Abstract
Background
This study evaluated the anti-allergic activity of corilagin and also postulates the possible mechanism of its action.
Material/Methods
Corilagin was given orally at dose of 10, 20, and 40 mg/kg/day. All the animals (guinea pigs, rats, and mice) were sensitized for allergy such as eosinophilia and leukocytosis induced by milk; degranulation of mast cell by compound 48/80; and passive and active anaphylaxis. Moreover, the antagonistic effect was determined by estimating the effect of corilagin on contraction of guinea pig tracheal chain and ileum induced by Ach and histamine, respectively.
Result
There was a significant decrease in the leukocyte and eosinophil counts in the corilagin-treated group compared to the negative control group. Treatment with corilagin significantly protects the degranulation of mast cells, and it also has significant anti-muscarinic and antihistaminic activity by reducing the muscle contraction induced by Acetylcholine (Ach) and histamine in guinea pig tracheal chain and ileum.
Conclusions
Corilagin possess anti-anaphylactic and anti-allergic activity by inhibiting the release of mediators from mast cells and by decreasing the serum concentration of immunoglobulin E (IgE).
MeSH Keywords: Allergy and Immunology; Anaphylaxis; Medicine, Chinese Traditional
Background
Anaphylactic reaction is a type I hypersensitivity reaction that occurs in an antigen-sensitized individual in which antigen antibody reaction activates the mast cells [1]. Activated mast cells degranulate and release histamine, which contributes to the anaphylactic reaction [2]. However, degranulation of mast cells also occurs by non-immunologic reactions such as activation with certain drugs, complement components, basic compounds, and neuropeptides [3]. In anaphylactic reaction, IgE bind with the receptor located on the cell membrane and thereby initiates the release of prostaglandin, histamine, and interleukin in the systemic circulation [4,5]. These chemicals alter the physiological function and contribute to the anaphylactic reaction.
Reports in the literature suggest that several natural products show promising effects in the management of anaphylactic reactions. Corilagin is isolated from the herb Caesalpinia coriaria and is chemically known as ellagitannin [6]. Previous reports suggested that corilagin has potent antioxidant, hepatoprotective, anti-inflammatory, analgesic, antihypertensive, antitumor, and carbonic anhydrase inhibitor properties [7–10]. It was reported that corilagin possesses analgesic activity by altering the glutaminergic system and anti-inflammatory activity by decreasing the production of pro-inflammatory cytokines and mediates tumor necrosis factor-α (TNF-α), IL-1β, IL-6, NO (iNOS), and cycloxygenase-2 (COX-2) at both the protein and gene level by blocking NF-κB nuclear translocation [11,12]. Thus, the present investigation evaluated the effect of corilagin against anaphylactic reaction.
Material and Methods
Chemicals
Histamine Hcl and Compound 48/80 were procured from Sigma Aldrich, USA. Horse serum and egg albumin were purchased from Loba Chemicals, India. Ketotifen and dexamethasone was procured from Pfizer Ltd., USA.
Animals
Dunkin Hartley guinea pigs (320–400 g), Swiss albino mice (20–25 g), and Sprague Dawley rats (180–200 g) were procured from Shanghai Changzheng Hospital, China. All the animals were kept in a well-maintained and controlled environment according to experimental animal care guidelines. The protocols used in this study were approved by Institutional Animal Ethics Committee of the Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine (SUTCM/2016/20) and we followed the guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC) for experimentation and animal use.
Estimation of anti-allergic effect of corilagin
Estimation of effect of corilagin on eosinophilia and leukocytosis
All the mice were separated into 6 groups (n=6): the control group received solvent, the negative control group received subcutaneous injection of milk (4 ml/kg), the standard (STD) group received intraperitoneal injection of dexamethasone (0.27 mg/kg), and the corilagin group received corilagin 10, 20, and 40 mg/kg orally. Leukocyte and eosinophil counts in the blood were estimated before and after administration of milk [14].
Passive cutaneous anaphylaxis
Subcutaneous injection of egg albumin with aluminum hydroxide was used to sensitize the rats on days 1, 3, and 5 of the protocol. Serum was separated from the blood drawn on day 11. All the animals were separated into 6 groups: the control group, the negative control group, the STD group, and the corilagin 10, 20, and 40 mg/kg groups. We shaved all the rats and subcutaneously injected 100 μl of homologous antiserum. A mixture of EA (100 μg) and Evan’s blue (0.5% w/v) at a ratio of 1: 1 was injected intravenously to each group of rats and used a Vernier caliper was used to measure the area of dye leakage [15].
