Abstract
Background
Insulin resistance (IR) and inflammation are associated with increased risk of complications in chronic kidney disease (CKD) patients. However, the relationship between IR and the important proinflammatory interleukin-1β (IL-1β) is unclear in CKD patients.
Material/Methods
We conducted a cross-sectional study including 79 non-diabetic patients who received hemodialysis after the exclusion process. Homeostasis model assessment (HOMA-IR) and leptin adiponectin ratio (LAR) were used to evaluate IR. Inflammation was assessed through C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and IL-1β evaluation. We tested associations of IR with IL-1β using logistic analysis and linear regression.
Results
Patients were divided into a HOMA-IR-positive group and a HOMA-IR-negative group. Although there were no differences between the 2 groups in terms of etiological causes, age, sex, BMI, triglyceride, cholesterol, ferritin, uric acid, and inflammatory indicators such as CRP, we found that IL-6, TNF-α, and IL-1β were significantly increased in the HOMA-IR-positive group compared with the HOMA-IR-negative group. Moreover, IL-1β contributed to HOMA-IR positivity and was positively correlated with LAR after adjusting for possible confounding factors.
Conclusions
Insulin resistance correlates positively with IL-1β among non-diabetic hemodialysis patients, which suggests that IL-1β may be involved in the pathogenesis of IR in this setting.
MeSH Keywords: Inflammation; Insulin Resistance; Kidney Failure, Chronic
Background
Globally, both the incidence and prevalence of chronic kidney disease (CKD) are rising at an alarming speed. Complications such as cardiovascular disease (CVD) and protein-energy wasting (PEW) are likely to develop in patients with CKD, especially those with end-stage renal disease (ESRD), which has significantly elevated all-cause mortality in the population [1]. Insulin insensitivity, or insulin resistance (IR), is common in patients with diabetic nephropathy as well as non-diabetic nephropathy. It is characterized by an inappropriate biological response of liver and skeletal muscle to insulin secretion, and is reportedly related to a decline in renal function [2]. Notably, several clinical consequences of ESRD have been associated with IR. It not only promotes endothelial dysfunction and increases cardiovascular mortality as a result, but IR also accelerates protein breakdown in skeletal muscle and consequently leads to PEW [3,4]. However, the etiology and mechanisms leading to IR are complex and involve many factors, including sedentary lifestyle, vitamin D deficiency, hyperactivity of the renin-angiotensin-aldosterone system (RAAS), and metabolic acidosis [5–7]. Thus, further study of IR is required in order to find therapeutic targets and improve outcomes in ESRD patients.
Inflammatory responses are induced by both infection and tissue damage to restore homeostasis, while excessive inflammation may aggravate tissue damage. It has been suggested that inflammation plays a key role in progression of CKD. It has also been demonstrated in hemodialysis patients that IR is positively correlated with serum levels of proinflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [8,9]. Recently, there has been considerable interest in the inflammasome, which recognizes both intracellular and extracellular danger signals. One of the most-studied inflammasomes is the nucleotide-binding domain and leucine-rich repeat containing PYD-3 (NLRP3). The NLRP3 inflammasome responds to signals by activating caspase-1 and subsequently cleaves pro-IL-1β to generate a mature, secreted form of the cytokine [10]. Recent studies have shown that IL-1β is involved in the pathogenesis of CKD and contributes to cardiovascular disease [11–14], but an association between the proinflammatory cytokine IL-1β and IR has not been reported in CKD patients.
In this study, we aimed to compare the relationship between insulin resistance and inflammation mediators in non-diabetic hemodialysis patients.
Material and Methods
In this study, 89 non-diabetic patients with ESRD undergoing hemodialysis in 1 hemodialysis center (Shanghai 9th Peoples Hospital, Shanghai Jiaotong University, Shanghai, PR China) were included in a cross-sectional manner. This study was approved by the Ethics Committee of Shanghai 9th Peoples Hospital at Shanghai Jiaotong University and all the patients provided written informed consent. Ten patients were excluded due to autoimmune and inflammatory diseases, heart failure, cancer, chronic liver disease, or acute infection. Other exclusion criteria were hemodialysis duration of less than 1 year and thyroid, parathyroid, and adrenal insufficiencies. All patients were clinically stable and free of obvious edema. After exclusion, 42 men and 37 women were enrolled in the study. Demographic and clinical data such as age, sex, height, body mass index (BMI), primary renal diagnosis, triglyceride, cholesterol, LDL, HDL, albumin, ferritin, uric acid, hemoglobin, C-reactive protein (CRP), and IL-6 and TNF-α levels were collected using the latest patient medical records.
