Abstract
Background
The -2518A/G (rs1024611) polymorphism of the CCL2 (C-C motif chemokine ligand 2), also known as MCP-1 (monocyte chemotactic protein-1) gene, has been reported to be associated with increased gynecological cancer risk, but the results are conflicting.
Methods
In this analysis, 1089 cases and 1553 controls from six publications were used to investigate the association between CCL2-2518A/G (rs1024611) polymorphism and the risk of gynecological cancer with a meta-analytic approach. Studies published on EBSCO, EMBASE, Web of Science, PubMed, SpringerLink, ScienceDirect, Weipu, and CNKI databases were identified (last update was on November 3, 2015). Six articles focused on the association between CCL2-2518A/G (rs1024611) polymorphism, and gynecological cancer risk was selected and data were extracted. The cancer type included endometrial cancer (n = 1), breast cancer (n = 2), ovarian cancer (n = 2), and cervical cancer (n = 1). All statistical analyses were performed using the STATA version 12.0 software.
Results
The meta-analysis showed that CCL2-2518A/G (rs1024611) polymorphism is associated with risk of gynecological cancer (GG vs AG + AA, OR = 1.55, 95%CI = 1.07–2.24, P < 0.05; AA vs GG, OR = 0.59 95%CI = 0.38–0.92, P < 0.05). Notably, the subgroup analysis demonstrated that the genotype AA is associated with a reduced gynecological cancer risk in Asians, but an increased risk when compared to AG in Europeans.
Conclusions
Our data demonstrated the CCL2-2518A/G (rs1024611) polymorphism is significantly associated with risk of gynecological cancer, and the association differs by ethnicity.
Keywords: Gynecological cancer, CCL2, CCL2-2518A/G, Polymorphism, Meta-analysis
Background
Gynecological cancer, including breast cancer (BC), endometrial carcinoma (EC), cervical cancer (CC), and ovarian cancer (OC), is the leading cause of cancer-related death in women. Currently, breast cancer is the most frequent among women with an estimated 1.67 million new cases diagnosed worldwide in 2012 [1]. Cervical cancer is the fourth most common cancer in women. According to GLOBOCAN 2012 statistics, there were approximately 266,000 women who died of cervical cancer worldwide [2].
Chemokines are proteins with low molecular weight (approximately 8–12 KD) that can induce leukocytes, including monocytes, neutrophil granulocytes, lymphocytes, tumor-associated macrophages (TAMs), natural killer (NK), and dendritic cells, into the area with infection. Chemokines and their receptors are associated with inflammation, cancer, allergy, autoimmunity, and AIDS [3]. Several studies showed that they play a critical role in pathological and physiological functions of the human body [4].
MCP-1 (monocyte chemotactic protein-1), also known as C-C motif chemokine ligand 2 (CCL2), is an inflammatory or inducible chemokine which is secreted by vascular endothelial cell (VEC) through exogenous lipopolysaccharide (LPS) and endogenous inflammatory factor (IL-1 and TNF-α) stimulation [5]. CCL2 and its receptor (CC chemokine receptor, CCR2) are pro-inflammatory mediators and chemoattractant that regulate the development and progression of tumor through migration and infiltration of monocytes or TAMs and CCL2/CCR2 axis. [6–8]. Epidemiology studies have shown the relationship between CCL2 level and atherosclerosis [9]. Animal model of CCL2 knockout mouse also showed decreased arterial lipid level and less infiltration of monocyte, which could alleviate the state of an illness or the risk of morbidity [10]. It has also been reported that the microenvironment regulated by chemokine was preferable to promote the proliferation of cancer cell and accelerate the process of cancerogenesis, which mechanisms include infiltrating into cancer tissue, promoting the proliferation of cancer cells through growth factor, inducing the neovascularization, and restraining the immune response during cancer [11].
