Abstract
Acute retinal necrosis (ARN) following herpes simplex encephalitis (HSE) in an immunocompetent patient is a rare condition. Quantitative real-time polymerase chain reaction (qPCR) has made it possible to identify and quantify viral genome. We report a case of ARN following HSE managed with the help of qPCR. A 45-year-old man developed ARN following HSE and was treated with intravenous acyclovir and intravitreal foscarnet. The retinitis did not respond initially and the qPCR demonstrated a rise in the number of copies of the HSV-1 viral genome. With continued treatment with intravenous acyclovir and intravitreal ganciclovir, the retinitis healed and the qPCR confirmed a reduction in the viral load. qPCR has a high sensitivity and specificity for HSV and is a useful tool for diagnosis and treatment of viral retinitis.
Keywords: Acute retinal necrosis, herpes simplex encephalitis, herpes simplex virus, real-time polymerase chain reaction
Acute retinal necrosis (ARN) following herpes simplex encephalitis (HSE) in an immunocompetent patient is relatively rare. The most common causative virus in ARN is VZV and herpes simplex virus 1 (HSV-1) in older patients and HSV-2 in younger patients.[1,2] Advances in polymerase chain reaction (PCR) techniques have made it possible to accurately identify and quantify viral genome using quantitative real-time PCR (qPCR). However, it is still not clear whether quantitative analysis of viral DNA in ocular specimen correlates with clinical course of ARN. There are rare reports of changes in the viral DNA loads with antiviral treatment in ARN due to VZV but none on HSV in ARN.[3] We report a case of ARN due to HSV-1 following encephalitis and the evolution of viral loads with treatment as demonstrated by qPCR.
Case Report
A 45-year-old Asian Indian male came with complaints of decreased vision in the left eye following a history of viral encephalitis 10 months back. He was diagnosed to have viral encephalitis based on magnetic resonance imaging (MRI) showing focal temporal lobe enhancement, persistent seizures, abnormal cerebrospinal fluid, and MRI improvement with antivirals. He was a diabetic and hypertensive with hypothyroidism.
His vision at presentation was 6/6 N6 in the right eye and 6/24 N12 in the left eye. The left eye had 3+ cells in the anterior chamber and small keratic precipitates, and the fundus showed moderate vitritis with retinal hemorrhages and retinitis suggestive of ARN [Fig. 1]. He was advised intravenous acyclovir 500 mg, 8 hourly. Anterior chamber tap was positive for HSV-1 by semi-nested PCR with primers flanking the glycoprotein D gene of HSV genome (HSV-1 and HSV-2). PCR for the detection of HSV was carried out as described by us earlier.[4] The viral load was estimated in the DNA extracts of all test samples using a commercial kit – “Geno-sen's HSV-1 and HSV-2 real-time PCR kit (Genome Diagnostics Pvt., Ltd.,), and the assay was performed on Rotor-Gene (Hilden, Germany) real-time PCR equipment based on TaqMan principle. Quantification of HSV genome DNA by qPCR detected 788,874 copies/ml of DNA.
Figure 1.
