Abstract
Background
Osteoporosis is associated with 17β-estradiol deficiency. The G protein-coupled receptor 30 (GPR30) is known to be an estrogen-responsive receptor, but its role in the degradation of mitochondria in osteoblasts by autophagy, or mitophagy, remains unclear. The aim of this in vitro study was to evaluate the effects of 17β-estradiol, GPR30, and its signaling pathway, on mitophagy in the murine MC3T3-E1 osteoblast cell line.
Material/Methods
In the murine MC3T3-E1 osteoblast cell line, cells were treated with 17β-estradiol, or G15, a selective GPR30 antagonist, or U0126, a mitogen-activated protein (MAP) kinase (ERK1/2) inhibitor, or with vehicle as control. The expression of GPR30 was determined by Western blot, reverse transcription-polymerase chain reaction (RT-PCR), and confocal immunofluorescence imaging. Cell morphology and mitochondrial autophagosomes were identified using transmission electron microscopy (TEM). Phosphorylation of the mitophagy markers, heat shock protein 60 (Hsp60), translocase of outer membrane (Tom)20, and microtubule-associated protein 1A/1B-light chain 3 (LC3) were determined by Western blot, and cell proliferation was determined using the bromodeoxyuridine (BrdU) assay.
Results
The optimum concentration of 17β-estradiol that resulted in GPR30 expression in MC3T3-E1 cells was 10−7 M, which led to the accumulation of mitochondrial autophagosomes and increased protein phosphorylation levels of Hsp60, Tom20, and LC3. In cells pretreated with G15 or U0126, 17β-estradiol treatment did not increase mitophagy in MC3T3-E1 cells.
Conclusions
In murine osteoblasts cultured in vitro, treatment with 17β-estradiol resulted in the expression of GPR30 and enhanced mitophagy through the GPR30 and ERK1/2 signaling pathway.
MeSH Keywords: Estradiol; MAP Kinase Signaling System; Mitochondrial Degradation; Receptors, G-Protein-Coupled
Background
Osteoporosis is a metabolic bone disease that is associated with a reduction in bone volume, with the main clinical features including fracture and bone pain. With the increasingly aging population worldwide, the incidence of morbidity and mortality from osteoporosis, and the associated economic burden of this disease continue to increase annually. Therefore, there is increasing interest in research into osteoporosis and its prevention.
Pathways involved in mitochondrial autophagy, or mitophagy, have been studied in cell lines in vitro, and have been shown to have important regulatory effects in the development and progression of several major diseases, including neurodegenerative diseases, heart disease, and diabetes mellitus [1–5]. Therefore, the factors that affect mitochondrial autophagy, or mitophagy, may be an emerging research area of interest in osteoporosis.
Primary osteoporosis more prevalent in postmenopausal women than in men; as the level of estrogen, or 17β-estradiol, decreases, bone loss increases and therefore osteoporosis increasingly occurs in perimenopausal women [1–5]. Estrogen replacement therapy is the main treatment for postmenopausal osteoporosis. Estrogen levels may be the main cause of osteoporosis, but the molecular mechanisms underlying osteoporosis caused by estrogen reduction remain unknown. Therefore, continued studies on the effects of estrogen on bone metabolism, particularly on osteoblast function, may provide information to improve the treatment of osteoporosis and other metabolic bone diseases. Therefore, the relationship between 17β-estradiol and mitophagy in osteoblasts is an important area of research that may lead to findings that affect clinical treatment [1–5].
Estradiol, or 17β-estradiol, may protect osteoblasts from apoptosis, autophagy, and mitophagy [6]. In 1977, a study identified an estrogen membrane receptor coupled to a G protein, G protein-coupled receptor 30 (GPR30), in human breast cancer cells [6]. GPR30 was first extracted from B-cells and confirmed as seven-span transmembrane protein, which is now known to exist in a variety of cells, including macrophages, and neuroglial cells [7]. In 2000, a study showed that mitogen-activated protein (MAP) kinase (ERK1/2) was activated by estrogen in breast cancer cell lines expressing GPR30, but not in cell lines lacking the receptor [8,9]. Studies on breast cancer have reported that estrogen activates ERK not by classic estrogen receptors, ER-α or ER-β, but by GPR30, similar to the rapid but transient activation of mitogen-activated protein kinase (MAPK) [9,10]. In 2005, Thomas et al. confirmed that GPR30 was an estrogen membrane receptor [10]. The action of GPR30 results in pleiotropic effects in metabolically active tissues, such as the pancreas, liver, and skeletal muscle [11]. Although GPR30 was initially considered to be a G protein-coupled estrogen receptor, it was recently reported to be expressed in osteoblasts of human bone [12]. However, the function of GPR30 in bone remains unknown [13].
