Abstract
Background
COPD is a complex, heterogeneous disease characterised by progressive development of airflow limitation. Spirometry provides little information about key aspects of pathology and is poorly related to clinical outcome, so other tools are required to investigate the disease. We sought to explore the relationships between quantitative CT analysis with functional, inflammatory and infective assessments of disease to identify the utility of imaging to stratify disease to better predict outcomes and disease response.
Methods
Patients from the AERIS study with moderate-very severe COPD underwent HRCT, with image analysis determining the quantity of emphysema (%LAA<− 950), small airways disease (E/I MLD) and bronchial wall thickening (Pi10). At enrolment subjects underwent lung function testing, six-minute walk testing (6MWT), blood sampling for inflammatory markers and sputum sampling for white cell differential and microbiological culture and PCR.
Results
122 subjects were included in this analysis. Emphysema and small airways disease had independent associations with airflow obstruction (β = − 0.34, p < 0.001 and β = − 0.56, p < 0.001). %LAA<− 950 had independent associations with gas transfer (β = − 0.37, p < 0.001) and E/I MLD with RV/TLC (β = 0.30, p =0.003). The distance walked during the 6MWT was not associated with CT parameters, but exertional desaturation was independently associated with emphysema (β = 0.73, p < 0.001). Pi10 did not show any independent associations with lung function or functional parameters.
No CT parameters had any associations with sputum inflammatory cells. Greater emphysema was associated with lower levels of systemic inflammation (CRP β = − 0.34, p < 0.001 and fibrinogen β = − 0.28, p =0.003). There was no significant difference in any of the CT parameters between subjects where potentially pathogenic bacteria were detected in sputum and those where it was not.
Conclusions
This study provides further validation for the use of quantitative CT measures of emphysema and small airways disease in COPD as they showed strong associations with pulmonary physiology and functional status. In contrast to this quantitative CT measures showed few convincing associations with biological measures of disease, suggesting it is not an effective tool at measuring disease activity.
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0734-y) contains supplementary material, which is available to authorized users.
Keywords: COPD, CT, Imaging, Phenotyping, Emphysema
Background
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease characterised by the progressive development of airflow limitation, leading to functional impairment and associated symptoms [1]. The clinical features and natural history of the condition vary considerably and traditional methods of measuring airflow obstruction do not reflect the complexity of the condition or the underlying pulmonary pathology. This has limited our understanding of COPD and consequently, there have not been the improvements in outcome seen in other chronic diseases and there are no significant disease-modifying medications. Other tools are required to help explain the heterogeneity and provide further insights into approaches to study and manage the condition.
Computed tomography (CT) can image the key pathological changes seen in COPD, including emphysema and remodelling of the large and small airways [2]. These pathologies both contribute to airflow obstruction and therefore CT has the potential to provide vital insights into the exact nature of the underlying pathophysiology. Newly developed automated image assessment techniques enable segmentation of the lung parenchyma and airways from the chest wall and surrounding structures, thus allowing quantitative analysis of emphysema, bronchial wall dimensions and small airways disease to characterise this heterogeneous disease more effectively.
Emphysema can be visualised as low attenuation areas on CT and by applying a density mask to the lung parenchyma, the percentage of voxels below − 950 Hounsfield (%LAA<− 950) can be used to assess the quantity of emphysema. This method shows strong associations with histological measures of emphysema as well as physiological markers of disease [3–5]. Using CT imaging, three-dimensional reconstructions of the bronchial tree can be generated down to the fifth or sixth generation airway, allowing analysis of airway wall dimensions. Thus far, studies have shown equivocal results, with some demonstrating thicker airway walls in COPD [6–8] and others demonstrating thinner walls [3, 9]. This may be partly explained by the variation in methods used to describe airway wall dimensions and therefore a standardised parameter called Pi10 has been developed. This predicts the square root of the wall area for a hypothetical airway with an internal perimeter of 10 mm, giving a single value for bronchial wall thickening of the entire bronchial tree and has shown an inverse correlation with forced expiratory volume in one second (FEV1) [3]. Due to limited resolution, CT cannot image the small airways directly and instead quantification of air trapping can be used as a surrogate marker. A number of CT-derived methods exist to measure air trapping with one of the most widely used being the ratio of the mean lung density (MLD) in expiration to inspiration. In COPD, this measure has been found to correlate with a number of physiological and functional parameters [10–12].
Recent analysis from large COPD cohort studies have added significantly to our understanding of the use of quantitative CT imaging in COPD, in particular exploring the relationships with pulmonary physiology [3, 13], functional ability [14, 15] and tracking the longitudinal progression of disease over time [13]. However, these large-scale studies allow only limited characterisation of their cohorts, with fewer detailed assessments possible. Furthermore, few studies have assessed biological markers of COPD, including measures of inflammation and infection. This further work is required to confirm CT analysis as an imaging biomarker of disease that can be a useful research tool to provide insights into the disease and as a clinical measure to guide management of patients with COPD. In the present study we performed quantitative CT analysis on a well-characterised cohort of patients with moderate to very severe COPD and explored the relationship with multiple clinical, physiological, microbiological and inflammatory indices to identify the utility of imaging to stratify disease to better predict outcomes and disease response.
Methods
Study design and study population
The Acute Exacerbation and Respiratory Infections in COPD (AERIS) study (ClinicalTrials.gov: NCT01360398, GSK study number 114378) is a prospective, observational cohort study and has been described in detail elsewhere [16, 17]. Briefly, subjects with moderate to very severe COPD, as defined by GOLD guidelines [1] were recruited. Subjects had at least a 10 pack year smoking history and had suffered at least one moderate or severe exacerbation in the preceding 12 months. Exclusion criteria included a history of other pulmonary disease, long-term antibiotics/systemic steroids or an exacerbation within the month prior to recruitment. All Subjects gave written informed consent and the study was approved by the South Central - Southampton B NRES Committee. We report a secondary analysis focusing on CT results at enrolment.
Procedures
At enrolment subjects had full clinical assessment, CT imaging, pulmonary function testing, six-minute walk testing, blood and sputum sampling.
CT scanning and quantitative image analysis
Subjects underwent volumetric CT scans of the chest using a Siemens Sensation 64 CT scanner. The imaging protocol consisted of; slice thickness 0.75 mm, slice separation 0.5 mm, tube voltage 120KV, effective mAs 90mAs (using dose modulation), collimation 0.6 mm and a pitch of 1. Subjects were scanned at full inspiration and at maximum expiration. These images were assessed by an experienced thoracic radiologist who visually reported the presence of bronchiectasis. This was defined as either a non-tapering bronchus with an internal diameter over 110% than the adjacent pulmonary artery or the presence of a visible bronchi within 1 cm of the pleural surface. The bronchiectasis score was determined in each lobe using a previously validated scoring system [18]; 0 if no bronchiectasis was present, 1 if less than 25% of bronchi were bronchiectatic, 2 for 25–49%, 3 for 50–74% and 4 for over 75%. An overall score was calculated out of maximum of 24 (the lingual was included as a separate lobe). Images reconstructed with the B35 kernel were used for image analysis using Apollo Software (VIDA Diagnostics). Emphysema was quantified by the percent of lung voxels on the inspiratory scan with attenuation values below -950HU (%LAA<− 950). Bronchial wall thickening was quantified using the standardised parameter Pi10, which is the square root of the wall area of a hypothetical airway with a 10 mm internal perimeter. A surrogate marker for small airways disease was measured using the ratio of mean lung attenuation on expiratory and inspiratory scans (E/I MLD).