Blood samples for insulin, glucose, IL-1β, adiponectin, and leptin were drawn in the morning after overnight fasting. Insulin was measured using a human insulin ELISA kit (Multi Sciences, China). Blood glucose was measured using blood glucose test strips (Tencent, China). IL-1β was measured using a human IL-1β ELISA kit (Raybio, Norcross, GA). Adiponectin was measured using a human adiponectin ELISA kit (Multi Sciences, China). Leptin was measured using a human leptin ELISA kit (Multi Sciences, China). IR was assessed using the homeostasis model assessment of insulin resistance (HOMA-IR) and leptin adiponectin ratio (LAR) as previously described [15]. HOMA-IR was calculated using the following formula: HOMA-IR (mg/dl ×μU/ml)=glucose (mg/dl)×insulin (μU/ml)/405. LAR was calculated as leptin (pg/ml)/adiponectin (pg/ml). The reference value for insulin resistance in the general population is HOMA-IR ≥2.6 [16].
Statistical analysis
The data are presented as means ±standard deviation or median with interquartile range depending on whether they were normally distributed or not. Differences between HOMA-IR (+) and HOMA-IR (−) groups were analyzed using the t test or Mann-Whitney test, as appropriate. Fisher’s exact test was used for comparisons of categorical data. Spearman correlation coefficient was applied for the correlation analysis of nonparametric data.
In logistic analysis of HOMA-IR, odds ratios (OR) and 95% confidence intervals (CI) were expressed per 0.01 pg/ml increase in IL-1β as well as for the IL-1β high group, which was categorized by IL-1β median, with the low group used as the reference range. Model 1 was adjusted for age and sex; model 2 was adjusted for model 1 variables, BMI, hypertension, and cardiovascular disease; and model 3 was adjusted for model 2 variables and laboratory findings (hemoglobin, ferritin, and C-reactive protein). Linear regression analysis of LAR was adjusted for the variables of logistic regression analysis and determined the correlations of IL-1β (per 0.01 pg/ml increase) and LAR. The coefficient β and 95% CI were calculated.
All statistical analysis was performed utilizing SPSS, Version 20.0 (IBM, New York, USA). Statistical significance was defined as a p value <0.05.
Results
Basic characteristics
Of the patients who were included in this study, 42 of 79 (53.2%) were male, 37 of 79 (46.8%) were female, and patients had an average age of 55.72±2.31 years. The average age for the 43 HOMA-IR-positive patients (24 male, 19 female) and 36 HOMA-IR-negative patients (18 male, 18 female) was 56.81±2.05 years and 54.64±2.44 years, respectively.
The etiological causes of CKD are shown for study participants in Table 1. There was no significant difference between the HOMA-IR-positive and HOMA-IR-negative group in terms of etiological causes (p>0.05). The characteristics of HOMA-IR-positive and HOMA-IR-negative patients such as age, sex, BMI, and average or median levels of triglyceride, cholesterol, LDL, HDL, albumin, ferritin, uric acid, hemoglobin, and inflammatory markers (CRP, IL-6, TNF-α, and IL-1β) are shown in Table 2. Although there was no significant difference between the HOMA-IR-positive group and HOMA-IR-negative group in regard to age, sex, BMI, triglyceride, cholesterol, ferritin, and uric acid, we found that inflammatory indicators such as CRP, IL-6, TNF-α, and IL-1β were significantly increased in the HOMA-IR-positive group compared with the HOMA-IR-negative group. The distribution of IL-1β is demonstrated in Figure 1.
Table 1.
Primary disease of the patients.
| Primary diseases | HOMA-IR (+) | HOMA-IR (−) |
|---|---|---|
| Hypertensive nephropathy | 12 | 10 |
| Chronic glomerulonephritis | 17 | 14 |
| Chronic pyelonephritis | 3 | 2 |
| Obstructive nephropathy | 1 | 1 |
| Polycystic renal disease | 3 | 4 |
| IgA nephropathy | 1 | 2 |
| Etiology unknown | 6 | 3 |
HOMA-IR – homeostasis model assessment of insulin resistance.
Table 2.