Mitochondria play an important role in the metabolism of living organisms, and provide energy for metabolic activity, produce reactive oxygen species (ROS), and have roles in signal transduction and gene regulation [14]. Damaged mitochondria can be degraded by mitophagy, and dysfunction of mitophagy is closely related to the pathogenesis of some diseases [15–17]. Impaired mitochondria release ROS and apoptosis factors, which lead to cell damage or apoptosis by autophagy, with the elimination of the damaged mitochondria. Estradiol, especially 17β-estradiol, has been proposed to protect osteoblasts against apoptosis [18]. However, whether mitophagy is involved in the protective effect of 17β-estradiol in osteoblasts, or whether 17β-estradiol induces mitophagy through its special membrane receptor, GPR30 and its signaling pathway, remain unknown.
Therefore, the aim of this in vitro study was to evaluate the effects of 17β-estradiol, GPR30, and its signaling pathway, on mitophagy in the murine MC3T3-E1 osteoblast cell line.
Material and Methods
Chemicals and reagents
Cell culture media, minimum essential medium-alpha (α-MEM) and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). 17β-estradiol was obtained from Sigma-Aldrich (St. Louis, MO, USA). The G protein-coupled receptor 30 (GPR30) antagonist, G15, was purchased from Tocris Bioscience (Minneapolis, MA, USA). The ERK inhibitor (U0126), phenylmethylsulfonyl fluoride, radioimmunoprecipitation assay buffer, and 4′, 6-diamidino-2-phenylindole (DAPI), were purchased from Beyotime Biotechnology (Jiangsu, China). Phosphatase inhibitors were purchased from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). The polyclonal, rabbit anti-mouse primary GPR30 antibody (sc-48524-R) (1: 200 for Western blot; 1: 50 for immunofluorescence), the polyclonal rabbit anti-mouse primary translocase of outer membrane (Tom)20 antibody (sc-11415) (1: 200 for Western blot), the monoclonal mouse anti-mouse microtubule-associated protein 1A/1B-light chain 3 (LC3) antibody (sc-376404) (1: 100 for Western blot), and the polyclonal, goat anti-mouse heat shock protein 60 (Hsp60) antibody (sc-1052) (1: 200 for Western blot) were all obtained from Santa Cruz Biotechnology (California, USA). Polyclonal anti-mouse anti-total and phospho-ERK antibody was obtained from Abcam (Cambridge, UK). Monoclonal, mouse anti-mouse antibody to β-actin (AF0003) (1: 1000) was purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Trizol reagent was purchased Life Technologies Co. (Carlsbad, CA, USA). TaqMan reagents were purchased from Takara (Otsu, Japan). The GPR30 and GAPDH primers for reverse transcription-polymerase chain reaction (RT-PCR) were provided by Shanghai Invitrogen Agent (Shanghai, China). The immunofluorescence staining kit was purchased from Beyotime Biotechnology (Jiangsu, China). The bromodeoxyuridine (BrdU) assay kit was purchased from Abcam (Cambridge, UK).
Cell culture of MC3T3-E1 murine osteoblasts
MC3T3-E1 cells were purchased from the XIEHE Cell Repository in Beijing, China. Cells were subcultured in α-MEM with 10% FBS and grown in a humidified atmosphere containing 5% CO2 at 37°C. Cells were subjected to different concentrations of 17β-estradiol (0 M, 10−9 M, 10−8 M and 10−7 M) for 48 h. G15, a selective GPR30 antagonist, or U0126, a mitogen-activated protein (MAP) kinase (ERK1/2) inhibitor, or vehicle as control, were also used to treat the cells.
Protein preparation and western blot analysis
MC3T3-E1 cells were washed with cold phosphate-buffered saline (PBS), and harvested in radio-immunoprecipitation assay (RIPA) buffer containing protease inhibitors, including phenylmethylsulfonyl fluoride and phosphatase inhibitors. The cells were incubated with lysates on ice for 30 min, and centrifuged at 12,000×g for 10 min at 4°C. The supernatants were collected and mixed with 5× loading buffer, and were denatured by boiling for 10 min. For Western blot, 50 μg of total proteins underwent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was transferred to polyvinylidene fluoride (PVDF) membranes using a transfer buffer (20 mM Tris, 150 mM glycine, and 20% methanol) at 70 V for 1.5 h, according to the molecular weight of the tested proteins. The membranes were incubated with TBST (50 mM Tris, 200 mM NaCl, 0.2% Tween 20) and 5% bovine serum albumin (BSA) for 2 h. After washing three times with TBST for 10 min each, the blot was incubated with the corresponding primary antibody overnight at 4°C, followed by horseradish peroxidase (HRP)-labeled secondary antibody for 1.5 h. The membranes were washed with TBST for 30 min and proteins were visualized using the BeyoECL plus kit (Beyotime Biotechnology, China).