Pulmonary function and six-minute walk tests
Pre and post-bronchodilator spirometry, plethysmography and gas transfer were performed in accordance with ATS guidelines [19]. The 6MWT was performed on a standard 30-m course and the patient was instructed to walk round the course at their own pace for 6 min and the number of laps walked in 6 min recorded. Finger-tip pulse oximetry was used to measure baseline and lowest saturations.
Sputum and blood testing
Sputum samples were obtained by spontaneous expectoration or by induction and were processed according to standard methods, described previously [20]. Briefly, sputum was collected in a petri dish and processed within 2 h. Sputum plugs were separated from saliva using tweezers and samples were divided for biomarker and microbiology analysis. Samples were sent to the Public Health England laboratory for culture-based microbiology. Potential bacterial respiratory pathogens, including Haemophilus influenzae (NTHI), Moraxella catarrhalis (MCAT), Streptococcus pneumoniae (SP), Pseudomonas aeruginosa (PA), and Staphylococcus aureus (SA) were identified using conventional culture techniques and PCR, described previosuly [17]. Sputum for white cell differential analysis was filtered through 100 μm filters and centrifuged at 400 g for 10 min at 4 °C. The resulting cell pellet was resuspended in PBS at a concentration of 5 x 105 cells/ml and were loaded onto poly-L-lysine coated glass slides using a cytocentrifuge. These were stained with Rapid Romanowsky stain (Raymond Lamb Ltd., Eastbourne, UK) and differential cell counts were obtained by counting 400 cells using light microscopy. For quality control purposes, only sputum samples with fewer than 30% squamous cells were included in the analysis.
Phlebotomy was performed and samples were processed by conventional methods for full blood count, C-reactive protein (CRP), fibrinogen and pro-calcitonin (PCT).
Statistical analysis
Statistical analyses were performed using SPSS version 23. The differences in demographic, physiological, biological and CT parameters between GOLD groups (2010, spirometric criteria) were tested using the Kruskal-Wallis test. Univariate associations between these parameters were assessed using Spearman’s correlation with rho and p values presented. FEV1%, TLCO%, RV/TLC, 6MWD, desaturation on exertion, CRP, fibrinogen, and sputum neutrophils/eosinophils were analysed via multiple linear regression on dependent variables such as CT parameters %LAA<− 950, E/I MLD and demographic variables (age, gender, current smoking status, pack years and BMI). Only variables that made a significant difference to the model are included in the results, with variables chosen using forward selection. Differences in CT parameters and FEV1% between subjects who could walk more or less than 350 m or did/did not desaturate at the 6MWT were tested using the Mann Whitney U test and logistic regression was used to conduct multivariate analysis. Mann Whitney U test was also used to assess the differences in CT parameters between subjects who did or did not culture bacteria in their sputum. Throughout the analysis a p-value of < 0.05 was considered statistically significant. Since the comparisons presented here are not part of the primary and secondary objectives of the AERIS study, the results should be considered as post hoc.
Results
Of 152 patients screened, 127 were enrolled onto the study. 122 of these had complete CT data as 5 of the scans could not be quantitatively analysed fully for technical reasons. All 122 had complete data for demographics, spirometry and CRP. 117 subjects had gas transfer data, 113 had plethysmography data, 120 had 6MWT parameters, 121 blood white cells and procalcitonin and 109 had fibrinogen. 106 subjects had sufficient sputum collected for microbiology culture while 98 had microbiology PCR performed. As sputum was prioritised for microbial analysis, only 75 subjects had enough for white cell differential and of these, 66 were deemed good quality samples (< 30% squamous cells). Baseline data for demographics, pulmonary physiology, biological and CT data are shown in Table 1. The majority of patients had moderate or severe airflow obstruction. There was no significant difference in age, gender, BMI or smoking status between the GOLD groups. As expected there was significantly lower FEV1% and FEF75–25% in the more severe GOLD groups. There was also significantly lower gas transfer and higher RV and RV/TLC ratio in subjects with severe and very severe COPD compared to moderate COPD. Subjects with very severe COPD had significantly lower 6MWD than subjects with either moderate or severe COPD. Subjects with severe and very severe COPD desaturated during exertion significantly more than those with moderate disease. There was no significant difference in sputum or blood inflammatory markers between the GOLD groups.
Table 1.