Characteristics of participants, stratified by HOMA-IR.
| Clinical and biochemical values | HOMA-IR (+) | HOMA-IR (−) | P value |
|---|---|---|---|
| Age (years) | 56.81±2.05 | 54.64±2.44 | NS |
| Gender (male) | 55.81% (24/43) | 50.0% (18/36) | NS |
| BMI (kg/m2) | 22.22±0.81 | 22.92±0.71 | NS |
| Triglyceride (mmol/L) | 1.78 (1.15, 2.53) | 1.56 (1.36, 2.79) | NS |
| Cholesterol (mmol/L) | 3.52 (3.15, 4.24) | 3.63 (2.96, 4.23) | NS |
| LDL (mmol/L) | 2.05±0.11 | 2.07±0.13 | NS |
| HDL (mmol/L) | 0.94±0.05 | 0.94±0.04 | NS |
| Albumin (g/L) | 37.31±0.37 | 37.95±0.36 | NS |
| Ferritin (ng/ml) | 249.27 (44.85, 427.32) | 232.23 (61.06, 744.33) | NS |
| Uric acid (umol/L) | 493.0 (449.0, 553.0) | 485.5 (414.0, 544.0) | NS |
| Hemoglobin (g/L) | 100.86±2.61 | 103.39±2.78 | NS |
| CRP (mg/L) | 10.36 (5.44, 12.68) | 2.58 (0.89, 4.30) | 0.036 |
| IL-6 (pg/ml) | 7.28 (4.96, 9.81) | 3.27 (2.61, 5.7) | 0.028 |
| TNF-α (pg/ml) | 19.95 (17.90, 22.10) | 17.15 (14.95, 20.28) | 0.047 |
| IL-1β (pg/ml) | 0.80 (0.69, 1.01) | 0.63 (0.45, 0.67) | <0.001 |
Mean ±SD values presented for variables with normal distribution while median (IQR) values presented for variables with non-parametric distribution. Values were missing for ferritin (n=1) and CRP (n=2).
Figure 1.
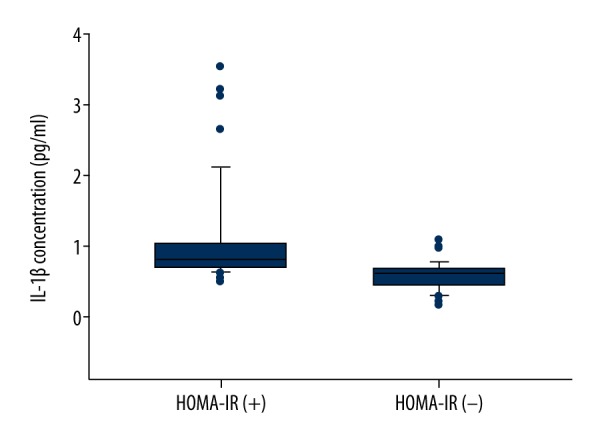
Distribution of IL-1β in HOMA-IR-positive group and HOMA-IR-negative group. HOMA-IR, homeostasis model assessment of insulin resistance.
HOMA-IR and IL-1β
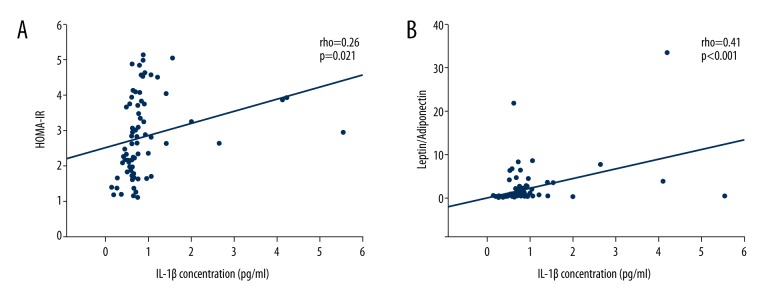
A significant positive correlation was found between HOMA-IR and IL-1β (rho=0.26, p=0.021) as shown in Figure 2A. Using multivariate logistic regression analysis, insulin resistance was associated with higher IL-1β for each 0.01 pg/ml IL-1β increment in model 3 (OR,1.096; 95%CI 1.036–1.161; p<0.001; see Table 3). This association became strong after using IL-1β group analysis. The higher IL-1β group contributed to insulin resistance compared with the lower group (OR 12.553, 95%CI 4.185–37.654; OR 13.438, 95%CI 4.265–42.343; OR 26.096, 95%CI 4.009–169.871 in models 1,2 and 3, respectively; shown in Table 3).