Real-time polymerase chain reaction (RT-PCR)
The RT-PCR method was used to extract RNA with trizol, reverse-transcribed mRNA to cDNA, amplified cDNA with PCR amplifications. Total RNA was extracted from MC3T3-E1 cells using trizol reagent. The expression of GPR30 mRNA was detected by real-time PCR using TaqMan reagents. The synthesis of primers was provided by Shanghai Invitrogen Agent.
The GPR30 forward primer was: 5′-AACAGAGCAGCGATCTGGAC-3′.
The GPR30 reverse primer was: 5′-GCAGAGTCCTTGGATGGCTT-3′.
The forward GAPDH primer was: 5′-AACAGAGCAGCGATCTGGAC-3′.
The reverse GAPDH primer was: 5′-GCAGAGTCCTTGGATGGCTT-3′.
The PCR reactions were carried out using the following conditions: 95°C for 30 s, then 40 cycles of 95°C for 5 s, and 60°C for 30 s. All primers and TaqMan probes specific for GPR30 and GAPDH were designed using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA, USA). The band to GAPDH ratio was deemed as the relative expression of GPR30 mRNA.
Confocal immunofluorescence microscopy
MC3T3-E1 cells were seeded into six-well plates and incubated for 24 h. Then, cells were washed once with ice-cold PBS and fixed with 4% paraformaldehyde for 30 min at 4°C. Following washing with PBS three times, the cells were blocked at non-specific antibody sites by 5% BSA in TBST for 30 min. The MC3T3-E1 murine osteoblast cells were incubated with specific primary rabbit anti-GPR30 antibodies (1: 50) overnight at 4°C. The next day, the MC3T3-E1 cells were washed with PBS, and then incubated with the secondary antibody for 30 min using a donkey anti-rabbit IgG (1: 1000). Then, these cells were stained with DAPI for 5 min. After washing with PBS for 15 min, the immunofluorescence-stained cells were viewed using an Olympus FV1000 confocal laser-scanning microscope with a peak emission wavelength of 518 nm (green) and 565 nm (red).
Transmission electron microscopy (TEM)
The MC3T3-E1 cells were harvested and fixed in 2.5% glutaraldehyde PBS for 2 h at room temperature. The cells were then post-fixed in 1% osmium tetroxide in water for 1 h, and stained in 2% uranyl acetate in water for 1 h in the dark. After dehydration in an ascending series of ethanol, the samples were embedded, sectioned, double-stained with uranyl acetate and lead citrate, and analyzed using a JEM-1200EX transmission electron microscope (TEM) (JEOL, Tokyo, Japan).
Cell proliferation using the bromodeoxyuridine (BrdU) assay
The effect of 17β-estradiol on cell proliferation was analyzed by measuring the incorporation of bromodeoxyuridine (BrdU) into genomic DNA. MC3T3-E1 cells were seeded into 96-well microtiter plates. After culture with 10−7 M 17β-estradiol for 48 h, with or without G15/U0126, 10 mM BrdU was added to the culture medium for incorporation into the DNA of replicating cells. After 2 hours, cells were fixed in 4% paraformaldehyde. BrdU incorporation was determined by a cell proliferation enzyme-linked immunosorbent assay (ELISA) BrdU kit.
Statistical analysis
Data were expressed as the mean ± the standard errors of the mean (SEM), and the differences between the means were analyzed by one-way analysis of variance (ANOVA). A P-value <0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 15.0 software package (SPSS, Inc., Chicago, IL, USA).
