Abstract
Patient: Male, 60
Final Diagnosis: Plasmacytoid urothelial carcinoma of ureter
Symptoms: Constipation • epigastric pain • microscopic hematuria • nausea • vomiting
Medication: —
Clinical Procedure: —
Specialty: Oncology
Objective:
Rare disease
Background:
Plasmacytoid is a rare histological variant of urothelial carcinoma (UC). Since the first reported case of plasmacytoid urothelial carcinoma (PUC), in 1991, only about 100 cases have since been reported, with most cases involving the bladder. Urothelial carcinomas of the upper urinary tract represent only 5% of urothelial cancers. To the best of our knowledge, there has only been 1 reported case of PUC of the ureter. PUC is a highly aggressive disease, with a poor prognosis. We present a rare biopsy-proven case of PUC of the ureter with retroperitoneal metastasis.
Case Report:
A 60-year-old man came into the hospital with complaints of a 5-day history of generalized abdominal pain, nausea, and vomiting, with no associated urinary symptoms prior to admission. CT demonstrated small bowel obstruction (SBO) and obstructive uropathy due to a right ureteric mass. Exploratory laparotomy, small bowel resection, gastrostomy tube placement, and umbilical hernia repair were all done. Histology and immunohistochemistry were compatible with plasmacytoid variant of urothelial cancer. He underwent a cystouretoscopy and a right ureteral stent placement with a right ureteroscopy. Final CT abdomen/pelvis revealed recurrent SBO before the ileocecal valve, possibly due to carcinomatosis. Ileocecal resection with end ileostomy placement was done. Systemic treatment will begin as an outpatient.
Conclusions:
PUC arising from the ureter is rare, and retroperitoneal metastatic disease has not been reported previously. Here, we compare the clinical manifestations of the more common PUC of the bladder with our case. From this we are able to learn more about the disease and its presentation.
MeSH Keywords: Plasmacytoma, Retroperitoneal Neoplasms, Ureteral Neoplasms, Ureteral Obstruction, Urinary Bladder Neoplasms
Background
Urothelial carcinomas of the upper urinary tract are relatively uncommon, representing only 5% of urothelial cancers. Plasmacytoid urothelial carcinoma (PUC) is an extremely rare variant of this epithelial tumor, which has proven to be highly aggressive. According to a recent study, since the first report in 1991 by Sahin et al., there have been less than 100 cases of PUC reported in the literature [1]. In addition, it was not until 2004 that the WHO recognized this histological variant under the classification of urothelial neoplasms. Nearly all plasmacytoid UC occur in the bladder; however, in 2013 the first case of PUC in the ureter was reported [2]. PUC is a highly aggressive disease, with a poor prognosis. We present a rare biopsy-proven case of PUC of the ureter with retroperitoneal metastasis.
The first reported case of PUC by Sahin et al. was characterized by lytic tumors involving the ribs and skull, which was mistaken for multiple myeloma [1]. The plasmacytoid pathological appearance was originally thought to be diagnostic of B cell lymphoma and plasmacytoma, which is why there have been many misdiagnoses. However, it is now known that the cells of non-B cell hematopoietic neoplasm and various nonhematopoietic neoplasm derivatives can also have a plasmacytoid appearance [3]. In 2006, two noninvasive bladder tumors resembling plasmacytoma were reported by Coyne and Sim [4]. The importance of obtaining immunohistochemistry in the differential diagnosis of neoplasm etiology in order to differentiate these tumors is emphasized.
Hematuria is a typical presenting characteristic of urological cancers. As a result, the major diagnostic pitfall is the deficiency of specific clinical features to differentiate PUC from other types of bladder cancers. PUC is often diagnosed late because of the absence of hematuria and lack of an identifiable tumor in the bladder, which is an unusual characteristic compared to conventional bladder cancers [3,5]. When PUC is identified, it is usually a high-grade aggressive tumor, often with advanced stage that results in worse outcomes than with conventional urothelial carcinomas.
Here, we report a rare case of PUC of the ureter, which presented as a small bowel obstruction as a result of a periduodenal mass extending from the ureter, with resultant hydronephrosis and SBO at the ileum. With no genitourinary symptoms or signs, it was surprising to find this rare variant of UC. We will explore the clinical and pathological differences we found in our case compared with other known cases of PUC.