Numerous studies that investigated the role of the -2518A/G (rs1026611) polymorphism in the MCP-1 (CCL2) gene have suggested that it is associated with cancer susceptibility, including breast cancer, cervical cancer, endometrial carcinoma, cervical cancer, and ovarian cancer, and might be a risk factor for cancer [12]. The rs1024611 is located in the promoter region of CCL2, and its function is complex. Previous study [13] reported that the rs1024611 is associated with allelic expression imbalance of CCL2, and there was higher level of CCL2 expression associated with the rs1024611G. However, the results reported so far remain inconclusive. We conducted a meta-analysis to further explore the association between CCL2 polymorphism and gynecological cancer risk.
Methods
This systematic review was conducted and reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) [14].
Study identification and selection
A systematic literature search was initiated in the EBSCO, EMBASE, Web of science, PubMed, SpringerLink, ScienceDirect, Weipu, and Chinese National Knowledge Infrastructure (CNKI) databases to identify articles that evaluated the association between polymorphism of the CCL2 gene and gynecological cancer risk (last update was on November 3, 2015). The search keywords were as follows: “cancer or tumor or carcinoma” in combination with “SNP or allele or polymorphism” and in combination with “CCL2 or chemokine (C-C motif) ligand 2 or MCP-1 or Monocyte chemoattractant protein 1.” There was a limit on the language of the article.
The process of the article selection was shown in Fig. 1, which included a hierarchical approach based on title, abstract, and full-text reading. Inclusion criteria of the eligible studies were as follows: case-control studies, cases were patients with gynecologic cancer (inclusive BC, EC, UCC, OC), controls consisted of healthy individuals, articles were used to evaluate the association between CCL2-2518A/G (rs1024611) polymorphism and gynecologic cancer (tumor) risk, and OR and 95% CI of the genotype or allele were reported in the studies. Reviews, duplicate book chapters, conference abstracts, and animal studies were excluded. The quality of these studies was assessed according to the Ottawa scale [15].
Fig. 1.
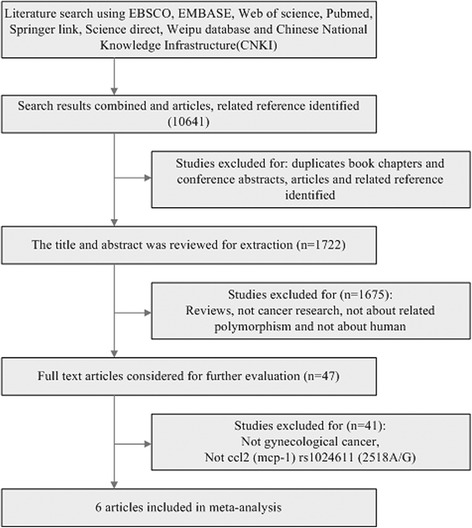
The flow chart of the article screening process
Data extraction
Two investigators rigorously assessed all eligible studies based on the inclusion criteria. Date extraction was independently performed by both investigators and included the name of first author, year of publication, study country, ethnicity of the study subjects, age of controls and cases, number of controls and cases, definition of case, genotyping methods, genotype, and allele.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) in controls was examined by asymptotic Pearson’s chi-square test. The association between CCL2-2518A/G (rs1024611) polymorphism and risk of cancer was estimated with OR (odds ratio) and 95% CI in individual case-control study. Five genetic models including recessive genotype (AA vs AG + GG), dominant genotype (GG vs AG + AA), heterozygous genotype (AA vs AG), homozygote genotype (AA vs GG), and allele genotype (A vs G) were explored to estimate the effects of the MCP-1-2518A/G (rs1024611) polymorphisms on cancer risk.
The heterogeneity among studies was assessed by a χ2-based Q test, and the pooled OR was calculated with a fixed-effects or random-effects model according to the heterogeneity test result. The pooled OR was calculated with a fixed-effects model when the Phet > 0.05. In contrast, a random-effects model was used when the Phet ≤ 0.05. Publication bias was assessed with Begg’s funnel plots (Pr > |z|) and Egger’s test (Pr > |t|). Subgroup analysis by ethnic group was also performed to evaluate the ethnicity-specific effect. All statistical analyses were performed using the STATA version 12.0 software.