Results
Expression of G protein-coupled receptor 30 (GPR30) in MC3T3-E1 cells
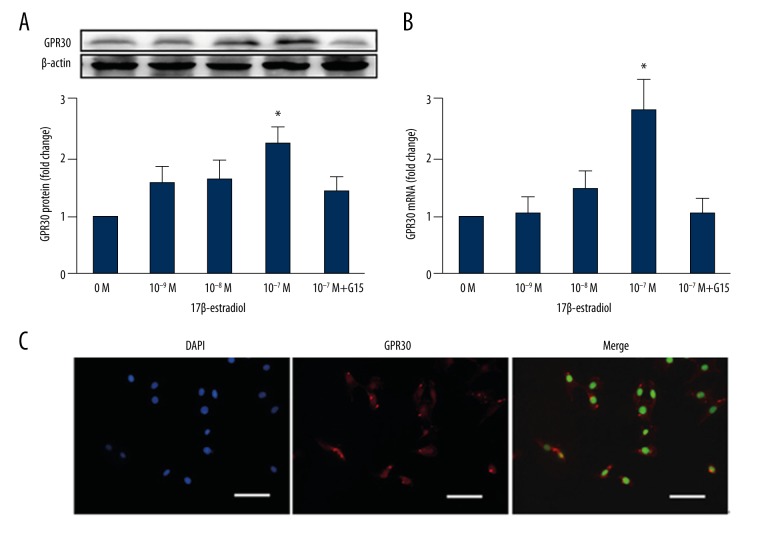
The expression of G protein-coupled receptor 30 (GPR30) in MC3T3-E1 murine osteoblast cells by real-time polymerase chain reaction (RT-PCR) and Western blot to determine whether 17β-estradiol was expressed in osteoblasts through GPR30. MC3T3-E1 cells were subjected to different concentrations of 17β-estradiol (0 M, 10−9 M, 10−8 M and 10−7 M) for 48 h in the presence or absence of G15 (a GPR30 inhibitor). Within 48 h, the protein expression of GPR30 increased with the addition of 17β-estradiol as shown by Western blot (Figure 1). The expression of GPR30 proteins in MC3T3-E1 cells increased in a dose-dependent manner with the increasing concentration of 17β-estradiol. Similar results were obtained by detecting the expression of mRNA by RT-PCR (Figure 1). According to the above results, the optimal concentration of 17β-estradiol required for receptor expression was 10−7 M. Then, 17β-estradiol expression in osteoblasts through GPR30 was identified by confocal immunofluorescence (Figure 1).
Figure 1.
G protein-coupled receptor 30 (GPR30) was expressed in murine MC3T3-E1 osteoblast cells treated with 17β-estradiol in a dose-dependent manner. MC3T3-E1 cells were treated with different concentrations (0 M, 10−9 M, 10−8 M, and 10−7 M) of 17β-estradiol, with or without 15 μM of G15 for 48 h. (A) Protein expression of GPR30 was measured by Western blot. *P<0.05 compared with the control. (B) mRNA expression was measured by real-time polymerase chain reaction (RT-PCR) analysis. * P<0.05 compared with the control. (C) Confocal immunofluorescence staining of MC3T3-E1 cells, stained for GPR30 (red). Nuclei are stained with 4′, 6-diamidino-2-phenylindole (DAPI) (blue). The white bar represents 50 μm. GPR30 is shown to be expressed in MC3T3-E1 cells and the optimal concentration of 17β-estradiol required for GPR30 expression is 10−7 M.
17β-estradiol induced mitophagy in osteoblasts in vitro
Mitophagy was shown to occur in the murine MC3T3-E1 osteoblast cell line by treatment with 17β-estradiol. MC3T3-E1 cells were treated with 10−7 M 17β-estradiol for 48 h, and with heat shock protein 60 (Hsp60), translocase of outer membrane (Tom)20, and microtubule-associated protein 1A/1B-light chain 3 (LC3), which were markers of mitophagy. Protein phosphorylation levels were measured by Western blot and compared with the control group (Figure 2). Treatment with 17β-estradiol led to further accumulation of Hsp60, Tom20 and LC3 in MC3T3-E1 cells, supporting an increase in mitophagy. Also, transmission electronmicroscopy (TEM) showed increased mitochondrial autophagosomes after 48 h of 17β-estradiol treatment (Figure 3). Also, after treatment with 10−7 M 17β-estradiol for 48 h, the mitochondrial autophagosomes increased in number, and the number of viable cells increased with the addition of 17β-estradiol.
Figure 2.
(A–D) Treatment with 17β-estradiol induced mitophagy in murine MC3T3-E1 osteoblast cells via the G protein-coupled receptor 30 (GPR30)-ERK1/2 signaling pathway. Western blot for phosphorylation of microtubule-associated protein 1A/1B-light chain 3 (LC3), heat shock protein 60 (Hsp60), translocase of outer membrane (Tom)20, p-ERK1/2, t-ERK1/2, and β-actin proteins. Murine MC3T3-E1 osteoblast cells were pretreated with 15 μM G15 or 20 μM U0126 for 1 hour, and then incubated with or without 10−8 M 17β-estradiol for 48 h. MC3T3-E1 cells cultured in vehicle were used as controls and β-actin was used as the protein loading control. The phosphorylated levels of mitophagy-associated proteins (LC3, Hsp60, and Tom20) were increased by the treatment of 17β-estradiol alone, but the increased levels were blocked by G15 and could not be reversed by 17β-estradiol. U0126 could partly inhibit mitophagy by suppressing phosphorylation of ERK (p-ERK) and 17β-estradiol did not override this effect. Phosphorylated levels of ERK (p-ERK) in MC3T3-E1 cells after 48 h exposure to 17β-estradiol alone were significantly increased. There were increased phosphorylated levels of ERK (p-ERK), which were inhibited when MC3T3-E1 cells were pre-treated with G15 (GPR30 antagonist). * P<0.05 compared with the control.
Figure 3.