Estimation of serum IgE
Following the procedure described earlier, we sensitized the rats and separated them into 4 different groups with 6 rats in each group: control, STD, and corilagin 20 and 40 mg/kg treated groups. Treatment was given between day 4 and day 10 of the protocol. Blood was drawn on days 6, 8, and 11 of the protocol and the concentration of IgE in the serum was estimated using anti-rat IgE antibody by ELISA method. The concentration of standard IgE used was 0.15–20 ng/ml.
Estimation of anaphylaxis reaction
Horse serum (0.5 ml) was subcutaneously injected to sensitize the rats and we separated them into 6 different groups with 6 rats in each group: control, negative control, STD, and corilagin 10, 20, and 40 mg/kg treated groups for the duration of 10 days. After the treatment, horse serum (0.25 ml) was injected intravenously and we observed symptoms such as cyanosis, dyspnea, enhanced respiratory rate, and respiratory distress of anaphylactic reactions. Anaphylactic reaction was scaled from 0 to 20 on the basis of its severity [16].
Assessment of paw edema
All the rats were separated into 6 different groups with 6 rats in each group: control, negative control, STD (ibuprofen 50 mg/kg, p.o.), and corilagin 10, 20, and 40 mg/kg. Histamine 1% w/v was injected into the planter region of the right paw and the drug was administered 30 min later. A plethysmometer was used to assess the paw volume at 2 and 3 h after the injection [17].
Estimation of mast cell degranulation
Subcutaneous injection of compound 48/80 (1 mg/kg) was used to sensitized the rats and we separated them into 6 different groups with 6 rats in each group: control, negative control, STD (ketotifen fumarate, 1 mg/kg, i.p.), and corilagin 10, 20, and 40 mg/kg treated groups for 7 days. Phosphate buffer saline was injected intraperitoneally on day 7 of the protocol and we massaged the abdomen gently. Thereafter, all the rats were sacrificed and peritoneal fluid was collected in a tube containing phosphate buffer. Percoll density centrifugation method was used for the purification of mast cells. Mast cells were incubated for 10 min at 37°C with 5 μg/ml concentration of compound 48/80 after resuspending it into phosphate buffer solution. Later, cells were separated out by centrifugation and stained with 0.1% Toluidine blue. A high-power microscope was used for the estimation of degranulated cells [18].
Effect of corilagin on the contraction of guinea pig ileum
Guinea pigs were fasted overnight and the ileum was isolated after sacrifice. Tissue was mounted in Tyrode solution containing the organ bath maintained at 37°C. IC50 value was calculated by taking the concentration response curve of histamine on ileum in presence or absence of corilagin.
Effect of corilagin on guinea pig tracheal chain
The trachea was isolated from the guinea pigs and we tied them together to prepare the chain from it. Krebs solution was used to mount the chain and CRC of Ach was carried out in the presence and absence of corilagin and atropine to calculate IC50 value [18].
Statistical analysis
Values of the given data are represented as mean ±SD (n=6) and the data were statistically analyzed by one-way ANOVA (Dunnett post hoc test). Level of significance was considered as p<0.05.
Results
Determination of the effect of corilagin on leukocytosis and eosinophilia
The effect of corilagin on milk-induced leukocytosis and eosinophilia is shown in Table 1. Differential leucocytes, WBCs, and eosinophil counts in the blood were significantly enhanced in the negative control group compared to the control group due to administration of milk. Treatment with corilagin significantly (p<0.01) decreased the count of differential leucocytes, WBCs, and eosinophils compared to the negative control group. We observed that corilagin attenuates the leukocytosis and eosinophilia in a dose-dependent manner.
Table 1.
Effect of corilagin on milk induced leukocytosis and eosinophilia.
| Sr. No. | Group | Eosinophilis per cu. mm (×10 c/μl)a | Differential Leukocytes (×10 c/μl)b | Total Leukocytes per cu. mmc | |
|---|---|---|---|---|---|
| Monocytes | Neutrophils | ||||
| 1 | Control | 62.83±4.72 | 34.22±2.16 | 3.35±0.21 | 6310±29.52 |
| 2 | Negative control | 151.26±11.29## | 48.92±1.94## | 10.48±0.87## | 9880±31.82## |
| 3 | Corilagin 10 mg/kg | 109.32±8.64** | 42.71±1.37* | 7.93±0.63** | 9138±18.74* |
| 4 | Corilagin 20 mg/kg | 91.78±7.92** | 38.89±1.53** | 6.11±0.71** | 7640±20.15** |
| 5 | Corilagin 40 mg/kg | 82.51±3.84** | 35.72±1.27** | 4.82±0.26** | 6844±12.62** |
| 6 | STD | 79.13±6.32** | 36.69±1.46** | 4.29±0.32** | 6519±14.79** |
Means ±SD (n=6),
p<0.01 than control,
p<0.05,
p<0.01 than negative control.