Case Report
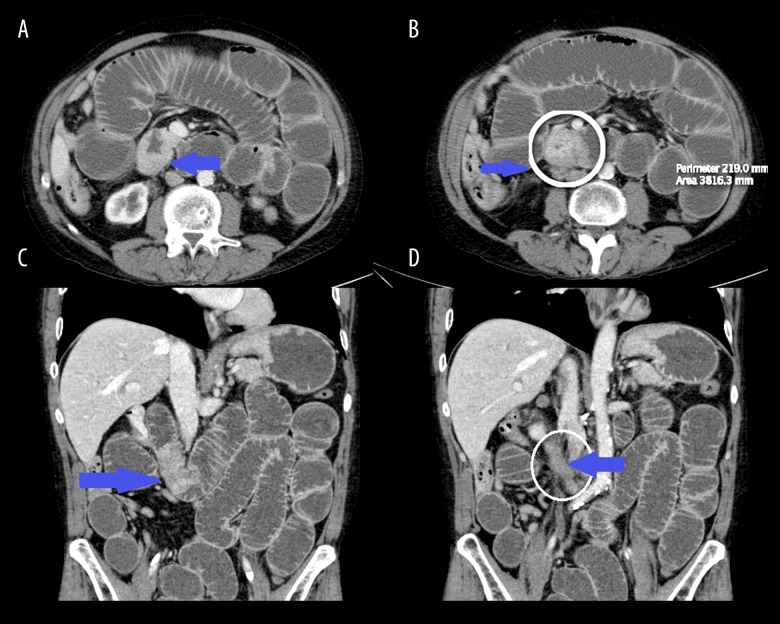
A 60-year-old white man was admitted with complaints of a 5-day history of sharp epigastric abdominal pain, with nausea and vomiting. The patient had been unable to pass stool or flatus for 1 week. There was absence of other symptoms such as gross hematuria, urgency, or frequency prior to admission. His abdomen was tender and distended. His past medical history was significant for EtOH abuse, uncontrolled hypertension, and a 44-pack-year history of smoking. He denied any personal or family history of cancer. Urine sent for urine analysis revealed microscopic hematuria. CA 19-9 was >5000, which is concerning for pancreaticobiliary origin. Computerized tomography (CT) demonstrated a right ureteric mass in continuity with a high-grade distal small bowel obstruction with transition points at the level of the proximal ileum and distal small and large bowel collapse, right obstructive uropathy and hydronephrosis, and bilateral dense renal lesions (suspicious for hemorrhagic cysts) (Figure 1).
Figure 1.
Axial sections (A, B) show focal thickening and enhancement of proximal transverse duodenum. Coronal sections (C, D) show extension of mass for approximately 5 cm and the continuous involvement of adjacent right ureter resulting in obstructive uropathy and hydronephrosis (seen in A).
A CT chest was done to evaluate for metastasis and there were no acute abnormalities or lymphadenopathy seen. The patient underwent an exploratory laparotomy, small bowel resection (SBR), gastrostomy tube placement, and umbilical hernia repair. Surgical pathology of the SBR revealed a poorly differentiated carcinoma with extensive lymphovascular invasion arising from the serosal surface with extensive involvement of the muscularis propria and submucosa. Mucosa demonstrated reactive changes. The biopsy revealed metastatic poorly differentiated carcinoma, most consistent with plasmacytoid variant urothelial carcinoma (Figure 2). The pathological staging of the UC was tumor stage 4 TXNXM1. Immunohistochemical profiles of the tumor cells were strongly diffusely positive for CK20 and CK7, positive for Gata-3 and AE1/3, but negative for TTF1, S100, and CDX2. Immunostains for CK20 and CK7 performed on lymph node sections demonstrated 1 of 2 lymph nodes with metastatic carcinoma. Extensive lymphvascular invasion was noted on surgical pathology.
Figure 2.
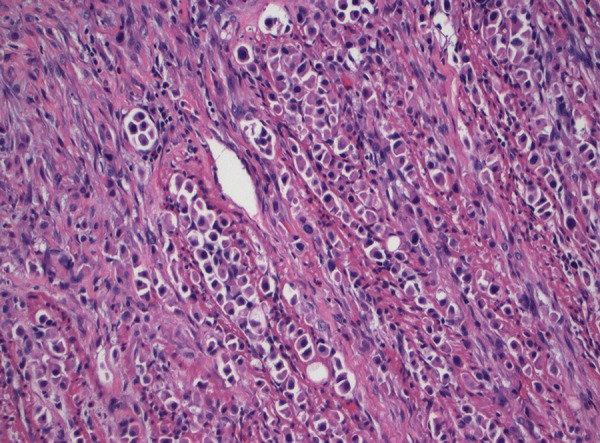
High-magnification pathological biopsy of segment of resected small intestine showing high-grade urothelial carcinoma with plasmacytoid features, characterized by abundant cytoplasm, eccentric nuclei, and discohesive invasive pattern.