17β-estradiol upregulated mitophagy to protect osteoblasts through G protein-coupled receptor 30 (GPR30) in MC3T3-E1 cells. Transmission electronmicroscopy (TEM) images show the presence of more mitochondrial autophagosomes following treatment with 10−8 M of 17β-estradiol for 48 h. The increased mitophagy was inhibited by G15 (1 h prior to 17β-estradiol treatment) and the activity of MC3T3-E1 cells was reduced followed by detection of damaged osteoblast cell membrane, and nuclear membrane. The arrows indicate autophagosomes.
MC3T3-E1 cells were treated with 10−7 M 17β-estradiol for 48 h. Cell proliferation was evaluated by using the bromodeoxyuridine (BrdU) assay, which showed that the cell proliferation of the 17β-estradiol-treated cells was significantly increased compared with that of the untreated control group (Figure 4). Transmission electron microscopy (TEM) showed that autophagic characteristics, including pyknotic chromatin, disintegrated nuclear membrane, and cytosolic autophasosomes, were abundant in the cells without 17β-estradiol treatment (Figure 3). The above data suggested that 17β-estradiol induced mitophagy in MC3T3-E1 cells in vitro.
Figure 4.

17β-estradiol protected MC3T3-E1 cells via the G protein-coupled receptor 30 (GPR30) -ERK1/2 signaling pathway. Cell proliferation detected by the bromodeoxyuridine (BrdU) assay. MC3T3-E1 cells were pretreated with or without 15 μM of G15 or 20 μM of U0126 for 1 h, and then incubated with or without 10−8 M 17β-estradiol for 48 h. The findings show that 17β-estradiol treatment upregulated cell proliferation, which was inhibited by G15 or U0126.
17β-estradiol protected osteoblasts by inducing mitophagy through the GPR30/ERK signaling pathway
Western blot was used to investigate the signaling pathway and underlying molecular mechanism by which 17β-estradiol enhanced mitophagy. To determine whether 17β-estradiol receptor, GPR30, was required for estradiol-enhanced mitophagy, osteoblasts were pretreated with the GPR30 inhibitor, G15 for 1 h and then stimulated with 17β-estradiol for 48 h or with vehicle. Western blot analysis showed that 17β-estradiol significantly increased the phosphorylation of HSP60, Tom20, and LC3 proteins in MC3T3-E1 cells after 48 h, but did not enhance mitophagy in the osteoblasts pretreated with G15 (Figure 2). Because LC3-II is converted from LC3-I and serves as a typical marker of completed autophagy, these results demonstrated that 17β-estradiol induced mitophagy via GPR30.
Extracellular signal-related kinase-1/2 (ERK1/2) activation promotes autophagy in different cell lines
To examine the relationships between 17β-estradiol and osteoblast mitophagy, the protein levels of phospo-ERK1/2 and total-ERK1/2 were assessed by Western blot (Figure 2). The effect of 17β-estradiol on the activation of the signaling pathway, which regulated several normal cellular functions that were critical for cell proliferation, growth and survival, were further investigated. The results showed that the expression was similar to the expression of mitophagy marker proteins. 17β-estradiol significantly upregulated p-ERK1/2 in MC3T3-E1 cells, but not when the cells were pretreated with the selective GPR30 antagonist, G15. Therefore, 17β-estradiol expressed in vitro in osteoblasts regulated the expression of p-ERK1/2. The mitogen-activated protein (MAP) kinase (ERK1/2) inhibitor, U0126, was used to block the ERK signaling pathway and to detect the variation of mitophagy in osteoblasts. As shown in Figure 2, the activation of phospho-Hsp60, phospho-Tom20 and phospho-LC3, induced by 17β-estradiol, were significantly reversed by treatment with U0126. These results suggested that treatment with 17β-estradiol induced osteoblast mitophagy via the GPR30-ERK1/2 signaling pathway.
When MC3T3-E1 cells were pretreated with G15 or U0126, the cell proliferation rate was reduced and could not be reversed by 17β-estradiol (Figure 4). Therefore, 17β-estradiol-induced cell proliferation could be blocked by G15 or U0126, and 17β-estradiol had a protective effect on murine osteoblasts in vitro through the GPR30-ERK1/2 signaling pathway.
Discussion
Estrogen deficiency is the leading cause of postmenopausal osteoporosis, and estrogen replacement therapy is effective for improving the bone mineral density in osteoporotic postmenopausal women. Although cellular and molecular mechanisms underlying the anti-osteoporosis effect of estrogen have been extensively studied, the specific mechanisms in osteoblasts are not fully understood. Estrogen, and 17β-estradiol, has significant impact on bone mineral metabolism.