Determination of effect of corilagin on anaphylactic reaction
Table 2 shows the effect of corilagin on passive cutaneous and active anaphylaxis and degranulation of mast cells. There was significant reduction in the anaphylactic reactions (passive cutaneous and active) in the corilagin-treated group compared to the negative control group. We observed that corilagin attenuated the anaphylactic reactions such as cyanosis, dyspnea, respiratory rate, and score in a dose-dependent manner. Moreover, the corilagin-treated group showed a significant reduction in the compound 48/80-induced degranulation of mast cells compared to the negative control group.
Table 2.
Effect of corilagin on passive cutaneous anaphylaxis, active anaphylaxis and degranulation of mast cell.
| Sr. No. | Group | Passive cutaneous anaphylaxis | Active anaphylaxis | Mast cell degranulation | |||
|---|---|---|---|---|---|---|---|
| Blueing reaction mm3 | Behavioural symptoms (% of mortality) | Respiratory score | Intact | Degranulated | |||
| 1 | Control | 00 | Normal | 00 (00) | 100 | 00 | |
| 2 | Negative control | 78.39±3.15 | Respiratory distress/collapse 3–5 min (70%) | 8.80±5.2 (65%) | 22.62±2.94## | 79.32±4.72## | |
| 3 | Corilagin 10 mg/kg | 69.17±3.09 | Dysponea 5–8 min (40%) | 6.25±3.15 (40%) | 31.72±2.45* | 67.37±4.31** | |
| 4 | Corilagin 20 mg/kg | 54.92±2.90 | Dysponea 15–20 min (25%) | 4.10±1.28 (25%) | 43.89±3.56** | 56.18±3.78** | |
| 5 | Corilagin 40 mg/kg | 47.36±1.97 | Normal | 2.74±1.45 (15%) | 55.73±4.71** | 44.27±3.26** | |
| 6 | STD | Dexomethasone | 43.18±2.69 | Normal | 2.36±0.75 (15%) | – | – |
| Ketotifen | – | – | – | 62.39±4.26** | 38.29±2.46** | ||
Means ±SD (n=6),
p<0.01 than control,
p<0.05,
p<0.01 than negative control.
Determination of effect of corilagin on the serum concentration of IgE
Figure 1 shows the effect of corilagin on the serum concentration of IgE in sensitized rats. The serum concentration of IgE was significantly decreased (p<0.01) in the corilagin-treated group compared to the negative control group at 8 and 11 h after treatment.
Figure 1.
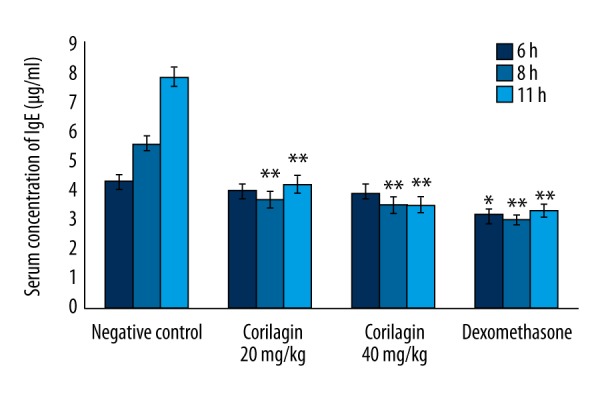
Effect of corilagin on the serum concentration of IgE in sensitized rats. Means ±SD (n=6), * p<0.05, ** p<0.01 compared to negative control.
Determination of effect of corilagin on IC50 value of Ach and histamine
Table 3 shows the effect of corilagin on Ach- and histamine-induced contraction of guinea pig tracheal chain and ileum, respectively. Treatment with corilagin significantly inhibited the Ach- and histamine-induced contraction of guinea pig tracheal chain and ileum, respectively. This inhibition of contraction was found to be in a dose-dependent manner. The IC50 value of corilagin for histamine was 415 (382–429) μg/ml and Ach was 261 (239–290) μg/ml.