Results
Study inclusion and characteristics
The search and selection criteria were showed in Fig. 1. A total of 10,641 articles were identified through an initial search of databases including EBSCO, EMBASE, Web of Science, PubMed, SpringerLink, ScienceDirect, Weipu, and CNKI. Once the duplicate articles, book chapters, and conference abstracts were excluded, 1722 articles remained. Then, we excluded those articles that were not cancer research, not related to polymorphisms in human, and 47 articles remained. Finally, 6 articles were selected into this study after 41 articles that were not related to CCL2-2518A/G (rs1024611) polymorphisms and gynecological cancer were excluded. The characteristics of the 6 studies included in this meta-analysis are shown in Table 1. There were 1 study of European population and 5 studies of Asian population, including a total of 1089 cases and 1553 controls. The cancer type included endometrial cancer (n = 1) [16], breast cancer (n = 2) [12, 17], ovarian cancer (n = 2) [18, 19], and cervical cancer (n = 1) [20]. In one study [16], the frequency of genotype in the control deviated from HWE (χ2 = 4.12, P < 0.05). Genotype and allele distribution of CCL2-2518A/G (rs1024611) polymorphisms in gynecologic cancer patients and control are showed in Table 2. The quality scores of these studies were between 5 and 7.
Table 1.
Characteristics of the studies included in the analysis
| Authors | Year of publication | Country | Host ethnicity | Age, years mean ± SD | Sample n | Genotyping | ||
|---|---|---|---|---|---|---|---|---|
| Case | Controls | Case | Controls | |||||
| Rukset A.et al | 2010 | Turkish | Asian | 57.75 ± 4.98 | 55.23 ± 6.55 | 50 | 211 | PCR-RFLP |
| Vasudha S.et al | 2015 | India | Asian | 49.05 ± 11.70 | 49.03 ± 11.69 | 200 | 200 | PCR |
| Łukasz K.et al | 2011 | Poland | European | 60.9 ± 10.1 | 59.7 ± 11.2 | 160 | 323 | PCR |
| Xin W.et al | 2014 | China | Asian | 54.6 ± 12.2 | 53.2 ± 11.7 | 257 | 273 | PCR-RFLP |
| Wu H.et al | 2010 | China | Asian | 54.2 ± 12.1 | 147 | 253 | PCR-RFLP | |
| Li L.et al | 2015 | China | Asian | 55.6 ± 12.8 | 54.9 ± 13.7 | 275 | 293 | PCR-RFLP |
SD standard deviation, PCR-RFLP polymerase chain reaction-restriction fragment length polymorphism
Table 2.
