical Co., Tokyo, Japan).
Results
Clinical findings
To explore the surgical approach, RRD was created in 20 rabbits as described and transscleral injections of either saline of DS hydrogel were performed. The area of the detachment was dramatically reduced 3 days posttreatment and absorbed by the 14th day, in both groups regardless to the treatment. In 2/12 rabbits in the experimental group, the eyes became hypotonic following induction of RRD, thus making it difficult to inject the hydrogel. However, once the pressure in the eyes was restored, transscleral injection of the hydrogel was easily feasible. In addition, these two eyes had minor and local hemorrhage immediately after the detachment procedure, which resolved between 1 and 3 weeks postoperatively [Fig. 2]. Furthermore, two eyes in each group developed a fibrotic membrane within 14 days postoperatively, thus hindering our ability to perform additional follow-up examinations of the fundus.
Figure 2.
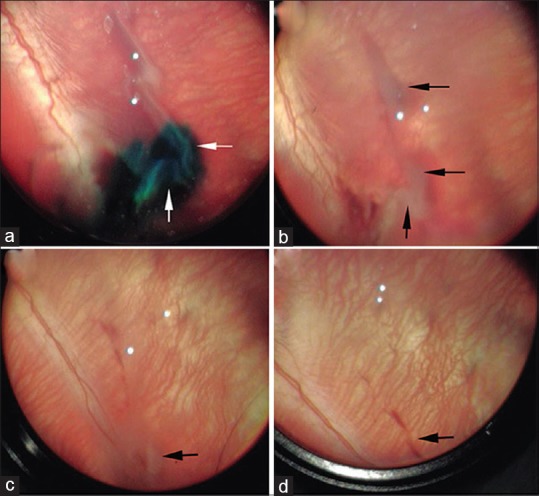
Three-week follow-up of a representative DuraSeal™ hydrogel-treated eye. (a) A detachment with DuraSeal™ localized to a small area under the retina (dark blue, white arrows). A major reduction in the size of the detachment was seen 3-days postsurgery (b), which almost completely cleared 14 days postoperatively (c), a small area of detachment is visible (c black arrow). By the end of the third week, however, there is no sign of a detachment nor a retinal tear (d). A local scar was observed (white arrow)
Electroretinography
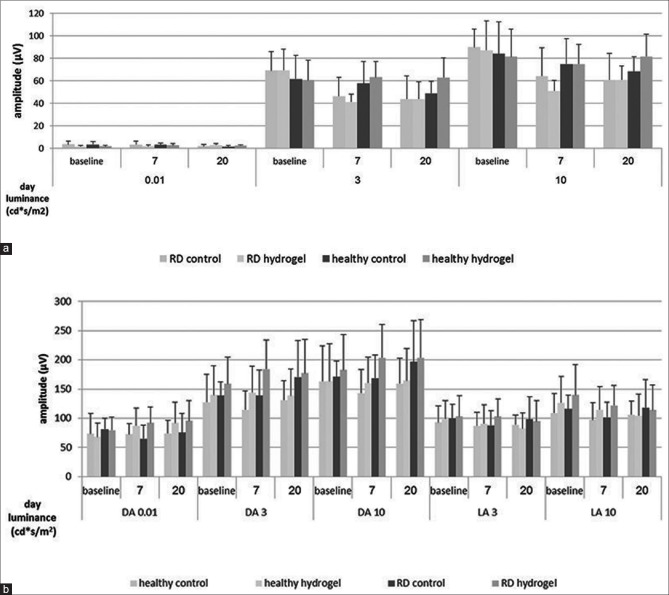
ERG was used to study retinal function in ten saline-treated and ten DS-treated rabbits. Fig. 3 shows the a-[Fig 3a] and b-[Fig. 3b] wave amplitudes of intact (i.e., the contralateral, not operated eyes) and retinal detachment eyes before surgery and at 7 and 20 days postsurgery. The time-dependent changes in the a-wave amplitudes of operated eyes were found to be significant [Fig. 3a, ANOVA of repeated measures, P < 0.05] yet these changes were similar across hydrogel and saline treatment groups. Dark and light-adapted b-wave amplitudes remained unaffected regardless of treatment group [Fig. 3b, ANOVA with repeated measures, P > 0.30]. Although an interesting reduction in a sub b-wave was noted in both groups (manuscript in review).