Characteristics of study subjects included in the study
| N = 122 | GOLD 2 (N = 56) | GOLD 3 (N = 49) | GOLD 4 (N = 17) | P value | |
|---|---|---|---|---|---|
| Age (years) | 67.0 (11.0) | 67.5 (13) | 67.0 (10) | 65.0 (10) | 0.992 |
| Male | 67 | 31 | 25 | 11 | 0.618 |
| BMI (kg/m2) | 27.1 (6.9) | 27.7 (7.3) | 26.5 (8.0) | 25.4 (7.2) | 0.054 |
| Current smoker | 46 | 21 | 18 | 7 | 0.947 |
| Pulmonary Function | |||||
| FEV1% | 47.2 (23.7) | 59.1 (12.9) #^ | 38.8 (10.5)*^ | 26.2 (5.9)*# | < 0.001 |
| FEV1/FVC | 0.43 (0.12) | 0.50 (0.10) #^ | 0.38 (0.15) *^ | 0.29 (0.08) *# | < 0.001 |
| FEF75–25% | 13.2 (8.3) | 17.2 (6.4) #^ | 11.0 (4.7) *^ | 6.8 (2.2) *# | < 0.001 |
| TLCO% | 58.3 (28.7) | 70.4 (23.9) #^ | 53.0 (22.8)* | 44.6 (11.8)* | < 0.001 |
| RV% | 144.8 (59.2) | 133.8 (38.9) #^ | 159.2 (48.1)* | 202.7 (96.6)* | < 0.001 |
| RV/TLC | 0.54 (0.14) | 0.49 (0.14) #^ | 0.58 (0.10)* | 0.64 (0.16)* | < 0.001 |
| Functional Markers | |||||
| 6MWD (meters) | 303.5 (174) | 360.0 (155)^ | 300.0 (156)^ | 169.5 (129)*# | < 0.001 |
| Desaturation on Exertion (%) | 4.0 (5.0) | 3.0 (3.0) #^ | 5.0 (8.0)* | 6.0 (6.5)* | 0.001 |
| Blood Measures | |||||
| Blood neutrophils (109L) | 4.8 (1.6) | 4.8 (1.6) | 4.7 (2.0) | 4.8 (1.6) | 0.914 |
| Blood eosinophils (109L) | 0.20 (0.20) | 0.20 (0.20) | 0.20 (0.20) | 0.20 (0.20) | 0.082 |
| Fibrinogen (g/L) | 4.8 (1.1) | 4.8 (1.1) | 4.8 (1.0) | 5.0 (1.4) | 0.813 |
| CRP (mg/L) | 5.0 (7.0) | 5.0 (6.8) | 4.0 (6.5) | 6.0 (7.0) | 0.570 |
| Procalcitonin (μg/L) | 0.059 (0.03) | 0.056 (0.02) | 0.061 (0.03) | 0.073 (0.05) | 0.236 |
| Sputum Differential | |||||
| Sputum Neutrophils(%) | 45.9 (70.8) | 17.7 (62.6) | 44.3 (75.8) | 71.2 (39.3) | 0.110 |
| Sputum Eosinophils (%) | 2.02 (5.2) | 1.97 (4.0) | 2.20 (5.7) | 2.62 (4.8) | 0.596 |
| CT Measures | |||||
| Emphysema (%LAA<− 950) | 12.0 (20.7) | 9.6 (13.1)#^ | 15.8 (26.9)* | 24.8 (12.3)* | < 0.001 |
| Bronchial wall area (Pi10) | 3.80 (0.12) | 3.79 (0.13) | 3.78 (0.10)^ | 3.84 (0.05)# | 0.040 |
| Small airways disease (E/I MLD) | 0.92 (0.07) | 0.88 (0.07) #^ | 0.93 (0.05)* | 0.96 (0.03)* | < 0.001 |
| Bronchiectasis | 8 (6.6) | 1 (1.8) | 5 (10.2) | 2 (11.8) | 0.143 |
Values given as medians (IQR). Males, smoking status and bronchiectasis given as number of subjects. N = 122 for whole cohort. For gas transfer n = 117, plethysmography n = 113, 6MWT parameters n = 120, blood white cells and procalcitonin n = 121, fibrinogen n = 109, sputum white cell differential n = 66. P value indicates difference between GOLD groups where < 0.05 taken as significant. * significant difference vs. GOLD 2 group, # significant difference vs. GOLD 3 group and ^ significant difference vs. GOLD 4 group
When assessing the CT parameters, there was significantly more emphysema and air trapping in severe and very severe COPD subjects compared to subjects with moderate COPD (Table 1). Pi10 was significantly raised in very severe COPD compared to subjects with severe COPD. Only 8 subjects had clinically significant bronchiectasis present on their CT scans and even then this was relatively mild with a median bronchiectasis score of 2.5. Given this low number of subjects with bronchiectasis, further analysis was not possible on this CT parameter. There was a significant positive association between %LAA<− 950 and E/I MLD (rho = 0.47, p < 0.001) (Additional file 1: Figure S1). There was also an inverse relationship between %LAA<− 950 and Pi10 (rho = − 0.36, p < 0.001) and a much weaker association between E/I MLD and Pi10 (rho = 0.18, p 0.045).
Relationship between CT parameters, PFTs and functional markers of disease
There were strong negative associations between airflow obstruction and %LAA<− 950 and E/I MLD (Table 2 and Fig. 1). In contrast to this there was no significant association between FEV1% and Pi10. On multivariate analysis only %LAA<− 950, E/I MLD and BMI had independent associations with FEV1% (Table 3). %LAA<− 950 and E/I MLD also had significant associations with gas transfer. %LAA<− 950 was the only CT parameter which had significant independent associations with gas transfer on multivariate analysis. %LAA<− 950 and E/I MLD also had significant associations with RV% and RV/TLC ratio. On multivariate analysis only E/I MLD, FEV1% and gender significantly predicted RV/TLC ratio.
Table 2.
Spearman’s correlation analysis between CT parameters, pulmonary function tests, functional markers, blood and sputum markers
| %LAA<− 950 | E/I MLD | Pi10 | |
|---|---|---|---|
| Pulmonary Function Tests | |||
| FEV1% | −0.49*** | −0.63*** | − 0.11 |
| FVC% | 0.06 | −0.21* | − 0.31** |
| FEV1/FVC | −0.72*** | − 0.64*** | 0.12 |
| TLCO% | −0.51*** | −0.34*** | 0.01 |
| RV% | 0.36*** | 0.64*** | −0.05 |
| RV/TLC | 0.19* | 0.62*** | 0.16 |
| Functional Markers | |||
| 6MWD (meters) | −0.14 | −0.10 | − 0.11 |
| Desaturation on exertion (%) | 0.56*** | 0.27** | −0.11 |
| Blood Markers | |||
| Blood neutrophils (109L) | −0.06 | −0.11 | 0.15 |
| Blood eosinophils (109L) | 0.15 | 0.01 | 0.01 |
| Fibrinogen (g/L) | −0.20* | − 0.04 | 0.21* |
| CRP (mg/L) | −0.27** | −0.28** | 0.25* |
| Procalcitonin (μg/L) | −0.01 | −0.06 | 0.18* |
| Sputum Differential | |||
| %neutrophils | 0.17 | 0.19 | −0.10 |
| %eosinophils | 0.16 | 0.04 | −0.11 |
Spearman’s r values given. N = 122 apart from associations with gas transfer (n = 117), plethysmography (n = 113), 6MWT parameters (n = 120), blood white cells and procalcitonin (n = 121), fibrinogen (n = 109) and sputum white cell differential (n = 66). *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 1.