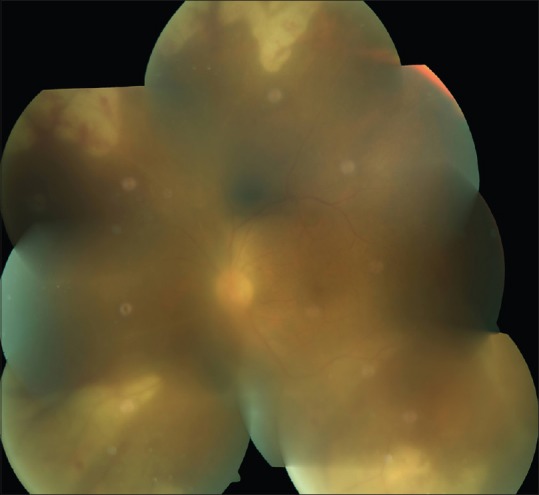
Left eye showing media haze due to vitritis, with peripheral retinal necrosis and haemorrhages
The patient received intravenous acyclovir and oral steroids (1 mg/kg body weight in tapering doses) along with intravitreal injections of foscarnet 2.4 mg every 3–4 days and a total of 3 such injections, but the retinitis did not respond. The qPCR from aqueous humor on day 12 showed increase in the copies of viral DNA to 12,212,384. The intravenous acyclovir was continued, but the subsequent intravitreal injections given were ganciclovir 2 mg since foscarnet was not financially viable. The PCR from aqueous humor was repeated after 1 week again, and the qPCR for HSV had further increased to 29,171,391 copies/ml of DNA. The intravenous acyclovir and the intravitreal ganciclovir were continued, and by day 21, the retinitis started healing. The intravenous acyclovir, which was continued to 21 days, was now shifted to oral valacyclovir. The retinitis healed well [Fig. 2], but the vitritis persisted. At 2 months from presentation, the retinitis had completely healed, but he had persistent vitreous condensation and opacities for which he underwent vitrectomy. The qPCR for HSV from the vitreous showed reduction in the copies to 243,436 copies/ml. Eight months after presentation, his vision in the left eye stabilized at 6/60, N24 with a quiet eye, attached retina, and partial optic atrophy.
Figure 2.
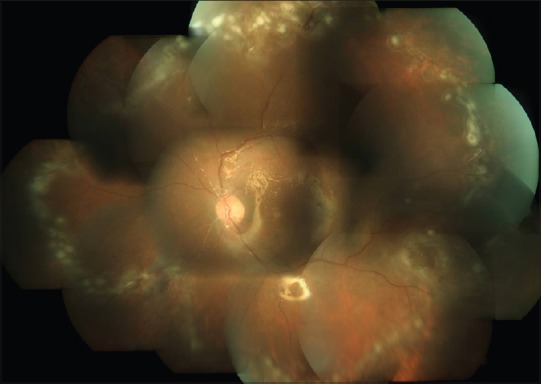
Healed retinitis with laser photocoagulation marks done to barrage the necrotic retina
Discussion
ARN after encephalitis although rare[5] has been reported to occur as early as 3 weeks[6] to as late as 20 years[7] after the encephalitis. HSV and VZV are the most commonly implicated viruses in ARN in the young immunocompetent patients. Of these, ARN caused by HSV-1 is seen in patients >25 years while HSV-2 is more often seen in children and neonates.[2] Diagnosis of the HSE is based on clinical features and radiological signs as was done in our patient. PCR or intrathecal antibodies are not essential in the diagnosis of HSE.
PCR is a highly specific and sensitive test[2] and has been used to detect the HSV genome in aqueous in ARN following HSE by Gain et al.[8] and Ganatra et al.[2] In our patient, the qPCR from aqueous was useful to ascertain the etiology of the ARN, especially because the retinitis did not respond initially. The qPCR showed increasing number of copies for HSV-1 in the first 3 weeks. Clinically, the retinitis showed onset of healing by the 3rd week from start of treatment, and reduction in viral load could be demonstrated only in the 8th week. This apparent lag time is because the vitreous qPCR was done from the sample acquired during vitrectomy done to reduce the vitreous opacities and condensation at the 8th week. In a study on the time profile of VZV DNA in ARN by Bernheim et al., the onset of reduction of viral load was delayed from onset of healing of retinal lesions in 2 patients.[3] In the rest, the decrease in viral load preceded the retinal healing. In our study also, based on the significant decrease in the number of copies, we can assume that the apparent lag time would have been lesser had we done the vitreous biopsy earlier. Their data also showed that conventional duration of 10 days of intravenous acyclovir could be insufficient in those patients who do not have rapid healing of the retinitis. Since the diagnosis was confirmed by qPCR in our patient, we increased the duration of the intravenous therapy and supplemented with intravitreal injections and followed it with oral valacyclovir. There is also no consensus in literature as to when the maintenance therapy can be stopped since low viral load may persist for several months.[3]
Therefore, when there is a clinical situation which suggests a differential diagnosis or there is an inadequate response to treatment, qPCR can be a useful tool in viral diseases of the eye,[9] particularly retinitis. Furthermore, anterior chamber tap is a safer and less invasive technique as compared to vitreous biopsy. In ARN, it is also convenient since it can be done before intravitreal injection in the affected eye and could be used for the early diagnosis and treatment of infectious retinitis.[10]
The immune status of our patient was assessed and found to be immunocompetent which was also seen in majority of the reported cases of unilateral ARN after HSE.[5,8] Majority had a treatment with antivirals during HSE for 2 weeks which is a standard. However, considering that ARN is a potentially blinding condition, we recommend that oral antivirals should follow IV antiviral therapy given during the HSE, and it should be continued for 6 months at least on a maintenance dose. This, along with early diagnosis and treatment of HSE, may reduce the risk of ARN.