Figure 3.
Electroretinography analysis of saline-and DuraSeal™-treated eyes. electroretinography was recorded at baseline, 7-and 20-days postsurgery. Mean ± standard deviation amplitudes of the a-(a) and b-waves (b) are illustrated. No differences were found between intact and operated eyes in any parameter tested
Histology
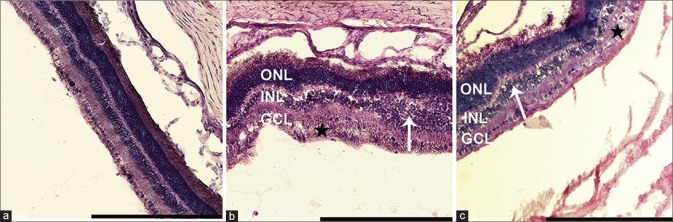
We examined the retinas for pathological changes that might be associated with the transscleral approach and for potential toxicity of DS. Histological analysis performed 21 days after RD revealed minor changes in the detached retinas of all operated eyes, regardless of the treatment group, including edema and vacuolar degeneration, mostly in the inner nuclear layer and ganglion cell layer. No major histopathological differences were observed between eyes of the two groups. In addition, no major cellular alternations were seen in the experimental eyes compared to the saline-treated eyes [Fig. 4].
Figure 4.
Histological comparison of control untreated, saline-and DuraSeal™-treated eyes. Control unoperated eyes exhibit a normal morphology (a). Edema and vacuolar degeneration were observed around the site of retinal detachment, mostly in the inner nuclear (white arrows) and ganglion cell (asterisk) layers of both saline-treated (b) and DuraSeal™-treated and (c) detached retinas. No differences were seen between saline-treated (b) and DuraSeal™-treated (c) eyes. Scale bar 100 μm
Discussion
Closure of retinal tears is a crucial step in the management and treatment of retinal detachment. Surgical techniques such as scleral buckling, laser, cryopexy, and vitrectomy are widely-used approaches to treat RRD by attaching the edge of the tear to the RPE. However, every technique has disadvantages and failure to close the break may results in formation of preretinal membrane, proliferative vitreoretinopaty, and RRD.
In the past few decades, tissue adhesives have been evaluated for closure of retinal tears including cyanoacrylate,[13,14,15,16,17,18,19] fibrin glue,[20,21] mussel glue,[22] transforming growth factor beta,[23] and m-Gelatin.[24] Results have been disappointing, mostly due to fast polymerization rate, low strength of adherence, or toxicity. The ideal intraocular adhesive should have biocompatibility, biodegradability, nontoxicity, good strength of adhesion, and long-term effect and should be easily delivered. Synthetic adhesives and liquid hydrogels fit these requirements and proved to be superior to previously tested adhesives, mainly due to their hydrophilic nature, biocompatibility, and safety. Seprafilm® and DS have been recently evaluated[6,7,8,9] for their potential use as intraocular adhesives to patch retinal breaks and have been found to be nontoxic, nonimmunogenic, and biocompatible.[6,7] We were considerably interested in the DS liquid hydrogel due to its water-uptake ability, which is a beneficial feature due to the pathophysiology of RRD. Since the course of RRD includes vitreous liquefaction, tearing of the retinal membrane and fluid inflow into the subretinal space, we hypothesized that the DS hydrogel is a good candidate for water absorption, reduction of subretinal fluid volume, and the formation of a tamponade to seal and flat out the retina. However, both Seprafilm and DS suffered from technical difficulties associated with delivery, which limited their full potential, and thus, there is a need to develop a new delivery method that would improve their outcome.