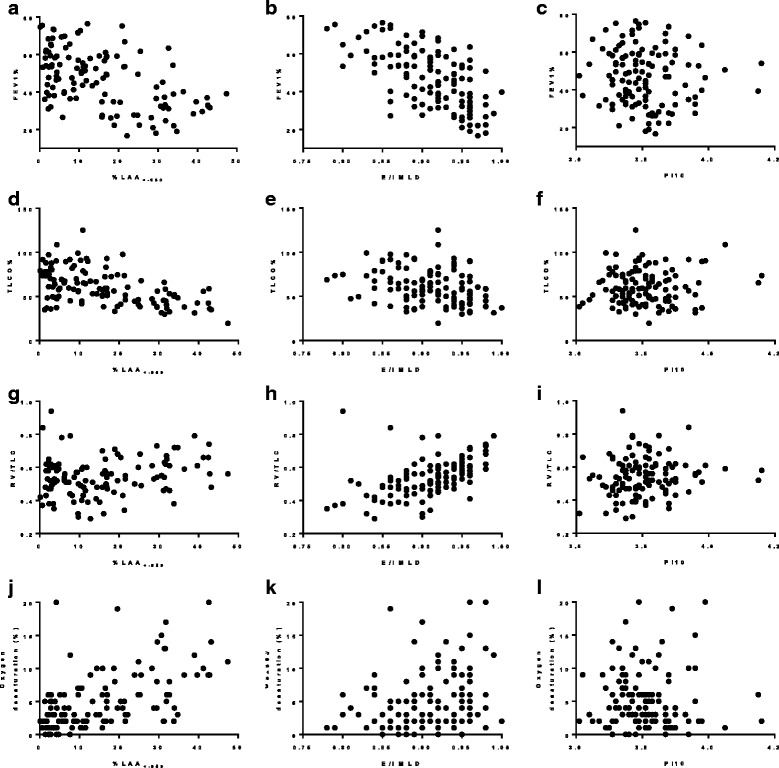
Scatterplots of (a) %LAA<−950 against FEV1% (rho −0.49***) (b) E/I MLD against FEV1% (rho −0.63***) (c) Pi10 against FEV1% (rho −0.11) (d) %LAA<− 950 against TLCO% (rho − 0.51***) (e) E/I MLD against TLCO% (rho − 0.34***) (f) Pi10 against TLCO% (rho 0.01) (g) %LAA<− 950 against RV/TLC (rho 0.19*) (h) E/I MLD against RV/TLC (rho 0.62***) (i) Pi10 against RV/TLC (rho 0.16) (j) %LAA<− 950 against oxygen desaturation (rho 0.56***) (k) E/I MLD against oxygen desaturation (rho 0.27**) (l) Pi10 against oxygen desaturation (rho − 0.11). For comparisons with FEV1% N = 122, for TLCO% N = 117, for RV/TLC N = 113 and for oxygen desaturation N = 120. * p < 0.05, ** p < 0.01, *** p < 0.001
Table 3.
Multiple regression analysis predicting FEV1%, TLCO%, RV/TLC, 6MWD, desaturation on exertion, CRP, fibrinogen and sputum neutrophils
| B coefficient | Standardised B coefficient | significance | |
|---|---|---|---|
| FEV1% | |||
| %LAA<− 950 | − 0.42 | − 0.34 | < 0.001 |
| E/I MLD | − 180.8 | − 0.56 | < 0.001 |
| BMI | −0.56 | − 0.20 | 0.010 |
| TLCO% | |||
| %LAA<− 950 | −0.62 | −0.37 | < 0.001 |
| FEV1% | 0.49 | −0.39 | < 0.001 |
| Gender | −7.5 | −0.19 | 0.014 |
| RV/TLC | |||
| E/I MLD | 0.75 | 0.30 | 0.003 |
| FEV1% | −0.003 | −0.32 | 0.002 |
| Gender | 0.07 | 0.28 | < 0.001 |
| 6MWD (metres) | |||
| FEV1% | 3.03 | 0.41 | < 0.001 |
| BMI | −5.19 | −0.26 | 0.003 |
| Gender | −38.2 | −0.17 | 0.044 |
| Desaturation on exertion (%) | |||
| %LAA<−950 | 0.041 | 0.73 | < 0.001 |
| BMI | 0.032 | 0.248 | 0.004 |
| Gender | 0.233 | 0.164 | 0.037 |
| CRP (mg/L) | |||
| %LAA<− 950 | −0.026 | −0.34 | < 0.001 |
| Fibrinogen (g/L) | |||
| %LAA<−950 | −0.020 | −0.28 | 0.003 |
| Age | 0.022 | 0.216 | 0.020 |
| Sputum Neutrophils (%) | |||
| Current smoker | −21.1 | −0.29 | 0.018 |
%LAA<−950, E/I MLD, PI10, bronchiectasis, FEV1% and demographics variables (age, gender, current smoking status, pack years and BMI) were combined into a regression model to predict each of the dependent variables. Only variables that made a significant difference to the model are included in the results. The natural log of CRP and desaturation on exertion (+ 1) were used for this analysis to normalise the distribution of the residuals
6MWD did not have any significant associations with any CT parameters (Table 2). On multivariate analysis only FEV1%, gender and BMI showed an independent association with 6MWD (Table 3). There was also no difference in CT parameters between subjects who could walk more than 350 m compared to those that could not (Table 4). On logistic regression analysis higher FEV1 and being male were the only variables that predicted being able to walk over 350 m (Table 6). Desaturation on exertion was significantly associated with both %LAA<− 950 and E/I MLD (Table 2 and Fig. 1). On multivariate analysis %LAA<− 950, BMI and gender were independently associated with desaturation (Table 3). Subjects who desaturated > 5% had a significantly raised %LAA<− 950 and E/I MLD and significantly lower FEV1%, but showed no difference in Pi10 (Table 5). On logistic regression analysis only higher %LAA<− 950 predicted whether a subject desaturated more than 5% (Table 6).
Table 4.
CT parameters and FEV1% in patient who could walk more or less than 350 m at the 6MWT
| < 350 m (N = 75) | > 350 m (N = 45) | P Value | |
|---|---|---|---|
| FEV1% | 40.1 (24.9) | 53.4 (14.9) | 0.002 |
| %LAA<− 950 | 15.8 (24.1) | 9.7 (13.9) | 0.027 |
| E/I MLD | 0.92 (0.07) | 0.91 (0.07) | 0.150 |
| Pi10 | 3.81 (0.12) | 3.79 (0.12) | 0.641 |
Values represent medians and IQR. P value tested using Mann Whitney U test
Table 6.
Logistic regression predicting which variables contributed to patients walking over 350 m or desaturating during the 6MWT
| Odds ratio | 95% Lower CI | 95% upper CI | significance | |
|---|---|---|---|---|
| 6MWD > 350 m | ||||
| FEV1% | 1.049 | 1.019 | 1.079 | 0.001 |
| Female | 0.379 | 0.165 | 0.871 | 0.022 |
| Desaturation > 5% | ||||
| %LAA<−950 | 1.101 | 1.059 | 1.143 | < 0.001 |
44 subjects desaturated > 5% and 76 subjects did not
45 subjects walked over 350 m and 75 subjects did not
%LAA<− 950, E/I MLD and PI10, FEV1% and demographics variables (age, gender, current smoking status, pack years and BMI) were combined into a regression model to predict each of the dependent variables. Only variables that made a significant difference to the model are included in the results
Table 5.