It is hypothesized that the cause of the ARN after HSE maybe an in situ reactivation of the latent virus or an anterograde axonal transport of the infection from the trigeminal ganglion to the retina. Ganatra et al.[2] showed that ARN with a history of CNS disease is often due to infection with HSV-1 and HSV-2 rather than VZV. HSV-1 is the likely cause if the patient had a history of encephalitis and HSV-2 if the patient had meningitis.[2]
Conclusion
Uveitis in a patient with a history of HSE should carry a high index of suspicion for ARN. Physicians should be aware that any patient with a history of HSE having ocular complaints must have a dilated retinal checkup with a specialist.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Van Gelder RN, Willig JL, Holland GN, Kaplan HJ. Herpes simplex virus type 2 as a cause of acute retinal necrosis syndrome in young patients. Ophthalmology. 2001;108:869–76. doi: 10.1016/s0161-6420(01)00556-5. [DOI] [PubMed] [Google Scholar]
- 2.Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129:166–72. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim D, Germi R, Labetoulle M, Romanet JP, Morand P, Chiquet C, et al. Time profile of viral DNA in aqueous humor samples of patients treated for varicella-zoster virus acute retinal necrosis by use of quantitative real-time PCR. J Clin Microbiol. 2013;51:2160–6. doi: 10.1128/JCM.00294-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madhavan HN, Priya K, Anand AR, Therese KL. Detection of herpes simplex virus (HSV) genome using polymerase chain reaction (PCR) in clinical samples comparison of PCR with standard laboratory methods for the detection of HSV. J Clin Virol. 1999;14:145–51. doi: 10.1016/s1386-6532(99)00047-5. [DOI] [PubMed] [Google Scholar]
- 5.Bristow EA, Cottrell DG, Pandit RJ. Bilateral acute retinal necrosis syndrome following herpes simplex type 1 encephalitis. Eye (Lond) 2006;20:1327–30. doi: 10.1038/sj.eye.6702196. [DOI] [PubMed] [Google Scholar]
- 6.Pepose JS, Kreiger AE, Tomiyasu U, Cancilla PA, Foos RY. Immunocytologic localization of herpes simplex type 1 viral antigens in herpetic retinitis and encephalitis in an adult. Ophthalmology. 1985;92:160–6. doi: 10.1016/s0161-6420(85)34077-0. [DOI] [PubMed] [Google Scholar]
- 7.Kamel OR, Galloway GD, Trew DR. Delayed onset acute retinal necrosis 20 years following herpetic encephalitis. Eye (Lond) 2000;14(Pt 5):788–9. doi: 10.1038/eye.2000.207. [DOI] [PubMed] [Google Scholar]
- 8.Gain P, Chiquet C, Thuret G, Drouet E, Antoine JC. Herpes simplex virus type 1 encephalitis associated with acute retinal necrosis syndrome in an immunocompetent patient. Acta Ophthalmol Scand. 2002;80:546–9. doi: 10.1034/j.1600-0420.2002.800517.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugita S, Shimizu N, Watanabe K, Mizukami M, Morio T, Sugamoto Y, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92:928–32. doi: 10.1136/bjo.2007.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenberger SD, Kim SJ, Thorne JE, Mruthyunjaya P, Yeh S, Bakri SJ, et al. Diagnosis and treatment of acute retinal necrosis: A report by the American Academy of Ophthalmology. Ophthalmology. 2017;124:382–92. doi: 10.1016/j.ophtha.2016.11.007. [DOI] [PubMed] [Google Scholar]