In our experimental setup, it was hard to evaluate the full potential of DS in closing retinal breaks due to the spontaneous absorption of the retinal detachment that was seen in the control group. Therefore, the efficacy of the DS has not be proven in this specific experimental study, and while the transscleral approach may be feasible, it was not easy to use. Indeed, many rabbit models for RRD use injection of hyaluronic acid solutions into the subretinal space to lift up the retina and ensure a longer duration of the detachment. We, however, wished to prevent any potential interactions between the hyaluronic acid and DS components that could have adversely affected the polymerization of the hydrogel and which may have led to failure of the study. However, our decision to induce RRD with saline became a two-edged sword, resulting in relatively fast, spontaneous absorption of the detachment without retinal pathologies, thus making it difficult to compare the two groups and evaluate the full potential of this adhesive. The feline eye serves as a good animal model for retinal detachment/reattachment surgeries, and recently, Wassmer et al.[25] developed a potentially more suitable surgical technique for the detachment in the feline model and should be taken into consideration when designing future experiments. While the spontaneous absorption in our study posed some limitations for the evaluation of the full potential of the hydrogel and only the safety can be stated, the rabbit eye is anatomically similar to the human eye[1] and therefore serves as a good model for the surgical techniques, in specific the transscleral delivery approach.
On another note, creation of a more bullous RD may not necessarily serve as a better model for RD as it induced an intraocular pressure and prevented a good outreach to the retinal tear (data not shown).
Histological analyses revealed minor pathological changes, including edema and vacuolar degeneration in the inner nuclear and ganglion cell layers of both DS-and saline-treated groups. As these changes were observed in both groups, we speculate that these changes may be due to retinal remodeling associated with the detachment.[2,26,27,28] Whether histological artifacts are present due to a technical procedure, it seems to equally exists in detached and DS-treated groups. It is important to note that no significant functional or pathological differences were observed between the groups [Fig. 3 and 4] and no sign of toxicity was seen in the DS group [Fig. 4]. The hydrodel, therefore, seems to be biocompatible to the eye, thought its potential to serve as a sealant in tissue repair in the clinic should be further evaluated using prolonged toxicological studies.
In this study, we assessed a new, transscleral delivery method that takes into consideration the anatomical and physiological constraints of the eye, the different aspects of retinal detachment, and the chemical properties of the hydrogel. We exploited the feasibility of the transscleral route while exploring the potential use of DS hydrogel to close retinal breaks. We found the transcleral approach for hydrogel injections to be feasible but moderately easy, safe, with minimal toxicity and reproducible.
It is important to note that while transvitreal approach is a gold standard in many ocular surgeries, it may not be suitable for the polymerization of adhesive sealants, such as DS, due to the gas of air-filed in the operated eyes which are used to prevent hypotony.
Although being relatively feasible approach in our setup, in the clinical setting the transcleral approach might be more suitable for RRDs without vitreous traction with tears allocated, especially in the upper half of the retina. The approach might therefore be proper for very selected RRDs.
Conclusion
Based on our observations, we believe that a transscleral delivery of an adhesive tamponade may be safe in the treatment of retinal detachment. However, further studies, with a more appropriate experimental animal model, are needed to evaluate the efficacy of DS hydrogel in closing retinal tears.
Financial support and sponsorship
This study was financially supported by a grant from the Claire and Amedee Maratier Institute for the Study of Blindness and Visual Disorders, Sackler Faculty of Medicine, Tel-Aviv University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Tamar Kadar for her assistance with the histological analysis.