CT parameters and FEV1% in patient who desaturated or not at 6MWT
| < 5% desaturation (N = 76) | > 5% desaturation (N = 44) | P Value | |
|---|---|---|---|
| FEV1% | 52.9 (19.2) | 36.5 (18.4) | < 0.001 |
| %LAA<− 950 | 7.9 (13.5) | 24.9 (19.3) | < 0.001 |
| E/I MLD | 0.91 (0.07) | 0.94 (0.06) | 0.010 |
| Pi10 | 3.91 (0.12) | 3.78 (0.11) | 0.232 |
Values represent medians and IQR. P value tested using Mann Whitney U test
Relationship between CT parameters and blood and sputum inflammatory markers and sputum microbiology
There were no significant associations between CT parameters and blood neutrophils and eosinophils (Table 2). Emphysema had a significant inverse relationship with CRP and fibrinogen, while E/I MLD had an inverse association with CRP. In addition, Pi10 had a positive association with fibrinogen, CRP and procalcitonin. On multivariate analysis only %LAA<− 950 had a weak independent relationship with CRP and fibrinogen (Table 3).
There were no associations between sputum neutrophils and eosinophils and any of the quantitative CT measures (Table 2 and Table 3) To ensure that only using good quality sputum samples (squamous cells < 30%) did not bias the results the analysis was repeated with all samples and similar results were found (Additional file 1: Table S1).
There were also few differences in emphysema, air trapping and Pi10 values between subjects who cultured bacteria in their sputum and those that did not (Table 7). The only exceptions to this was that Pi10 was significantly higher in those that did not have SA in their sputum compared to these that did and E/I MLD was significantly higher in those that cultured PA compared to those that didn’t. When repeating the analysis using PCR to detect the presence of bacteria in sputum the results were much the same, with only E/I MLD being significantly higher in those that had PA detected compared to those that did not (Additional file 1: Table S2).
Table 7.
CT parameters in subjects according to sputum microbiology
| Frequency | %LAA<−950 | E/I MLD | Pi10 | |
|---|---|---|---|---|
| Potentially Pathogenic Bacteria | ||||
| Culture positive | 55 | 10.9 (17.2) | 0.92 (0.07) | 3.79 (0.12) |
| Culture negative | 51 | 15.1 (24.0) | 0.90 (0.07) | 3.78 (0.09) |
| P value | – | 0.260 | 0.152 | 0.146 |
| Haemophilus influenzae | ||||
| Culture positive | 31 | 11.5 (18.0) | 0.92 (0.08) | 3.79 (0.11) |
| Culture negative | 75 | 13.3 (20.0) | 0.91 (0.07) | 3.79 (0.10) |
| P value | – | 0.370 | 0.207 | 0.197 |
| Moraxella catarrhalis | ||||
| Culture positive | 8 | 12.4 (23.1) | 0.93 (0.07) | 3.78 (0.10) |
| Culture negative | 98 | 12.5 (20.3) | 0.91 (0.07) | 3.79 (0.11) |
| P value | – | 0.981 | 0.720 | 0.549 |
| Streptococcus pneumoniae | ||||
| Culture positive | 18 | 10.2 (9.9) | 0.92 (0.05) | 3.83 (0.11) |
| Culture negative | 88 | 14.3 (24.9) | 0.91 (0.08) | 3.79 (0.09) |
| P value | – | 0.501 | 0.626 | 0.203 |
| Staphylococcus aureus | ||||
| Culture positive | 6 | 13.5 (22.4) | 0.91 (0.08) | 3.74 (0.05) |
| Culture negative | 100 | 12.5 (20.6) | 0.91 (0.07) | 3.80 (0.11) |
| P value | – | 0.667 | 0.682 | 0.039* |
| Pseudomonas aeruginosa | ||||
| Culture positive | 6 | 21.4 (27.5) | 0.95 (0.06) | 3.83 (0.16) |
| Culture negative | 100 | 12.0 (20.1) | 0.91 (0.07) | 3.79 (0.11) |
| P value | – | 0.367 | 0.034* | 0.498 |
Frequency given as number of subjects. For CT parameters, values represent medians and IQR. *P < 0.05 using Mann Whitney U test
Discussion
In the AERIS study we used sophisticated, cutting-edge techniques to explore the heterogeneity of COPD, resulting in an extremely well-characterised cohort of subjects. This was complemented by using CT analysis to understand the key structural changes identified in COPD and describing the relationship with a number of key physiological, functional and biological markers of disease. The main findings from the present analysis were the demonstration of a novel independent association between emphysema and desaturation on exertion, but interestingly not on exercise capacity. We also showed that CT-derived measurements of emphysema and small airways disease were independently associated with FEV1%. These CT measures also had strong associations with other pulmonary function tests, with emphysema having the strongest association with gas transfer and small airways disease having the strongest association with RV/TLC. None of the CT parameters were associated with sputum inflammatory cells or microbiology.
In this study, subjects exhibited a wide range of emphysema and small airways disease, with those in more severe GOLD categories having larger amounts, which is consistent with previous studies [3]. For dimensions of larger airways this was less clear-cut with only those in the most severe GOLD group having larger airway wall dimensions than severe COPD but not moderate COPD. Other studies have also shown inconsistent results when using Pi10 as a marker of bronchial wall thickening in COPD [3, 9, 21]. Bronchiectasis was not particularly common in this cohort, which is inconsistent with previous work that have shown higher rates in COPD populations [18, 22, 23]. The reasons for this are unknown, however subjects from this study were recruited from an area with low TB prevalence and most patients were recruited from specialist COPD clinics where clinically relevant bronchiectasis would have been detected and referred to specialist care. When assessing the association between these CT measures, a significant positive association was found between emphysema and small airways disease. However, the scatterplots demonstrated significant variability of small airways disease at lower levels of emphysema. It has been hypothesised that small airways disease may be a precursor to emphysema although more longitudinal work is required to understand this. There was also a weak inverse association between Pi10 and emphysema. Interestingly there was no real relationship between Pi10 and small airways disease, suggesting that structural abnormalities of the large and small airways are unrelated in COPD.
CT measures of emphysema and small airways disease had independent associations with airflow obstruction, which is in agreement with previous studies [3]. E/I MLD had the strongest association with FEV1%, suggesting small airways disease is the largest contributor to airflow obstruction in COPD. Emphysema and small airways disease also had significant associations with gas transfer, although in multivariate analysis %LAA<− 950 was the only CT parameter had independent associations. As gas transfer is a measure of the disruption of the alveolar-capillary membrane it is unsurprising that emphysema was the only CT measure that showed this association and is consistent with prior studies [11, 24–30]. E/I MLD was the only CT measure to independently predict RV% and RV/TLC ratio. RV and RV/TLC ratio are measures of pulmonary air trapping, therefore confirming E/I MLD as a an accurate marker of small airways disease, which has also been shown by other studies [10, 24, 31, 32]. Pi10 did not show an independent associations with any lung function parameters.