Several previously published studies have demonstrated the existence of membrane-associated estrogen receptor (ER), either similar to or distinct from the classical nuclear ER [19–21]. Besides the classical estrogen receptors (ER-α and ER-β), a transmembrane G-protein-coupled receptor, the G protein-coupled receptor 30 (GPR30) was shown to mediate estrogenic effects [11,22]. It has also previously been demonstrated that GPR30 was expressed in osteoblast, vascular endothelial cells, and pancreatic islet β-cells [23–26], but the function of GPR30, and the signaling pathways involved in bone in osteoblasts remains unknown. The findings of this study showed that GPR30 was expressed in the murine MC3T3-E1 osteoblast cells in vitro, and its expression changed in a dose-dependent and time-dependent manner with 17β-estradiol treatment (Figure 1). The optimum concentration of 17β-estradiol, for the expression of GPR30, was 10−7 M. Therefore, to further examine the role of GPR30 in 17β-estradiol-induced mitophagy, 10−7 M 17β-estradiol was added to the MC3T3-E1 cells, which were treated for 48 h.
Mitochondria play an important role in the metabolism of living organisms, provide energy for metabolic activity, produce reactive oxygen species (ROS), and are required for signal transduction and gene regulation. For this reason, dysfunction or damage of mitochondria has serious consequences, and can lead to cell death. Mitophagy is essential for cellular homeostasis, and previously published studies have demonstrated the involvement of mitophagy in physiological and pathological processes, including differentiation, development, defense against pathogens, in aging, apoptosis, and inflammation [27–30]. Previously published studies have shown that 17β-estradiol protected cellular homeostasis by inducing mitophagy in traumatic brain injury, alcoholic fatty liver, and osteosarcoma cells [31–33].
The findings of this study showed that in the murine MC3T3-E1 osteoblast cell line, mitophagy, detected by Western blot (Figure 2) and transmission electronmicroscopy (TEM) (Figure 3), was induced and increased by treatment with 17β-estradiol compared with the control group, as demonstrated by the increase in mitophagy markers heat shock protein 60 (Hsp60), translocase of outer membrane (Tom)20, and microtubule-associated protein 1A/1B-light chain 3 (LC3), and the accumulation of mitophagolysosomes. Also with the increase of the 17β-estradiol-induced mitophagy, the cell proliferation rate was also increased, which was detected by TEM and the bromodeoxyuridine (BrdU) assay (Figure 4), respectively.
A previously published study has shown that neither bone loss nor high bone turnover is detectable in ER-α single knockout or ER-α and ER-β double knockout mice, suggesting that another estrogen-binding receptor that may compensate for the lack of ER [34]. GPR30 mediates membrane-initiated estrogen signaling as a G-protein coupled ER [35]. Therefore, the maintenance of bone mass in the mice might be partly due to the complementary effect of GPR30 [34,35]. Also 17β-estradiol has been shown to protect rat mesothelium by inducing mitophagy via GPR30 [22], and 17β-estradiol has been shown to induce the proliferation of osteoblasts through GPR30 [23]. This study was undertaken to investigate the role of GPR30 in osteoblast mitophagy and used pretreatment with the GPR30 inhibitor, G15, and when GPR30 was inhibited, 17β-estradiol partly lost its ability to induce mitophagy (Figure 2).
To our knowledge, this was the first study to demonstrate that GPR30 is an effective receptor for 17β-estradiol-induced mitophagy in osteoblasts. When GPR30 was inhibited, the proliferation of osteoblasts was reduced (Figure 4). The activation of mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinases (ERK) regulates osteoblast differentiation in vitro and in vivo [36,37]. 17β-estradiol could modulate osteoblasts to control cell stress, apoptosis and cell death through the MAPK signaling pathway [38–40]. 17β-estradiol has been previously reported to induce mitophagy in the MDA-MB-231 human breast cancer cell line when transfected with GPR30, suggesting that estrogen can activate ERK through GPR30 [41]. It has also previously been shown that the binding of estrogen and GPR30 can trigger a cascade of MAPK signaling and activation of ERK, and thereby promote cell growth and cell proliferation [2]. Treatment of cells in vitro with U0126, a mitogen-activated protein (MAP) kinase (ERK1/2) inhibitor has been shown to attenuate phytoestrogenic effects [42], but this effect was not due to the effects of ER-α phosphorylation, but by blocking a GPR30-mediated increase in intracellular cAMP, which further demonstrates that estrogen binds to GPR30 through the ERK pathway. In the present study, the expression of phosphorylated ERK could be induced by 17β-estradiol and suppressed by G15 (Figure 2). Also, 17β-estradiol-induced mitophagy could be blocked by U0126. Therefore, 17β-estradiol appeared to protect murine osteoblasts by enhancing mitophagy through the GPR30/ERK pathway.