On post-op SBR day 4, the patient underwent a bilateral retrograde pyelogram, right uteroscopy, and a cystoscopy. Cystoscopy revealed a heavily trabeculated bladder with several diverticula but no evidence of stones or tumors. With a right pyelogram the ureter was noted to be quite tortuous and extremely stenotic from the mid-ureter up toward the renal pelvis and collecting system. This is where the stent was placed. The upper pole of the left ureter showed distortion with apparent mass affect but no tumor in the urinary collecting system itself. Umbilical hernia pathology reports show fibrovascular adipose tissue, consistent with a hernia, and no evidence of malignant tissue.
On post-op SBR day 10, a repeat abdominal/pelvis CT showed a recurrent SBO area before the ileocecal valve, possibly due to adhesion or carcinomatosis. We also saw a stable known tumor in the second and third portions of the duodenum, with metastasis in the right retroperitoneum obstructing the right ureter. The patient underwent a second exploratory laparotomy, including an ileocecal resection and end ileostomy bag application.
While an inpatient, our patient was medically managed for hypertension, hyponatremia, agitation, dietary supplementation, and pain control. The list of related medication are as follows: clonidine patch 0.2 mg, metoprolol 5 mg IV q6h, sodium chloride 1 gm tab PO BID, Ativan 0.5 mg IV push q8, Morphine 1 mg/ml PCA interval 8 min 4 h limit dose 30 mg IV, Robaxin 1000 mg PO q6 PRN, and hyperalimentation-ADULT 2160 mL IV over 24 h.
Although palliative care treatment is suggested due to the advanced disease in this patient, he has chosen to undergo chemotherapy. Adjuvant chemotherapy was not started immediately; he will be visiting an oncologist as an outpatient after being discharged. A cisplatin-based therapy will be initiated during this time. While the patient will be a candidate for chemotherapy followed by radiation or surgery, this disease is likely to be very aggressive. There is also high concern regarding compliance with further appointments due to social issues. These issues will be further explored and discussed with the patient prior to start of therapy.
Discussion
Plasmacytoid urothelial carcinoma is an extremely rare variant of UC. The histological feature for the tumor tissue is predominately manifested by characteristic plasmacytoid tumor cell morphology. This variant is a high-grade UC, which is usually diagnosed in the advanced pathological stage. The aggressive behavior of the PUC’s is further emphasized in previous published case reports of survival rate, which shows the tendency toward a shorter overall survival of the patients with PUC compared to those with conventional invasive UC of the bladder. However, the majority of the series in the literature are small, with the largest ones having 32 patients [6].
In our case, the patient has a poorly differentiated urothelial carcinoma plasmacytoid type of the ureter with retroperitoneal metastasis and small bowel obstruction. In a 2012 review by Ricardo-Gonzales & Nguyen et al., PUC had a predilection for intraperitoneal spread, which is unusual when compared to conventional UC of the bladder, presenting with discontinuous involvement of serosal surfaces [7]. However, our case did not demonstrate this; rather, we saw retroperitoneal metastasis with continuous spread.
Several authors have reported the lack of E-cadherin expression in most plasmacytoid tumors, which is a necessary protein for cell-to-cell adhesion. This provides support of the histological findings of loss of cellular differentiation, increased cellular invasiveness, and the overall discohesive growth of the tumor, which was also seen in our case [3,6,8].
Clinically, PUC is characterized by advanced stage at diagnosis and poor prognosis. The most common presenting symptom is hematuria, which can be associated with urgency, frequency, or lower abdominal pain. However, diagnosis is often delayed due to the absence of hematuria until late stage of the disease. In fact, there have been reported cases in which PUC was diagnosed late due to the lack of hematuria and absence of an identifiable tumor in the bladder. In cases like this, cystoscopy findings are usually thickened bladder wall and indu-rated mucosa [3,9]. The mean age of initial diagnosis is in the 60’s, and there is a male predominance (8: 1) [10]. In our case, there were no presenting symptoms to indicate urogenital concerns until a urine analysis showed microscopic hematuria.
Clinical management for PUC is not well-defined due to the rarity of the disease and the late presentation. Since most patients presenting with this disease have advanced stage at diagnosis, treatment in the past primarily consisted of both deep transurethral resection of tumor and partial or radical cystectomy. To date, radical cystectomy is considered the best initial choice for invasive or noninvasive PUC, followed by adjuvant cisplatin-based chemotherapy and radiation/surgery, with a median survival of 27.4 months [1,10]. Management of patients with metastatic disease generally includes systemic therapy due to the poor prognostic factors. Systemic treatment with gemcitabine and cisplatin will be the therapy of choice for our patient. An aggressive approach is recommended given the high invasive potential of PUC. Compared to conventional UC, PUC patients have a significantly worse overall survival rate; thus, more studies that include a larger number of patients are necessary to define the best treatment strategies for these patients.