It is vital to understand the pathologies that contribute to poor exercise in COPD, as lower exercise capacity during the 6MWT has been linked with increased mortality [33–37]. We found no associations between the 6MWD and CT parameters, with only FEV1%, BMI and gender showing an independent association. In addition, FEV1% was the only variable that significantly predicted which subjects walked less than 350 m. Previous work has suggested that subjects with increased emphysema [15, 27, 38, 39] and air trapping [11, 14, 38] walk shorter distances at the six minute walk test, although this is not a universal finding [40, 41]. The reasons for these apparent discrepancies are unknown but in our cohort there was significant variability in the 6MWD, which is likely to be influenced by a number of complex pulmonary and extra-pulmonary manifestations of disease. In addition, co-morbidities and psychological factors can have an effect on walking distance which were not investigated as part of this analysis. Desaturation during the 6MWT has also been linked with mortality in COPD patients [34, 42, 43]. On univariate analysis we found an association between desaturation and %LAA<− 950 and E/I MLD but not Pi10. On multivariate analysis this association only remained significant for %LAA<− 950. We also found that subjects who desaturated more than 5% on exertion had significantly lower FEV1% and higher %LAA<− 950 and on multivariate analysis, %LAA<− 950 was the only variable which independently predicted whether a subjects desaturated. We used > 5% to define significant desaturation as this has previously been linked to increasing mortality [43]. To the best of our knowledge the association between desaturation and CT parameters has not been previously investigated and our results suggest that emphysema, but not airways remodelling are directly linked to oxygen desaturation during exertion in COPD. This study does not provide any mechanistic explanations for this although a plausible suggestion would be the ventilation/perfusion mismatch that is caused by alveolar destruction seen in emphysema.
Few previous studies have investigated the link between CT parameters and biological markers of inflammation and microbiology. Although multiple studies have shown increased airway neutrophils in COPD, we found no association between any of the quantitative CT markers and sputum white cell differential. Previous studies have also demonstrated no link between emphysema and airway neutrophils [12, 32, 44, 45] and inconsistent results with sputum eosinophils [44, 46]. As opposed to this, a study using PET-CT demonstrated a relationship between pulmonary uptake of FDG, a marker of neutrophilic inflammation, and emphysema severity [47]. Prior work has also shown inconsistent results when investigating the link between CT parameters of gas trapping and airway neutrophil counts [12, 32], although these studies differed from ours as they utilised BAL sampling. Histological studies have also shown equivocal results regarding neutrophilia within the distal airways [48, 49]. The reason why our study and others fail to show a consistent link between neutrophils and emphysema or small airways disease is unknown, however it may be that the concentration of airway neutrophils at a single-time point is more influenced by other factors such as airway microbiology or smoking status. There was a suggestion of a negative relationship between emphysema and systemic inflammation, although the association was rather weak and so the relevance of this finding is unknown, which is further highlighted by a previous study showing increased fibrinogen but not CRP in emphysematous subjects [50].
We have previously shown in this cohort that colonisation of the airways with potentially pathogenic bacteria is important as it is related to exacerbations [17], making it vital to understand the underlying features which pre-dispose to this. We found few differences in CT parameters between subjects where bacteria were detected in sputum and those where it was not, by either culture or PCR. Few previous studies have investigated the link between structural changes identified on CT and microbiology, although one study supported our findings by demonstrating no difference in sputum bacterial culture between patients who had emphysema on CT and those who did not [46]. It is perhaps unsurprising that bacterial detection did not influence these structural changes as colonisation or invasion is likely to be due to abnormalities within the epithelial mucosal surface or immunological deficiencies that cannot be detected on CT. There are also obvious limitations on relying on sputum culture and PCR for bacterial detection and so further work is required to explore the relationship between the microbiome and structural changes within the lung.
There were a number of limitations to this study. Firstly, a number of different quantitative analysis techniques exist with which to measure each facet of structural lung disease, however we chose some of the most widely used and best-validated techniques. There is particular uncertainty for using Pi10 as a measure of airway wall dimensions, as simplifying the complexity of the bronchial tree into one number may be sub-optimal. Further work is required to understand which technique represents the best measure of airway wall dimensions in COPD. More recently, co-registration techniques such as parametric response mapping have been developed to estimate emphysema and small airways disease [51]. It has been proposed that this analysis method may be more accurate than those employed in this study although there is little direct evidence to support this currently. As is standard, we measured air trapping on CT by scanning subjects at maximal expiration. In some subjects, especially those with more severe COPD it may have been difficult to obtain residual volume which could have introduced errors. Furthermore, physiological and CT measurements of air trapping may not only reflect small airways disease but air trapping secondary to emphysema and therefore further work is required to validate CT measures by comparing them with more specific physiological measures of small airways disease. Another limitation is the multiple comparisons made in this study, however we found far more significant associations than would be expected by chance alone. For a CT study the sample size was relatively small, which may have limited the statistical power of the study, although the benefit of this was that it allowed us to perform substantial and in-depth phenotyping of the cohort. In addition, the results reported in this study were cross-sectional and some of the variables may vary over time and therefore further longitudinal studies are required to understand this.
Conclusions
In conclusion, CT-derived measurements of emphysema and E/I MLD had independent associations with FEV1%, providing further evidence that both of these structural abnormalities are directly associated with the airflow obstruction that defines COPD. We also provided further validation for their use, as CT-measures of emphysema showed strong associations with gas transfer and CT-measure of gas trapping with RV. Furthermore, quantitative CT measures of emphysema have independent associations with desaturation on exertion, a measure which has been linked to mortality and therefore may help explain the mechanism by which emphysema is related to mortality. In contrast to this, quantitative CT parameters did not show any convincing associations with biological markers of disease. This suggests that while quantitative CT measures of emphysema and small airways disease are useful tools at reflecting pulmonary physiology and functional status they are less effective at reflecting disease biology and therefore may not be a useful tool in assessing disease activity.
Additional file
Table S1. Spearman’s correlation analysis between CT parameters and sputum markers (all sputum samples). Table S2. CT parameters in subjects according to sputum Bacterial PCR Detection. Figure S1. Scatterplots of (A) %LAA<− 950 against Pi10 (rho − 0.36***, p < 0.001) (B) E/I MLD against Pi10 (rho 0.18*, p 0.045) (C) %LAA<− 950 against E/I MLD (rho 0.47***, p < 0.001). (DOCX 74 kb)
Acknowledgements
The authors would like to thank all the study volunteers for their invaluable contribution towards furthering COPD knowledge and each team member for their assistance conducting the study. We acknowledge all members of the AERIS study group. The authors would also like to thank Geraldine Drevon and Regis Azizieh (XPE Pharma & Science, on behalf of GSK Vaccines) for coordination and editorial support. The study was funded by GlaxoSmithKline Biologicals SA.