Figure 2.
Correlations between insulin resistance and IL-1β. (A) Positive correlation between HOMA-IR and IL-1β; (B) Positive correlation between LAR and IL-1β. HOMA-IR, homeostasis model assessment of insulin resistance; LAR, leptin adiponectin ratio.
Table 3.
Adjusted odds ratio for HOMA-IR-positive by IL-1β in 79 non-diabetic hemodialysis patients.
| Model 1a | Model 2 b | Model 3 c | ||||
|---|---|---|---|---|---|---|
| OR(95% CI) | p value | OR(95% CI) | p value | OR(95% CI) | p value | |
| Continuous: IL-1β each 0.01 pg/ml increase | ||||||
| 1.07 (1.03–1.12) | <0.001 | 1.07 (1.03–1.12) | <0.001 | 1.09 (1.04–1.16) | <0.001 | |
| Categorical: IL-1β lower group reference | ||||||
| IL-1β higher group | 12.55 (4.19–37.65) | <0.001 | 13.44 (4.27–42.34) | <0.001 | 26.09 (4.01–169.87) | <0.001 |
CI – confidence interval; OR – odds ratio.
Model 1 adjusted for age and gender;
Model 2 adjusted for model 1 variables; BMI – hypertension and cardiovascular disease;
Model 3 adjusted for model 2 variables, hemoglobin, C-reactive protein and ferritin.
IL-1β higher group and IL-1β lower group are stratified by IL-1β median.
LAR and IL-1β
As shown in Figure 2B, LAR was positively associated with IL-1β (r=0.41, p<0.001). Multiple linear regression analysis was used to determine the impact of IL-1β on LAR. The result indicated that after adjusting for confounding factors, IL-1β was positively correlated with LAR (adjusted β 0.389, 95%CI 0.178–0.595; adjusted β 0.341, 95%CI 0.130–0.548; adjusted β 0.334, 95%CI 0.114–0.551 in models 1, 2, and 3, respectively; shown in Table 4).
Table 4.
Linear regression analysis of IL-1β associated with LAR.
| Crude β (95% CI) | p Value | Adjusted β (95% CI) | p Value | |
|---|---|---|---|---|
| Model 1a | 2.15 (0.99, 3.31) | <0.001 | 0.39 (0.18, 0.59) | <0.001 |
| Model 2b | 1.89 (0.73, 3.05) | 0.002 | 0.34 (0.13, 0.55) | 0.002 |
| Model 3c | 1.85 (0.63, 3.07) | 0.003 | 0.33 (0.11, 0.55) | 0.003 |
LAR – leptin adiponectin ratio; CI – confidence interval.
Model 1 adjusted for age and gender;
Model 2 adjusted for model 1 variables, BMI, hypertension and cardiovascular disease;
Model 3 adjusted for model 2 variables, hemoglobin, C-reactive protein and ferritin.
Discussion
This is the first study to evaluate the association between insulin resistance and serum IL-1β in ESRD patients receiving hemodialysis. We demonstrate that non-diabetic patients receiving hemodialysis who exhibit insulin resistance tend to have higher levels of proinflammatory cytokines compared with the patients who are relatively insulin-sensitive. These findings indicate that elevated proinflammatory cytokines such as IL-1β may contribute to insulin resistance in non-diabetic patients receiving hemodialysis, which tends to promote the development of CKD complications.
Insulin is an important hormone that principally regulates glucose metabolism. Insulin resistance is an early alteration of metabolism in CKD patients. It happens even when the glomerular filtration rate is within the normal range, and becomes universal in ESRD patients [17]. Insulin resistance plays a key role in progression and prognosis of CKD and its complications. Shinohara and co-workers have suggested that IR is an independent predictor of cardiovascular mortality in non-diabetic patients with ESRD receiving maintenance hemodialysis [18]. Karakan et al. showed that IR is an independent risk factor for left ventricular hypertrophy in ESRD patients receiving hemodialysis, which is a very common complication in ESRD patients [19]. Zhou et al. demonstrated that IR is closely associated with carotid arterial stiffness in non-diabetic hemodialysis patients [20]. In addition, IR is also linked to PEW and malnutrition in ESRD patients [21,22]. Therefore, more research on IR in ESRD patients is required.