Our patient will follow up as an outpatient with urology/oncology for right ureteral stent removal, as well as to further discuss treatment options. No tumor was identified in the bladder; therefore, a cystectomy was not done as an inpatient. However, we question whether a nephrouterectomy with bladder cuff excision can be done as a definitive treatment option, as was done in the first case of ureteral PUC [2]. The treatment in the study resulted in a known 12-month survival period after diagnosis, without evidence of reoccurrence on follow-up. If after chemotherapy is completed there are no detectable masses, it will be interesting to follow this patient and see if there is any reoccurrence of PUC without a complete tumor resection. There is a strong predilection for reoccurrence along the peritoneal lining, and in some reported cases there is an initial surge in the serum CA-125 levels. This surge precedes radiological and symptomatic findings of progression [11]. Thus, follow-up for this patient can include measurements of serum CA-125, which may help recognize early disease progression and aid in initiating second-line therapies before the patient becomes symptomatic.
Conclusions
PUC continues to remain a rare variant of UC after the first discovery in 1991. The plasmacytoid variant proves to differ from conventional bladder cancers in a number of ways, including clinical presentation and course of metastasis. This brings up the question as to why we are treating it using the same conventional methods, when responses to treatments are only for a short duration of time before tumor recurrence. There is very little known about PUC and the course of this disease and best treatment management, and our case helps provide further insight into how this malignancy can present clinically and pathologically. Further studies based on early and late diagnoses of this tumor entity, with larger cohorts, are needed to develop new and effective identification and therapeutic strategies.
Footnotes
Conflict of Interest
None
References:
- 1.Wang YG, Perera M, Gleeson J. Plasmacytoid urothelial carcinoma of the bladder with extensive scrotal wall invasion. Urology Annals. 2016;8(3):381–83. doi: 10.4103/0974-7796.184897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang I-W, Hsu C-T, Huang C-Y, Tsai J-W. Plasmacytoid urothelial carcinoma: First case reported in the ureter. Pathol Int. 2013;63:73–76. doi: 10.1111/pin.12026. [DOI] [PubMed] [Google Scholar]
- 3.Fritsche HM, Burger M, Denzinger S, et al. Plasmacytoid urothelial carcinoma of the bladder: histological and clinical features of 5 cases. J Urol. 2008;180:1923–27. doi: 10.1016/j.juro.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 4.Coyne JD, Sim E. Urothelial neoplasia with plasmacytoid morphology. Histopathology. 2006;48(2):200–1. doi: 10.1111/j.1365-2559.2005.02153.x. [DOI] [PubMed] [Google Scholar]
- 5.Nabbout P, Fuur J, Parri M, Slobodov G. Plasmacytoid urothelial carcinoma of the bladder metastatic to the stomach: A case report. Case Rep Urol. 2012;2012:715951. doi: 10.1155/2012/715951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keck B, Stoehr R, Wach S, et al. The plasmacytoid carcinoma of the bladder – Rare variant of aggressive urothelial carcinoma. Int J Cancer. 2011;129:346–54. doi: 10.1002/ijc.25700. [DOI] [PubMed] [Google Scholar]
- 7.Ricardo-Gonzalaz RR, Nguyen M, Gokden N, et al. Plasmacytoid carcinoma of the bladder: A urothelial carcinoma variant with a predilection for intraperitoneal spread. J Urol. 2012;187(3):852–55. doi: 10.1016/j.juro.2011.10.145. [DOI] [PubMed] [Google Scholar]
- 8.Cai T, Piazzini M, Nesi G, et al. E-cadherin mRNA expression analysis in evaluating the natural history of urothelial bladder cell carcinoma: Results from a long-term follow-up study. Oncol Rep. 2007;17(4):925–30. [PubMed] [Google Scholar]
- 9.Mai KT, Park PC, Yazdi HM, et al. Plasmacytoid urothelial carcinoma of the urinary bladder report of seven new cases. Eur Urol. 2006;50:1111–14. doi: 10.1016/j.eururo.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 10.Keck B, Wach S, Stohr R, et al. Plasmacytoid variant of bladder cancer defines patients with poor prognosis if treated with cystectomy and adjuvant cisplatin-based chemotherapy. BMC Cancer. 2013;13:71. doi: 10.1186/1471-2407-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dayyani F, Czerniak BA, Sircar K, et al. Plasmacytoid urothelial carcinomas – a chemo-sensitive cancer with poor prognosis, and peritoneal carcinomatosis. J Urol. 2013;189(5):1656–61. doi: 10.1016/j.juro.2012.11.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eble JN, Sauter G, Epstein JI. WHO Classification of Tumors. Lyon: IARC Press; 2004. Pathology and genetics: Tumors of the urinary system and male genital organs. [Google Scholar]