The AERIS Study Group;
J.Alnajar, R Anderson, E Aris, WR Ballou, A Barton, S Bourne, M Caubet, SC Clarke, D Cleary, C Cohet, NA Coombs, K Cox, J-M Devaster, V Devine, N Devos, E Dineen, T Elliot, R Gladstone, S Harden, J Jefferies, V Kim, S Mesia-Vela, P Moris, K Ostridge, TG Pascal, M Peeters, S Schoonbroodt, KJ Staples, A Tuck, L Welsh, V Weynants, TMA Wilkinson, AP Williams, NP Williams, C Woelk, M Wojtas, S Wootton. All members of the AERIS Study Group were involved in the planning, conduct, and/or reporting of the work described in the article.
Funding
The study was funded by GlaxoSmithKline Biologicals SA. No restrictions were placed on authors regarding the statements made in the manuscript.
Availability of data and materials
Not applicable
Abbreviations
- %LAA<− 950
Lung voxels on the inspiratory scan with attenuation values below − 950 Hounsfield units
- 6MWD
Six-minute walk distance
- 6MWT
Six-minute walk test
- AERIS
The Acute Exacerbation and Respiratory Infections in COPD study
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- CT
Computed tomography
- E/I MLD
The ratio of the mean lung density, expiration/ inspiration
- FEF75–25%
The forced expiratory flow at 25–75% of forced vital capacity
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- MCAT
Moraxella catarrhalis
- NTHI
Non-typeable Haemophilus influenza
- PA
Pseudomonas aeruginosa
- PCT
Pro-calcitonin
- PPM
Potentially pathogenic bacteria
- RV
Residual volume
- SA
Staphylococcus aureus
- SP
Streptococcus pneumonia
- TLC
Total lung capacity
- TLCO
Carbon monoxide transfer factor
Authors’ contributions
KO had full access to the data and takes responsibility for the accuracy of the data analysis. JMD, SB, SW, AT, VK, SCC, AW and TMAW conceived and designed the AERIS study. EA, JMD, SB, SW, AT, NPW, KO, KJS, SCC, VK, AW, SH and TMAW collected or generated the data. EA, JMD, SB, SW, NPW, KO, KJS, SCC, NAC, VK, AW and TMAW analysed or interpreted the data, All authors contributed to the development of the manuscript and approved the final version.
Ethics approval and consent to participate
Patient consent was obtained and the study was approved by the South Central - Southampton B NRES Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12931-018-0734-y) contains supplementary material, which is available to authorized users.
Contributor Information
Kristoffer Ostridge, Email: k.ostridge@soton.ac.uk.
Nicholas P. Williams, Email: npw1m13@soton.ac.uk
Viktoriya Kim, Email: v.kim@soton.ac.uk.
Stephen Harden, Email: Stephen.harden@uhs.nhs.uk.
Simon Bourne, Email: simon@soton.ac.uk.
Stuart C. Clarke, Email: S.C.Clarke@soton.ac.uk
Emmanuel Aris, Email: EMMANUEL.X.ARIS@GSK.COM.
Sonia Mesia-Vela, Email: sonia.x.mesia-vela@gsk.com.
Jeanne-Marie Devaster, Email: JEANNE-MARIE.DEVASTER@GSK.COM.
Andrew Tuck, Email: A.C.Tuck@soton.ac.uk.
Anthony Williams, Email: A.P.Williams@soton.ac.uk.
Stephen Wootton, Email: s.wooton@soton.ac.uk.
Karl J. Staples, Email: k.staples@soton.ac.uk
Tom M. A. Wilkinson, Email: t.wilkinson@soton.ac.uk
References
- 1.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Ostridge K, Wilkinson TMAA. Present and future utility of computed tomography scanning in the assessment and management of COPD. Eur Respir J. 2016;48:216–228. doi: 10.1183/13993003.00041-2016. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder JD, McKenzie AS, J a Z, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201:W460–W470. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gevenois P a, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 5.Gevenois P a, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 6.Achenbach T, Weinheimer O, Biedermann A, et al. MDCT assessment of airway wall thickness in COPD patients using a new method: correlations with pulmonary function tests. Eur Radiol. 2008;18:2731–2738. doi: 10.1007/s00330-008-1089-4. [DOI] [PubMed] [Google Scholar]
- 7.Yamashiro T, Matsuoka S, Estépar RSJ, et al. Quantitative assessment of bronchial wall attenuation with thin-section CT: an indicator of airflow limitation in chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2010;195:363–369. doi: 10.2214/AJR.09.3653. [DOI] [PubMed] [Google Scholar]
- 8.Kim WJ, Silverman EK, Hoffman E, et al. CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136:396–404. doi: 10.1378/chest.08-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BM, Hoffman E a, Rabinowitz D, et al. Comparison of spatially matched airways reveals thinner airway walls in COPD. The multi-ethnic study of atherosclerosis (MESA) COPD study and the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax 2014;0:1–10. [DOI] [PMC free article] [PubMed]
- 10.Mets OM, Zanen P, Lammers J-WJ, et al. Early identification of small airways disease on lung cancer screening CT: comparison of current air trapping measures. Lung. 2012;190:629–633. doi: 10.1007/s00408-012-9422-8. [DOI] [PubMed] [Google Scholar]
- 11.Lee YK, Oh Y-M, Lee J-H, et al. Quantitative assessment of emphysema, air trapping, and airway thickening on computed tomography. Lung. 2008;186:157–165. doi: 10.1007/s00408-008-9071-0. [DOI] [PubMed] [Google Scholar]
- 12.Ostridge K, Williams N, Kim V, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71:126–132. doi: 10.1136/thoraxjnl-2015-207428. [DOI] [PubMed] [Google Scholar]
- 13.Bhatt S, Soler X, Wang X, et al. Association between functional small airways disease and FEV 1 decline in COPD. Am J Respir Crit Care Med. 2016;1164:201511–22219O. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehdi Rambod MD, Janos Porszasz MD, BJ PD, FCCP MMD, Crapo JD, MD FCCP, Casaburi R, PhD MD, CopdgI FCCP t, Rambod M, Porszasz J, et al. six-minute walk distance predictors, including CT scan measures, in the COPDGene cohort. Chest. 2012;141:867–875. doi: 10.1378/chest.11-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz AA, Bartholmai B, San R, et al. Relationship of emphysema and airway disease assessed by CT to exercise capacity in COPD. Respir Med. 2011;104:1145–1151. doi: 10.1016/j.rmed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourne S, Cohet C, Kim V, et al. Acute exacerbation and respiratory InfectionS in COPD (AERIS): protocol for a prospective, observational cohort study. BMJ Open. 2014;4:e004546. doi: 10.1136/bmjopen-2013-004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson TMA, Aris E, Bourne S, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72:919–927. doi: 10.1136/thoraxjnl-2016-209023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel IS, Vlahos I, TM a W, et al. Bronchiectasis, exacerbation indices, and inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:400–407. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 20.Vignola AM, SI R, FE H, et al. Standardised methodology of sputum induction and processing. Future directions. Eur Respir J Suppl. 2002;37:51s–55s. [PubMed] [Google Scholar]