Genotype and allele distribution of ccl2 polymorphisms in gynecologic cancer patients and controls
| SNP | Study | Case | Control | HWE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AG | GG | A | G | AA | AG | GG | A | G | X 2 | p | ||
| rs1024611 | RA et al. | 26 | 17 | 7 | 69 | 31 | 124 | 82 | 5 | 330 | 92 | 4.12 | 0.04 |
| VS et al. | 86 | 83 | 31 | 255 | 145 | 97 | 86 | 17 | 280 | 120 | 0.11 | 0.74 | |
| TK et al. | 89 | 54 | 17 | 232 | 88 | 154 | 145 | 24 | 453 | 193 | 1.65 | 0.2 | |
| Xin et al. | 30 | 115 | 112 | 175 | 339 | 47 | 135 | 91 | 229 | 317 | 0.06 | 0.8 | |
| Wu et al. | 23 | 81 | 43 | 127 | 167 | 33 | 132 | 88 | 198 | 308 | 2.29 | 0.13 | |
| Li et al. | 37 | 120 | 118 | 194 | 356 | 58 | 140 | 95 | 256 | 330 | 0.24 | 0.62 | |
SNP single nucleotide polymorphism, HWE Hardy-Weinberg equilibrium
Meta-analysis
Quantitative data synthesis and hypothesis testing
A meta-analysis of five comparative genetic models of -2518A/G polymorphisms and gynecological cancer risk was performed. To define the appropriate model to be used, we interrogated the heterogeneity of the five genetic models. The heterogeneity result of AA vs AG + GG, GG vs AG + AA, AA vs GG, and A vs G were significant, with Phet value < 0.05 (I2 = 53.90%), 0.01 (I2 = 67.50%), 0.02 (I2 = 63.50%), and 0.01 (I2 = 66.00%) respectively, and the random-effects model was used to synthesize the data. AA vs AG model used the fixed-effects model. Indeed, ORs of dominant GG vs AG + AA model and heterozygous AA vs GG model were 1.55 (95%CI = 1.07–2.24, P < 0.05) and 0.59 (95%CI = 0.38–0.92, P < 0.05) respectively, indicating a significant association between CCL2-2518A/G and gynecological cancer risk (Fig. 2a, b; Table 3). On the contrary, no association between CCL2-2518A/G and gynecological cancer risk was noted in the AA vs AG + GG (OR = 0.87, 95%CI = 0.65–1.16, P > 0.05), AA vs AG (OR = 1.01, 95%CI = 0.83–1.23, P > 0.05), and A vs G (OR = 0.83, 95%CI = 0.68–1.02, P > 0.05) models (Table 3).
Fig. 2.
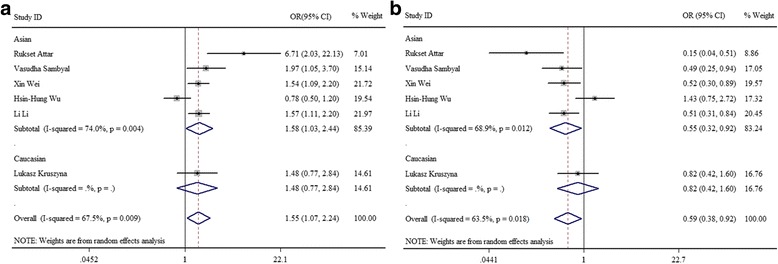
The forest plots of the association between CCL2-2518A/G polymorphism and gynecological cancer risk. a CCL2-2518A/G dominant (GG vs AG + AA) model. b Heterozygous (AA vs GG) model. CCL2 C-C motif chemokine ligand 2, ORs odds ratios
Table 3.
Meta-analysis of five comparative genetic models of -2518A/G polymorphisms on gynecological cancer risk
| Genetic model | OR (95% CI) | Z | P value | I2% | P het | Effect model | Begg’s test p > │z│ | Egger’s test p > │t│ |
|---|---|---|---|---|---|---|---|---|
| AA vs AG + GG | 0.87 (0.65, 1.16) | 0.96 | 0.34 | 53.90 | 0.05 | Random | 0.85 | 0.64 |
| GG vs AG + AA | 1.55 (1.07, 2.24) | 2.33 | 0.03 | 67.50 | 0.01 | Random | 0.57 | 0.31 |
| AA vs AG | 1.01 (0.83, 1.23) | 0.08 | 0.93 | 32.80 | 0.19 | Fixed | 0.85 | 0.61 |
| AA vs GG | 0.59 (0.38, 0.92) | 2.34 | 0.02 | 63.50 | 0.02 | Random | 0.85 | 0.53 |
| A vs G | 0.83 (0.68, 1.02) | 1.73 | 0.08 | 66.00 | 0.01 | Random | 0.35 | 0.95 |
OR odds ratio
Subgroup analysis
According to the study population, we also investigated the association by the ethnicity in the subgroup analysis with five genetic models. The results of subgroup analysis showed that AA vs AG + GG (OR = 0.77, 95%CI = 0.62–0.95, P < 0.05), AA vs GG (OR = 0.55, 95%CI = 0.32–0.92, P < 0.05), and A vs G (OR = 0.78, 95%CI = 0.64–0.97) of CCL2-2518A/G (rs1024611) were all associated with the reduced risk of gynecological cancer in Asians (Fig. 3a–e), There was also a reduced risk of gynecological cancer in AA genotype compared with GG (OR = 0.59, 95%CI = 0.38–0.92). In contrast, genetic models of GG vs AG + AA (OR = 1.58, 95%CI = 1.03–2.44, P < 0.05) in Asians and AA vs AG (OR = 1.55, 95%CI = 1.03–2.33, P < 0.05) in Europeans both showed an increased risk of gynecological cancer, and the overall population OR of the GG vs AG + AA was 1.55 (95%CI = 1.07–2.24, P < 0.05) (Fig. 3d, e).