This study identified an important role of 17β-estradiol, an inducer of osteoblast mitophagy in bone development, in murine MC3T3-E1 cells. The ablation of 17β-estradiol in osteoblasts inhibited proliferation by negatively affecting osteoblast mitophagy. These results provide novel insights into the detailed molecular mechanisms by which mitophagy regulates osteoblast functions. However, there are several remaining questions that require further study, including the regulatory mechanisms governing bone development and osteoblast differentiation by mitophagy. The roles of mitophagy in vivo or in human bone development remain to be investigated. The effects of aging on osteoblast mitophagy also remain to be investigated, in view of the increasing prevalence of osteoporosis in an increasingly aging population.
Conclusions
The findings of this in vitro study showed, that in a murine osteoblast cell line, the expression of G protein-coupled receptor 30 (GPR30) was dependent on 17β-estradiol, in a dose-dependent way. 17β-estradiol treatment enhanced mitophagy through the GPR30-ERK1/2 signaling axis. In the murine MC3T3-E1 osteoblast cell line, 17β-estradiol appeared to protect osteoblasts and promoted osteoblast proliferation via stimulation of the degradation of mitochondria mitophagy in murine osteoblasts in vitro.
Acknowledgements
The authors would like to thank The China Medical University Affiliated Hospital Laboratory Center for kindly providing the equipment
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of China (Grant Nos. 81470998, 81071460, and 81271996)
References
- 1.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 3.Kanda N, Watanabe S. 17-beta-estradiol inhibits oxidative stress induced apoptosis in keratinocytes by promoting Bcl-2 expression. J Invest Dermatol. 2003;121:1500–9. doi: 10.1111/j.1523-1747.2003.12617.x. [DOI] [PubMed] [Google Scholar]
- 4.Heino TJ, Chagin AS, Sävendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197:1–6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, Madungwe NB, da Cruz Junho CV, Bopassa JC. Activation of G protein-coupled oestrogen receptor 1 at the onset of reperfusion protects the myocardium against ischemia/reperfusion injury by reducing mitochondrial dysfunction and mitophagy. Br J Pharmacol. 2017;174:4329–44. doi: 10.1111/bph.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;14(23):624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 7.Ordonez MP. Defective mitophagy in human Niemann-Pick Type C1 neurons is due to abnormal autophagy activation. Autophagy. 2012;8:1157–58. doi: 10.4161/auto.20668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langer G, Bader B, Meoli L, et al. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:3–10. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Owman C, Blay P, Nilssion C, Lolait SJ. Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochem Biophys Res Commun. 1996;228:285–92. doi: 10.1006/bbrc.1996.1654. [DOI] [PubMed] [Google Scholar]
- 10.Novak I. Mitophagy: A complex mechanism of mitochondrial removal. Antioxid Redox Signal. 2012;17:794–802. doi: 10.1089/ars.2011.4407. [DOI] [PubMed] [Google Scholar]
- 11.Sharma G, Prossnitz ER. G-protein-coupled estrogen receptor (GPER) and sex-specific metabolic homeostasis. Adv Exp Med Biol. 2017;1043:427–53. doi: 10.1007/978-3-319-70178-3_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor R, Goldman SJ. Mitophagy and disease: New avenues for pharmacological intervention. Curr Pharm Des. 2011;17:2056–73. doi: 10.2174/138161211796904768. [DOI] [PubMed] [Google Scholar]
- 13.Mijaljica D, Prescott M, Devenish R. Mitophagy and mitoptosis in disease processes. Methods Mol Biol. 2010;648:93–106. doi: 10.1007/978-1-60761-756-3_6. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299:203–10. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuehua Y, Ke C, Bo L, et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER–ERK–mTOR pathway. Apoptosis. 2013;18:1363–75. doi: 10.1007/s10495-013-0867-x. [DOI] [PubMed] [Google Scholar]
- 16.Pietras RJ, Szeqo CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 17.Shaerzadeh F, Motamedi F, Minai-Tehrani D, Khodaqholi F. Monitoring of neuronal loss in the hippocampus of Aβ-injected rat: autophagy, mitophagy and mitochondrial biogenesis stand against apoptosis. Neuromolecular Med. 2014;16:175–90. doi: 10.1007/s12017-013-8272-8. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Ye B, Miller S, et al. Ablation of ALCAT1 mitigates hypertrophic cardiomyopathy through effects on oxidative stress and mitophagy. Mol Cell Biol. 2012;32:4493–504. doi: 10.1128/MCB.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: The second decade. Cell. 1995;83:5–9. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prossnitz ER, Arterburn JB, Sklar LA. GPR30: A G protein-coupled receptor for estrogen. Mol Cell Endocrinol. 2007;138:265–66. doi: 10.1016/j.mce.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:1–12. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- 22.Baloqh P, Szabo A, Liko I, et al. Autophagy may contribute to the recovery of rat mesothelium following acute inflammation in vivo. Cell Tissue Res. 2015;362(1):127–37. doi: 10.1007/s00441-015-2188-6. [DOI] [PubMed] [Google Scholar]