References
- 1.Jackson TL, Hillenkamp J, Williamson TH, Clarke KW, Almubarak AI, Marshall J, et al. An experimental model of rhegmatogenous retinal detachment: Surgical results and glial cell response. Invest Ophthalmol Vis Sci. 2003;44:4026–34. doi: 10.1167/iovs.02-1264. [DOI] [PubMed] [Google Scholar]
- 2.de Souza CF, Kalloniatis M, Polkinghorne PJ, McGhee CN, Acosta ML. Functional and anatomical remodeling in human retinal detachment. Exp Eye Res. 2012;97:73–89. doi: 10.1016/j.exer.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Tyagi P, Kadam RS, Kompella UB. Comparison of suprachoroidal drug delivery with subconjunctival and intravitreal routes using noninvasive fluorophotometry. PLoS One. 2012;7:48188. doi: 10.1371/journal.pone.0048188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jonas JB, Spandau UH, Schlichtenbrede F. Short-term complications of intravitreal injections of triamcinolone and bevacizumab. Eye (Lond) 2008;22:590–1. doi: 10.1038/eye.2008.10. [DOI] [PubMed] [Google Scholar]
- 5.Margalit E, Fujii GY, Lai JC, Gupta P, Chen SJ, Shyu JS, et al. Bioadhesives for intraocular use. Retina. 2000;20:469–77. doi: 10.1097/00006982-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Sueda J, Fukuchi T, Usumoto N, Okuno T, Arai M, Hirose T, et al. Intraocular use of hydrogel tissue adhesive in rabbit eyes. Jpn J Ophthalmol. 2007;51:89–95. doi: 10.1007/s10384-006-0405-2. [DOI] [PubMed] [Google Scholar]
- 7.Sueda J, Sakuma T, Nakamura H, Usumoto N, Okuno T, Arai M, et al. In vivo and in vitro feasibility studies of intraocular use of seprafilm to close retinal breaks in bovine and rabbit eyes. Invest Ophthalmol Vis Sci. 2006;47:1142–8. doi: 10.1167/iovs.05-0931. [DOI] [PubMed] [Google Scholar]
- 8.Teruya K, Sueda J, Arai M, Tsurumaru N, Yamakawa R, Hirata A, et al. Patching retinal breaks with seprafilm in experimental rhegmatogenous retinal detachment of rabbit eyes. Eye (Lond) 2009;23:2256–9. doi: 10.1038/eye.2008.403. [DOI] [PubMed] [Google Scholar]
- 9.Campbell PK, Bennett SL, Driscoll A, Sawhney AS. Evaluation of Absorbable Surgical Sealants: In vitro Testing. Covidien. [Last accessed on 2017 Oct 23]. Available from: http://www.covidien.com/imageServer.aspx/doc179399.pdf?contentID=14109&contenttype=application/pdf .
- 10.Gupta D, Ong J, Burton RL. Trans-scleral dye injection during vitreous surgery to identify clinically undetectable retinal breaks causing retinal detachment. Eye (Lond) 2011;25:1045–9. doi: 10.1038/eye.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A, Cholkar K, Agrahari V, Mitra AK. Ocular drug delivery systems: An overview. World J Pharmacol. 2013;2:47–64. doi: 10.5497/wjp.v2.i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA Executive Summary 2009-4437b 1-0 1. [Last accessed on 2017 Oct 23]. Available from: https://www.fda.gov/ohrms/dockets/ac/09/briefing/2009-4437b1-01%20FDA%20Executive%20Summary.pdf .