Fig. 3.
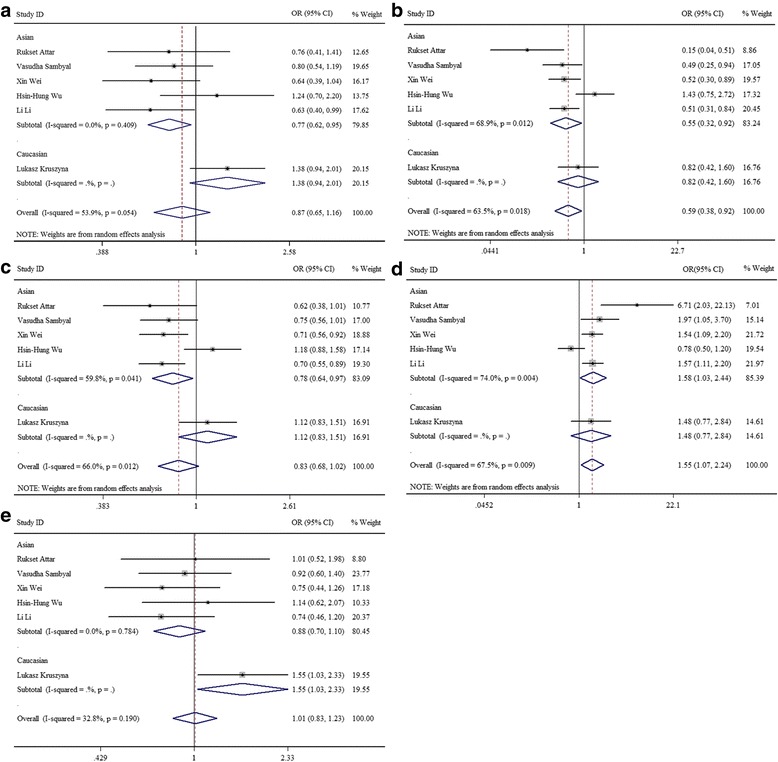
The forest plots of the association between CCL2-2518A/G polymorphism and gynecological cancer risk by ethnicity. a CCL2-2518A/G recessive (AA vs AG + GG) model. b CCL2-2518A/G homozygote (AA vs GG) model. c CCL2-2518A/G allele. d CCL2-2518A/G dominant (GG vs AG + AA) model. e CCL2-2518A/G heterozygous (AA vs AG) model. CCL2 C-C motif chemokine ligand 2, ORs odds ratios
Publication bias
Publication bias was assessed using the Begg’s test (P > │z│) and Egger’s test (P > │t│). The result suggested no significant publication bias (Fig. 4 and Table 3).
Fig. 4.