- 23.Seino HN, Sawada K, Hayakawa J, et al. Estradiol and reloxifene induce the proliferation of osteoblasts through G-protein-coupled receptor GPR30. J Endocrinol Invest. 2013;36:21–27. doi: 10.3275/8301. [DOI] [PubMed] [Google Scholar]
- 24.Zhou LY, Chen H, Mao X, et al. G-protein-coupled receptor 30 mediates the effects of estrogen on endothelial cell tube formation in vitro. Int J Molec Med. 2017;39:1461–67. doi: 10.3892/ijmm.2017.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding QY, Hussain J, Chorazyczewski R, et al. GPER-independent effects of estrogen in rat aortic vascular endothelial cells. Molec Cell Endocrinol. 2015;399:60–68. doi: 10.1016/j.mce.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Rajesh K, Alexander B, Stefan A, et al. Insulinotropic and antidiabetic effects of 17β-estradiol and GPR30 agonist G-1 on human pancreatic islets. Endocrinology. 2011;152(7):2568–79. doi: 10.1210/en.2010-1361. [DOI] [PubMed] [Google Scholar]
- 27.Toran-Allerand CD, Guan X, et al. ER-X: A novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:391–401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura T, Imai Y, Matsumoto T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:11–23. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Maggiolini M, Vivacqua A, Fasanella G, et al. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17β-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008–16. doi: 10.1074/jbc.M403588200. [DOI] [PubMed] [Google Scholar]
- 30.Kang K, Lee SB, Junq SH, et al. Tectoridin, a poor ligand of estrogen receptor alpha, exerts its estrogenic effects via an ERK-dependent pathway. Mol Cell. 2009;27:351–57. doi: 10.1007/s10059-009-0045-8. [DOI] [PubMed] [Google Scholar]
- 31.Cho HI, Seo MJ, Lee SM. 2-Methoxyestradiol protects against ischemia/reperfusion injury in alcoholic fatty liver by enhancing sirtuin 1-mediated autophagy. Biochem Pharmacol. 2017;131:40–51. doi: 10.1016/j.bcp.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Schaible EV, Windschuql J, Bobkiewicz W, et al. 2-Methoxyestradiol confers neuroprotection and inhibits a maladaptive HIF-1α response aftertraumatic brain injury in mice. J Neurochem. 2014;129(6):940–54. doi: 10.1111/jnc.12708. [DOI] [PubMed] [Google Scholar]
- 33.Bravo D, Shoqren KL, Zuo D, et al. 2-Methoxyestradiol-mediated induction of frzb contributes to cell death and autophagy in MG63 osteosarcoma cells. J Cell Biochem. 2017;118(6):1497–504. doi: 10.1002/jcb.25809. [DOI] [PubMed] [Google Scholar]
- 34.Jacquel A, Obba S, Boyer L, et al. Autophagy is required for CSF-1-induced macrophagic differentiation and acquisition of phagocytic functions. Blood. 2012;119:4527–31. doi: 10.1182/blood-2011-11-392167. [DOI] [PubMed] [Google Scholar]
- 35.Graziotto JJ, Cao K, Collins FS, Krainc D. Rapamycin activates autophagy in Hutchinson-Gilford progeria syndrome: Implications for normal aging and age-dependent neurodegenerative disorders. Autophagy. 2012;8:147–51. doi: 10.4161/auto.8.1.18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsushita T, Chan YY, Kawanami A, et al. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol Cell Biol. 2009;29(21):5843–57. doi: 10.1128/MCB.01549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Zhou J, Li Y, et al. Rap1A regulates osteoblastic differentiation via the ERK and p38 mediated signaling. PLoS One. 2015;10(11):e0143777. doi: 10.1371/journal.pone.0143777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filardo E, Quinn J, Pang Y, et al. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:36–45. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- 39.Prouillet C, Maziere JC, Maziere C, et al. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67:1307–13. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 40.Chen JR, Plotkin LI, Aguirre JI, et al. Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem. 2005;280:4632–38. doi: 10.1074/jbc.M411530200. [DOI] [PubMed] [Google Scholar]
- 41.Aguirre JI, Plotkin LI, Gortazar AR, et al. A novel ligand independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282:25501–8. doi: 10.1074/jbc.M702231200. [DOI] [PubMed] [Google Scholar]
- 42.Joubert PE, Werneke SW, de la Calle C, et al. Chikungunya virus-induced autophagy delays caspase-dependent cell death. J Exp Med. 2012;209:1029–47. doi: 10.1084/jem.20110996. [DOI] [PMC free article] [PubMed] [Google Scholar]