- 13.McCuen BW, 2nd, Hida T, Sheta SM, Isbey EK, 3rd, Hahn DK, Hickingbotham D, et al. Experimental transvitreal cyanoacrylate retinopexy. Am J Ophthalmol. 1986;102:199–207. doi: 10.1016/0002-9394(86)90145-5. [DOI] [PubMed] [Google Scholar]
- 14.Hida T, Sheta SM, Proia AD, McCuen BW., 2nd Retinal toxicity of cyanoacrylate tissue adhesive in the rabbit. Retina. 1988;8:148–53. doi: 10.1097/00006982-198808020-00013. [DOI] [PubMed] [Google Scholar]
- 15.McCuen BW, 2nd, Hida T, Sheta SM. Transvitreal cyanoacrylate retinopexy in the management of complicated retinal detachment. Am J Ophthalmol. 1987;104:127–32. doi: 10.1016/0002-9394(87)90003-1. [DOI] [PubMed] [Google Scholar]
- 16.Hartnett ME, Hirose T. Cyanoacrylate glue in the repair of retinal detachment associated with posterior retinal breaks in infants and children. Retina. 1998;18:125–9. doi: 10.1097/00006982-199818020-00005. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Hirakata A, Hida T. The management of retinal detachments associated with choroidal colobomas by vitrectomy with cyanoacrylate retinopexy. Jpn J Ophthalmol. 1998;42:323–6. doi: 10.1016/s0021-5155(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 18.Sheta SM, Hida T, McCuen BW., 2nd Cyanoacrylate tissue adhesive in the management of recurrent retinal detachment caused by macular hole. Am J Ophthalmol. 1990;109:28–32. doi: 10.1016/s0002-9394(14)75574-6. [DOI] [PubMed] [Google Scholar]
- 19.Faulborn J, Witschel H. Intraocular application of tissue adhesive (histoacryl) in retinal detachment surgery. A clinicopathologic report of two cases. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1978;207:15–20. doi: 10.1007/BF00414489. [DOI] [PubMed] [Google Scholar]
- 20.Nasaduke I, Peyman GA. The use of autologous rabbit fibrin sealant to plug retinal holes in experimental detachments. Ann Ophthalmol. 1986;18:324–7. [PubMed] [Google Scholar]
- 21.Coleman DJ, Lucas BC, Fleischman JA, Dennis PH, Jr, Chang S, Iwamoto T, et al. A biologic tissue adhesive for vitreoretinal surgery. Retina. 1988;8:250–6. doi: 10.1097/00006982-198808040-00006. [DOI] [PubMed] [Google Scholar]
- 22.Liggett PE, Cano M, Robin JB, Green RL, Lean JS. Intravitreal biocompatibility of mussel adhesive protein. A preliminary study. Retina. 1990;10:144–7. doi: 10.1097/00006982-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Smiddy WE, Glaser BM, Green WR, Connor TB, Jr, Roberts AB, Lucas R, et al. Transforming growth factor beta. A biologic chorioretinal glue. Arch Ophthalmol. 1989;107:577–80. doi: 10.1001/archopht.1989.01070010591036. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Hirata A, Ishikawa S, Ohta K, Nakamura K, Okinami S, et al. Feasibility of using gelatin-microbial transglutaminase complex to repair experimental retinal detachment in rabbit eyes. Graefes Arch Clin Exp Ophthalmol. 2013;251:1109–14. doi: 10.1007/s00417-012-2245-8. [DOI] [PubMed] [Google Scholar]
- 25.Wassmer S, Leonard BC, Coupland SG, Baker A, Hamilton J, Torlone R, et al. The development of a cat model of retinal detachment and re-attachment. Adv Exp Med Biol. 2016;854:315–21. doi: 10.1007/978-3-319-17121-0_42. [DOI] [PubMed] [Google Scholar]
- 26.Fisher S. Cellular Remodeling in Mammalian Retina Induced by Retinal Detachment. Webvision. [Last accessed on 2017 Oct 23]. Available from: http://www.webvision.med.utah.edu/book/part-xii-cell-biology-of-retinal-degenerations/cellular-remodeling-in-mammalian-retina-induced-by-retinal-detachment/ [PubMed]
- 27.Mandal N, Lewis GP, Fisher SK, Heegaard S, Prause JU, la Cour M, et al. Protein changes in the retina following experimental retinal detachment in rabbits. Mol Vis. 2011;17:2634–48. [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis GP, Sethi CS, Linberg KA, Charteris DG, Fisher SK. Experimental retinal reattachment: A new perspective. Mol Neurobiol. 2003;28:159–75. doi: 10.1385/MN:28:2:159. [DOI] [PubMed] [Google Scholar]