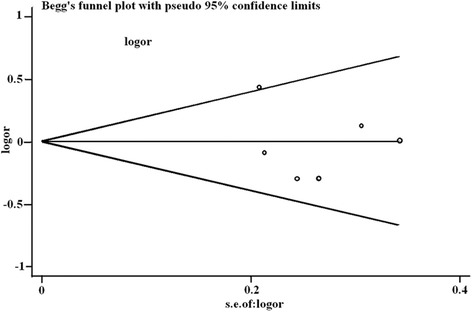
Begg’s funnel plot of the identified studies for the CCL2-2518A/G heterozygous (AA vs AG) model. CCL2, C-C motif chemokine ligand 2
Discussion
CCL2 is a member of the C-C chemokine family and involved in the tumorigenesis and metastasis of tumor by participating in mediating the tumor microenvironment [4]. CCL2 mediates the tumor generation and metastasis through multiple mechanisms, such as inducing tumor angiogenesis, mediating tumor immune response, promoting the tumor invasion and metastasis, and directly contributing to tumor progression [21]. The role of CCL2 in the development of tumor is mediated through its receptor CCR2. CCL2 recruits monocytes with CCR2 from the peripheral blood to tumor site and interacts with them. Subsequently, the recruited monocytes transform into TAMs stimulated by cell factors (like macrophage colony-stimulating factor (M-CSF), vascular permeability factor (VEGF)-1, IL-4, IL-10, IL-13), and then, tumor angiogenesis is induced by TAM-secreted angiogenesis factors (Fig. 5) [22, 23]. Of note, CCL2 stimulates tumor cell proliferation and migration by activating IP3-dependent Akt/PKB signal pathway [24]. And CCL2-CCR2 axis also promotes metastasis of tumor cell by activating ERK1/2-MMP2/9 signal pathway [25]. Previous researches have assessed the association between CCL2-2518A/G (rs1024611) polymorphism and cancer risk, including breast cancer, lung cancer, gastric cancer, bladder cancer, ovarian cancer, and others. Sambyal et al. showed that GG genotype of the CCL2-2518A/G polymorphism was a risk factor for breast cancer in Punjab [12]. However, Kruszyna et al. found that the CCL2-2518A/G polymorphism was not associated with the breast cancer risk [17]. Thus, we performed this meta-analysis to assess the association between CCL2-2518A/G and gynecological cancer risk.
Fig. 5.
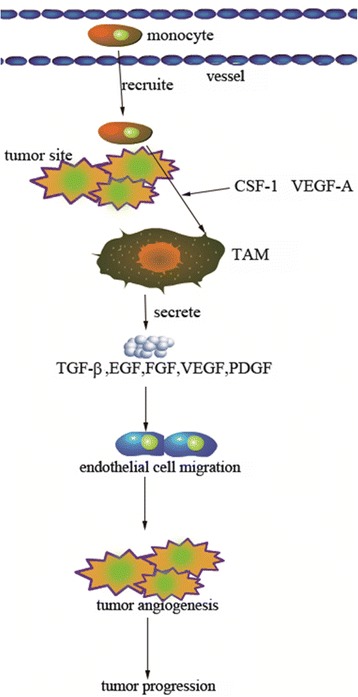
Schematic plot of CCL2 involved in tumor progression. The monocytes expressing CCL2 in blood vessel were recruited to tumor site through CCR2, TAMs when stimulated by cell factors (M-CSF-1, VEGF-A, IL-4, IL-10, IL-13), then TAMs induce endothelial cell migration by secreting TGF-β, EGF, FGF, VEGF, and PDGF, and these TAM-derived factors could promote tumor angiogenesis and CCL2-involved tumor progression. CCL2 chemokine (C-C motif) ligand 2, CCR2 CC chemokine receptor, TAMs tumor-associated macrophages, M-CSF macrophage colony-stimulating factor, VEGF vascular permeability factor, IL interleukin
This study identified CCL2-2518A/G genetic polymorphism is associated with gynecological cancer risk. The result showed that the homozygote GG, not AA or AG + GG, was a risk factor for gynecological cancer. In subgroup analysis by ethnicity, we found a distinct discrepancy between Asian and European population. Individuals carried AA or A genotype may have a decreased risk for gynecological cancer in Asians but AA genotype is a risk factor in Europeans, and the reason is unclear. This suggests that AA or A genotype may be a protective factor for gynecological cancer in Asians but AA genotype a risk factor in Europeans. In contrary to the finding of this study, Jia et al. conducted a meta-analysis of 11 studies on several types of cancer such as oral cancer, cervical cancer, breast cancer, and bladder cancer and demonstrated that the GG genotype was actually associated with the reduced overall cancer risk in Asian population [26]. However, this report only contained two (2/11) types of gynecological cancer, so we do not think the conclusion was applicable for all gynecological cancer types. These results suggested that the role of GG or G genotype in the incidence of gynecologic cancer in the Asians should be further investigated in future studies with sufficient sample size. Even so, the significant difference of A vs G in Asians indicated that allele A of rs1024611 seems to play a protective role comparing to G allele, and measures targeting this SNP may provide benefits in preventing the occurrence of gynecological cancer.