- 21.Dournes G, Laurent F, Coste F, et al. Computed tomographic measurement of airway remodeling and emphysema in advanced chronic obstructive pulmonary disease: correlation with pulmonary hypertension. Am J Respir Crit Care Med. 2015;191:63–70. doi: 10.1164/rccm.201408-1423OC. [DOI] [PubMed] [Google Scholar]
- 22.Martínez-García M-A, de la Rosa Carrillo D, Soler-Cataluña J-J, et al. Prognostic value of bronchiectasis in patients with moderate-to-severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:823–831. doi: 10.1164/rccm.201208-1518OC. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-García MÁ, Soler-Cataluña JJ, Donat Sanz Y, et al. Factors associated with bronchiectasis in patients with COPD. Chest. 2011;140:1130–1137. doi: 10.1378/chest.10-1758. [DOI] [PubMed] [Google Scholar]
- 24.Mets OM, Murphy K, Zanen P, et al. The relationship between lung function impairment and quantitative computed tomography in chronic obstructive pulmonary disease. Eur Radiol. 2012;22:120–128. doi: 10.1007/s00330-011-2237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa E, Nakano Y, Ohara T, et al. Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax. 2009;64:20–25. doi: 10.1136/thx.2008.097543. [DOI] [PubMed] [Google Scholar]
- 26.Cavigli E, Camiciottoli G, Diciotti S, et al. Whole-lung densitometry versus visual assessment of emphysema. Eur Radiol. 2009;19:1686–1692. doi: 10.1007/s00330-009-1320-y. [DOI] [PubMed] [Google Scholar]
- 27.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD. 2009;6:459–467. doi: 10.3109/15412550903341513. [DOI] [PubMed] [Google Scholar]
- 28.Washko GR, Criner GJ, Mohsenifar Z, et al. Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD. 2008;5:177–186. doi: 10.1080/15412550802093025. [DOI] [PubMed] [Google Scholar]
- 29.Desai SR, Hansell DM, Walker A, et al. Quantification of emphysema: a composite physiologic index derived from CT estimation of disease extent. Eur Radiol. 2007;17:911–918. doi: 10.1007/s00330-006-0369-0. [DOI] [PubMed] [Google Scholar]
- 30.Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology. 2006;238:1036–1043. doi: 10.1148/radiol.2382042196. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka S, Kurihara Y, Yagihashi K, et al. Quantitative assessment of air trapping in chronic obstructive pulmonary disease using inspiratory and expiratory volumetric MDCT. AJR Am J Roentgenol. 2008;190:762–769. doi: 10.2214/AJR.07.2820. [DOI] [PubMed] [Google Scholar]
- 32.R a O’D, Peebles C, J a W, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–842. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golpe R, Pérez-de-Llano L a, Méndez-Marote L, et al. Prognostic value of walk distance, work, oxygen saturation, and dyspnea during 6-minute walk test in COPD patients. Respir Care. 2013;58:1329–1334. doi: 10.4187/respcare.02290. [DOI] [PubMed] [Google Scholar]
- 34.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 35.Cote CG, Casanova C, Marín JM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 36.Cote CG, Pinto-Plata V, Kasprzyk K, et al. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007;132:1778–1785. doi: 10.1378/chest.07-2050. [DOI] [PubMed] [Google Scholar]
- 37.Pinto-Plata VM, Cote C, Cabral H, et al. The 6-min walk distance: change over time and value as a predictor of survival in severe COPD. Eur Respir J. 2004;23:28–33. doi: 10.1183/09031936.03.00034603. [DOI] [PubMed] [Google Scholar]
- 38.Hersh CP, Washko GR, Estépar RSJ, et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res. 2013;14:42. doi: 10.1186/1465-9921-14-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MeiLan K, Han MDM, Ella A, Kazerooni M, David A, Lynch M, et al. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mair G, Miller JJ, McAllister D, et al. Computed tomographic emphysema distribution: relationship to clinical features in a cohort of smokers. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2009;33:536–542. doi: 10.1183/09031936.00111808. [DOI] [PubMed] [Google Scholar]
- 41.Camiciottoli G, Bigazzi F, Bartolucci M, et al. BODE-index, modified BODE-index and ADO-score in chronic obstructive pulmonary disease: relationship with COPD phenotypes and CT lung density changes. COPD. 2012;9:297–304. doi: 10.3109/15412555.2012.661000. [DOI] [PubMed] [Google Scholar]
- 42.Waatevik M, Johannessen A, Gomez Real F, et al. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur Respir J. 2016;48(1):82–91. doi: 10.1183/13993003.00975-2015. [DOI] [PubMed] [Google Scholar]
- 43.Takigawa N, Tada A, Soda R, et al. Distance and oxygen desaturation in 6-min walk test predict prognosis in COPD patients. Respir Med. 2007;101:561–567. doi: 10.1016/j.rmed.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Boschetto P, Quintavalle S, Zeni E, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh D, Edwards L, Tal-Singer R, et al. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res. 2010;11:77. doi: 10.1186/1465-9921-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bafadhel M, Umar I, Gupta S, et al. The role of CT scanning in multidimensional phenotyping of COPD. Chest. 2011;140:634–642. doi: 10.1378/chest.10-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian DR, Jenkins L, Edgar R, et al. Assessment of pulmonary neutrophilic inflammation in emphysema by quantitative positron emission tomography. Am J Respir Crit Care Med. 2012;186:1125–1132. doi: 10.1164/rccm.201201-0051OC. [DOI] [PubMed] [Google Scholar]
- 48.Baraldo S, Turato G, Badin C, et al. Neutrophilic infiltration within the airway smooth muscle in patients with COPD. Thorax. 2004;59:308–312. doi: 10.1136/thx.2003.012146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.James C, Hogg MD, Fanny Chu B. Sc., Soraya Utokaparch, b.Sc., Ryan woods, m.Sc., W. Mark Elliott PD, Liliana Buzatu, M.D., Ruben M. Cherniack, M.D., Robert M. Rogers, M.D., frank C. Sciurba MD, Harvey O. Coxson, Ph.D., and Peter D. Paré MD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. [DOI] [PubMed]
- 50.Papaioannou AI, Mazioti A, Kiropoulos T, et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir Med. 2010;104:275–282. doi: 10.1016/j.rmed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, FJ M, BD R. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1716. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman’s correlation analysis between CT parameters and sputum markers (all sputum samples). Table S2. CT parameters in subjects according to sputum Bacterial PCR Detection. Figure S1. Scatterplots of (A) %LAA<− 950 against Pi10 (rho − 0.36***, p < 0.001) (B) E/I MLD against Pi10 (rho 0.18*, p 0.045) (C) %LAA<− 950 against E/I MLD (rho 0.47***, p < 0.001). (DOCX 74 kb)
Data Availability Statement
Not applicable