Table 3.
Effect of corilagin on IC50 value of Ach and histamine in guinea pig tracheal chain and ileum.
| Sr. No. | Antagonists | IC50 values | |
|---|---|---|---|
| Response of Histamine in presence of antagonist | Response of Ach in presence of antagonist | ||
| 1 | Nil | 16.5 (14.15–17.46) μg/ml | 162 (142–176) μg/ml |
| 2 | Chlorpheniramine maleate | 2.75 (2.21–2.89) μg/ml | |
| 3 | Atropine maleate | 2.79 (2.55–3.10) μg/ml | |
| 4 | Corilagin | 415 (382–429) μg/ml | 261 (239–290) μg/ml |
Determination of effect of corilagin on paw edema
The effect of corilagin on the histamine-induced paw edema in rats is shown in Figure 2. There was significantly increased paw edema volume in the negative control group of rats compared to the control group at 1 and 3 h after drug administration. However, treatment with corilagin significantly (p<0.05, p<0.01) decreased the paw edema compared to the negative control group, in a dose-dependent manner.
Figure 2.
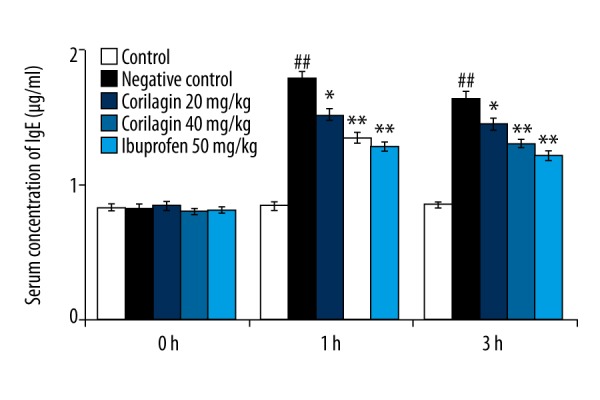
Effect of corilagin on histamine-induced paw edema. Means ±SD (n=6), ## p<0.01 compared to control, * p<0.05, ** p<0.01 compared to negative control.
Discussion
The present study investigated the effect of corilagin against anaphylactic and allergic reaction. Eosinophilia and leukocytosis were induced by injecting milk into mice and anaphylactic reaction was induced by sensitizing rats with albumin. Moreover, degranulation of mast cells was induced by 48/80 compound in rats. We estimated the concentration of IgE in the serum and IC50 value of corilagin was estimated against histamine- and Ach-induced contraction of tracheal chain and ileum, respectively.
The literature reveals that degranulation of eosinophils results into immunological reactions, and in various allergic conditions, the eosinophil count and leucocytosis significantly increases in allergy/asthma [19]. Allergic reaction involves the release of several inflammatory mediators such as cytokines, D4, C4, leukotrienes, and histamine, and the result of this study suggest that treatment with corilagin significantly reduces the count of leucocytes and eosinophils compared to the negative control group [20–22].
Reports reveal that immunoglobin (IgE) is mainly responsible for the anaphylactic reaction and to the degranulation of mast cells [23]. Our results suggest that corilagin significantly attenuates the anaphylactic reaction and degranulation of mast cells. Moreover, it also decreases the concentration of IgE in the blood. Several studies suggest that lipid layer permeability is significantly enhanced by compound 40/80 and results in the release of mediators [24]. This compound enhances the level of cAMP in the cells that stimulates Ca influx and thereby activates the mast cells for degranulation. Corilagin attenuates the anaphylaxis and allergic reaction, probably by activating G-protein, membrane stabilization, and releasing the intracellular Ca+.
Treatment with corilagin significantly decreased the Ach- and histamine-induced contraction in guinea pig tracheal chain and ileum, suggesting the antagonistic activity of corilagin on histamine and muscarinic receptors.
Conclusions
Our study shows that corilagin has anti-anaphylactic and anti-allergic activity by inhibiting the release of mediators from mast cells and by decreasing the IgE serum concentration.
Footnotes
Conflicts of interest
None.