Although this meta-analysis included five genetic models, there are several limitations. First, because this study was used to analyze the association between CCL2 and gynecological cancer risk, the number of articles identified is relatively smaller than other researches that explored all types of cancers. Second, one article showed genotype frequency in the control group deviated from HWE, which might have affected the results of the original study and this meta-analysis. Third, while there were five studies conducted in Asian countries, there was only one study in Europeans. Thus, the subgroup analysis results may not be reliable.
Conclusion
The meta-analysis demonstrated a noteworthy association between CCL2-2518A/G polymorphism and the risk of gynecological cancer, and the association varied by ethnicity. In the overall analysis, the allele GG of rs1024611 was associated with an increased risk of gynecological cancer when compared to AA or AG + AA. However, in subgroup analysis, AA or A genotype was associated with a decreased risk of gynecological cancer in Asians compared with AG + GG, GG, and G, while AA was associated with an increased risk when compared with AG in Europeans. To further confirm these associations, studies with large sample size, involving different cancer types and multiple ethnic groups, should be considered.
Acknowledgements
None
Funding
None
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author.
Abbreviations
- BC
Breast cancer
- CCL2
Chemokine (C-C motif) ligand 2
- CCR2
CC chemokine receptor
- CNKI
Chinese National Knowledge Infrastructure
- EC
Endometrial carcinoma
- HWE
Hardy-Weinberg equilibrium
- IL
Interleukin
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein-1
- M-CSF
Macrophage colony-stimulating factor
- NK
Natural killer
- OC
Ovarian cancer
- ORs
Odds ratios
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
- SNP
Single nucleotide polymorphism
- TAMs
Tumor-associated macrophages
- UCC
Uterine cervix cancer
- VEC
Vascular endothelial cell
- VEGF
Vascular permeability factor
Authors’ contributions
SYH contributed to the project development, data collection, data analysis, and manuscript writing. XZZ contributed to the project development and data collection. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of China XD Group Hospital.