Many methods have been applied to quantify insulin resistance. HOMA-IR is a widely-used statical test to estimate IR from fasting glucose and insulin concentration. However, due to the raised insulin concentration in CKD patients as a result of impaired renal clearance, statical indices based on fasting insulin concentration are imperfect estimates in CKD patients [17]. Therefore, we use additional indices such as LAR to evaluate IR in hemodialysis patients. Leptin and adiponectin, the 2 major adipokines, are both elevated owing to decreased renal clearance. Leptin promotes secretion of IL-6 and TNF-α and increases production and accumulation of reactive oxygen species, which contributes to insulin resistance [23,24]. On the other hand, adiponectin improves endothelial function and increases insulin sensitivity [25]. According to recent studies, LAR correlates of IR measured by the criterion standard hyperinsulinemic euglycemic clamp (HEGC) are better than HOMA-IR in dialysis patients [15,26]. Therefore, it is reasonable that we use LAR to assess IR in our hemodialysis patients.
Chronic inflammation is an important comorbid condition, which is associated with clinical outcomes of CKD patients [27]. According to previous reports, inflammation plays a vital role in development of insulin resistance in hemodialysis patients, as demonstrated by positive associations between HOMA-IR and serum levels of inflammatory indicators such as circulating fibrinogen, CRP, IL-6, and TNF-α [8,9]. Our study also showed higher levels of serum CRP, IL-6, and TNF-α in HOMA-IR-positive patients, which is consistent with previous reports. Furthermore, recent studies have linked proinflammatory IL-1β with insulin resistance [28]. Release of IL-1β often follows the activation of NLRP3 inflammasome and subsequent cleavage of caspase 1, which is not the case for IL-6 and TNF-α. Caspase 1 induces inflammation by binding with interleukin-1 receptor type I (IL-1RI) and activating many other proinflammatory chemokines and cytokines, including IL-6, IL-8, and CXCL1 [29,30]. After the generation of inflammation, this key regulator promotes impairment of insulin secretion in β cells of the pancreatic islets. Moreover, IL-1β also induces inflammation in peripheral tissues, which consequently decreases the ability of peripheral tissues to utilize insulin in response to glucose, and finally results in the development of IR in peripheral tissues. However, few reports have examined the relationship between IR and IL-1β in CKD patients. We demonstrate for the first time that IR in non-diabetic ESRD patients receiving hemodialysis correlates positively with the serum IL-1β level. Since IL-1β antagonism has been shown to reduce hyperglycemia and improve both insulin sensitivity and pancreatic islet function in type 2 diabetes mellitus [31], IL-1β may be a potential therapeutic target to improve insulin sensitivity and decrease morbidity and mortality in hemodialysis patients. However, this is a key cytokine, so any attempt to inhibit this should be considered very carefully.
Some limitations should be considered in our study. First, some important causes of IR in hemodialysis patients, such as vitamin D deficiency and metabolic acidosis, were not included in the study. Second, the number of investigated patients was limited to a small sample size. Third, use of HEGC may be necessary to further determine the association between IR and IL-1β.