Source of support: Innovation Funds of Pudong New Area Science and Technology Commission of Shanghai (Grant NO. PKJ2015-Y11), and Qi Ming Xing Training Program of Seventh People’s Hospital of Shanghai University of Traditional Chinese Medicine (Grant NO. QMX2016-03)
References
- 1.Xie H, He SH. Roles of histamine and its receptors in allergic and inflammatory bowel diseases. World J Gastroenterol. 2005;11:2851–57. doi: 10.3748/wjg.v11.i19.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–23. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalher-Machado SC, Rosas EC, de Brito FA, et al. The anti-allergic activity of the acetate fraction of Schinus terebinthifolius leaves in IgE induced mice paw edema and pleurisy. Int Immunopharmacol. 2008;8:1552–60. doi: 10.1016/j.intimp.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1:157–67. doi: 10.5415/apallergy.2011.1.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 Suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 7.Rios JL, Recio MC, Giner RM, Máñez S. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–93. [Google Scholar]
- 8.Hosseinzadeh H, Talebzadeh F. Anticonvulsant evaluation of safranal and crocin from Crocus sativus in mice. Fitoterapia. 2005;76:722–24. doi: 10.1016/j.fitote.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativusstigma extracts and its constituents, crocin, and safranal, in mice. J Med Plants. 2004;3:48–58. [Google Scholar]
- 10.Nemati H, Boskabady MH, Ahmadzadef Vostakolaei H. Stimulatory effect of Crocus sativus (saffron) on beta2-adrenoceptors of guinea pig tracheal chains. Phytomedicine. 2008;15:1038–45. doi: 10.1016/j.phymed.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Abdullaev FI, Espinosa-Aguirre JJ. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev. 2004;28:426–32. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm PharmSci. 2005;8:387–93. [PubMed] [Google Scholar]
- 13.Bhargava KP, Singh N. Anti-stress activity of Ocimum sanctum Linn. Indian J Med Res. 1981;73:443–51. [PubMed] [Google Scholar]
- 14.Xu W, Wu H, Ma M, et al. Correlation between peripheral white blood cell counts and hyperglycemic emergencies. Int J Med Sci. 2013;10(6):758–65. doi: 10.7150/ijms.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taur DJ, Patil RY. Mast cell stabilizing and antiallergic activity of Abrus precatorius in the management of asthma. Asian Pac J Trop Med. 2011;4(1):46–49. doi: 10.1016/S1995-7645(11)60030-8. [DOI] [PubMed] [Google Scholar]
- 16.Das B, Chauhan R. Anti-histaminic and mast cell stabilizing activity of a Fern-Lygodium flexuosum. Int J LifeSc Bt Pharm Res. 2013;2:152–62. [Google Scholar]
- 17.Andhare RN, Raut MK, Naik SR. Evaluation of antiallergic and anti-anaphylactic activity of ethanolic extract of Sanseveiria trifasciata leaves (EEST) in rodents. J Ethnopharmacol. 2012;142:627–33. doi: 10.1016/j.jep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 18.El-Awady MS, Said E. Vardenafil ameliorates immunologic- and non-immunologic-induced allergic reactions. Can J Physiol Pharmacol. 2014;92:175–80. doi: 10.1139/cjpp-2013-0316. [DOI] [PubMed] [Google Scholar]
- 19.Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Imm. 2003;131:46–52. doi: 10.1159/000070434. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, But PPH, Chu LM, Chan YP. Inhibitory effects of Selaginella tamariscina on immediate allergic reactions. Am J Chinese Med. 2005;33:957–66. doi: 10.1142/S0192415X05003442. [DOI] [PubMed] [Google Scholar]
- 21.Binmadi N, Almazrooa S. Dermographism in the oral cavity. Am J Case Rep. 2016;17:421–24. doi: 10.12659/AJCR.898247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin VJ, Shreffler WG, Yuan Q. Presumed allergic proctocolitis resolves with probiotic monotherapy: A report of 4 cases. Am J Case Rep. 2016;17:621–24. doi: 10.12659/AJCR.898490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shin TY. Inhibition of immunologic and nonimmunologic stimulation-mediated anaphylactic reactions by the aqueous extract of Mentha arvensis. Immunopharmacol immunotoxicol. 2003;25:273–83. doi: 10.1081/iph-120020475. [DOI] [PubMed] [Google Scholar]
- 24.Han SJ, Bae EA, Trinh HT, et al. Magnolol and honokiol: Inhibitors against mouse passive cutaneous anaphylaxis reaction and scratching behaviors. Biol Pharm Bull. 2007;30:2201–3. doi: 10.1248/bpb.30.2201. [DOI] [PubMed] [Google Scholar]