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mendes D, Alves C, Afonso N, Cardoso F, Passos-Coelho JL, Costa L, Andrade S, Batel-Marques F. The benefit of HER2-targeted therapies on overall survival of patients with metastatic HER2-positive breast cancer—a systematic review. Breast Cancer Res. 2015;17:140. doi: 10.1186/s13058-015-0648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan D, Wei K, Ling Y, Su S, Zhu M, Chen G. The prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysis. Med Sci Monit. 2015;21:882–889. doi: 10.12659/MSM.893714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95(9):858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21(1):41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribatti D, Nico B, Crivellato E, Vacca A. Macrophages and tumor angiogenesis. Leukemia. 2007;21(10):2085–2089. doi: 10.1038/sj.leu.2404900. [DOI] [PubMed] [Google Scholar]
- 6.Graves DT. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 1999;28(3):482–490. doi: 10.1086/515178. [DOI] [PubMed] [Google Scholar]
- 7.O'Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. The Biochemical journal. 2008;409(3):635–649. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 8.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10(4):329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, Paloscia L, Materazzo G, D'Annunzio E, Conti P. Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol. 2001;21(6):1090–1091. doi: 10.1161/01.ATV.21.6.1090. [DOI] [PubMed] [Google Scholar]
- 10.Daly CRB. Monnocyte chemoattractant protein-1(CCL2) in inflamaatory of disease and adaptive immunity,therapeutic opportunities and controversies. Microcirculation. 2003;10(3–4):247–257. doi: 10.1080/mic.10.3-4.247.257. [DOI] [PubMed] [Google Scholar]
- 11.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256(2):137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sambyal V, Guleria K, Kapahi R, Manjari M, Sudan M, Uppal MS, Singh NR. Association of the -2518 A/G polymorphism of MCP-1 with breast cancer in Punjab, North-West India. Asian Pac J Cancer Prev. 2015;16(16):7243–7248. doi: 10.7314/APJCP.2015.16.16.7243. [DOI] [PubMed] [Google Scholar]
- 13.Pham MHT, Bonello GB, Castiblanco J, Le T, Sigala J, He W, Mummidi S. The rs1024611 regulatory region polymorphism is associated with CCL2 allelic expression imbalance. PLoS One. 2012;7(11):e49498. doi: 10.1371/journal.pone.0049498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea BJ, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non-randomized studies in meta-analysis[J] Appl Eng Agric. 2014;18(6):727–34. [Google Scholar]
- 16.Attar R, Agachan B, Kuran SB, Cacina C, Sozen S, Yurdum LM, Attar E, Isbir T. Association of CCL2 and CCR2 gene variants with endometrial cancer in Turkish women. In vivo. 2010;24(2):243–248. [PubMed] [Google Scholar]
- 17.Kruszyna L, Lianeri M, Rubis B, Knula H, Rybczynska M, Grodecka-Gazdecka S, Jagodzinski PP. CCL2 -2518 A/G single nucleotide polymorphism as a risk factor for breast cancer. Mol Biol Rep. 2011;38(2):1263–1267. doi: 10.1007/s11033-010-0225-9. [DOI] [PubMed] [Google Scholar]
- 18.Wei X, Tian Y, Lu W, Li W, Zhang M, Lu X, Liu Y. Functional polymorphisms in monocyte chemoattractant protein-1 are associated with increased susceptibility to ovarian cancer. DNA Cell Biol. 2015;34(1):37–42. doi: 10.1089/dna.2014.2644. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Zhang J, Weng X, Wen G. Genetic variations in monocyte chemoattractant protein-1 and susceptibility to ovarian cancer. Tumour Biol. 2015;36(1):233–238. doi: 10.1007/s13277-014-2619-0. [DOI] [PubMed] [Google Scholar]
- 20.Wu HH, Lee TH, Tee YT, Chen SC, Yang SF, Lee SK, Ko JL, Wang PH. Relationships of single nucleotide polymorphisms of monocyte chemoattractant protein 1 and chemokine receptor 2 with susceptibility and clinicopathologic characteristics of neoplasia of uterine cervix in Taiwan women. Reprod Sci. 2013;20(10):1175–1183. doi: 10.1177/1933719113477481. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102(8):522–528. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14(3):149–154. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267(2):271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Lv X, Chen J, et al. CCL2–CCR2 axis promotes metastasis of nasopharyngeal carcinoma by activating ERK1/2-MMP2/9 pathway:[J] Oncotarget. 2016;7(13):15632–47. doi: 10.18632/oncotarget.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia LQ, Shen YC, Guo SJ, Hu QJ, Pang CS, Wang T, Chen L, Wen FQ. The 2518 A/G polymorphism in the MCP-1 gene and cancer risk: a meta-analysis. Asian Pac J Cancer Prev. 2013;14(6):3575–3579. doi: 10.7314/APJCP.2013.14.6.3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author.