Conclusions
Our study demonstrated a positive correlation between insulin resistance and IL-1β in non-diabetic ESRD patients receiving hemodialysis, which may shed new light on the pathogenesis of CKD complications and provide new therapeutic prospects for ESRD patients. A larger population-based study is necessary to further determine the relationship between insulin resistance and IL-1β.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by grants from the Funds for Distinguished Young Scholars of the Ninth People’s Hospital (jyyq09201701)
References
- 1.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Boer IH, Zelnick L, Afkarian M, et al. Impaired glucose and insulin homeostasis in moderate-severe CKD. J Am Soc Nephrol. 2016;27:2861–71. doi: 10.1681/ASN.2015070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teta D. Insulin resistance as a therapeutic target for chronic kidney disease. J Ren Nutr. 2015;25:226–29. doi: 10.1053/j.jrn.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Garibotto G, Sofia A, Russo R, et al. Insulin sensitivity of muscle protein metabolism is altered in patients with chronic kidney disease and metabolic acidosis. Kidney Int. 2015;88:1419–26. doi: 10.1038/ki.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellasi A, Di Micco L, Santoro D, et al. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 2016;17:158. doi: 10.1186/s12882-016-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stefikova K, Spustova V, Krivosikova Z, et al. Insulin resistance and vitamin D deficiency in patients with chronic kidney disease stage 2–3. Physiol Res. 2011;60:149–55. doi: 10.33549/physiolres.931814. [DOI] [PubMed] [Google Scholar]
- 7.Bowlby W, Zelnick LR, Henry C, et al. Physical activity and metabolic health in chronic kidney disease: A cross-sectional study. BMC Nephrol. 2016;17:187. doi: 10.1186/s12882-016-0400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borazan A, Binici DN. Relationship between insulin resistance and inflamation markers in hemodialysis patients. Ren Fail. 2010;32:198–202. doi: 10.3109/08860220903491232. [DOI] [PubMed] [Google Scholar]
- 9.Kursat S, Colak HB, Toraman A, et al. Relationship of insulin resistance in chronic haemodialysis patients with inflammatory indicators, malnutrition, echocardiographic parameters and 24 hour ambulatory blood pressure monitoring. Scand J Urol Nephrol. 2010;44:257–64. doi: 10.3109/00365591003733682. [DOI] [PubMed] [Google Scholar]
- 10.Artlett CM, Thacker JD. Molecular activation of the NLRP3 Inflammasome in fibrosis: Common threads linking divergent fibrogenic diseases. Antioxid Redox Signal. 2015;22:1162–75. doi: 10.1089/ars.2014.6148. [DOI] [PubMed] [Google Scholar]
- 11.Vilaysane A, Chun J, Seamone ME, et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol. 2010;21:1732–44. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granata S, Masola V, Zoratti E, et al. NLRP3 inflammasome activation in dialyzed chronic kidney disease patients. PLoS One. 2015;10:e122272. doi: 10.1371/journal.pone.0122272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding W, Guo H, Xu C, et al. Mitochondrial reactive oxygen species-mediated NLRP3 inflammasome activation contributes to aldosterone-induced renal tubular cells injury. Oncotarget. 2016;7:17479–91. doi: 10.18632/oncotarget.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader DJ. IL-1 and atherosclerosis: a murine twist to an evolving human story. J Clin Invest. 2012;122:27–30. doi: 10.1172/JCI61163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung AM, Sundell MB, Egbert P, et al. A comparison of novel and commonly-used indices of insulin sensitivity in African American chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:767–74. doi: 10.2215/CJN.08070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascaso JF, Pardo S, Real JT, et al. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–25. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 17.Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: A systematic review. Am J Physiol Renal Physiol. 2016;311:F1087–108. doi: 10.1152/ajprenal.00340.2016. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:1894–900. doi: 10.1097/01.asn.0000019900.87535.43. [DOI] [PubMed] [Google Scholar]
- 19.Karakan S, Sezer S, Ozdemir AF. Insulin resistance and left ventricular mass in non-diabetic hemodialysis patients. Curr Ther Res Clin Exp. 2012;73:165–73. doi: 10.1016/j.curtheres.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Yu Z, Jia H, et al. Association between insulin resistance and carotid arterial stiffness in nondiabetic hemodialysis patients. Blood Purif. 2009;28:193–99. doi: 10.1159/000230810. [DOI] [PubMed] [Google Scholar]
- 21.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–98. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 22.Barazzoni R, Gortan CG, Zanetti M, et al. Ghrelin and muscle metabolism in chronic uremia. J Ren Nutr. 2012;22:171–75. doi: 10.1053/j.jrn.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 24.Yamagishi SI, Edelstein D, Du XL, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 25.Berg AH, Combs TP, Du X, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 26.King-Morris KR, Deger SM, Hung AM, et al. Measurement and correlation of indices of insulin resistance in patients on peritoneal dialysis. Perit Dial Int. 2016;36:433–41. doi: 10.3747/pdi.2013.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Impellizzeri D, Esposito E, Attley J, et al. Targeting inflammation: New therapeutic approaches in chronic kidney disease (CKD) Pharmacol Res. 2014;81:91–102. doi: 10.1016/j.phrs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J Biomed Sci. 2016;23:87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donath MY, Boni-Schnetzler M, Ellingsgaard H, et al. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–31. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 30.Akash MS, Shen Q, Rehman K, et al. Interleukin-1 receptor antagonist: A new therapy for type 2 diabetes mellitus. J Pharm Sci. 2012;101:1647–58. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 31.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]