Abstract
Tau protein participates in microtubule stabilization, axonal transport, and protein trafficking. Loss of normal tau function will exert a negative effect. However, current knowledge on the impact of tau deficiency on the motor behavior and related neurobiological changes is controversial. In this study, we examined motor functions and analyzed several proteins implicated in the maintenance of midbrain dopaminergic (DA) neurons (mDANs) function of adult and aged tau+/+, tau+/−, tau−/− mice. We found tau deficiency could not induce significant motor disorders. However, we discovered lower expression levels of transcription factors Orthodenticle homeobox 2 (OTX2) of mDANs in older aged mice. Compared with age-matched tau+/+ mice, there were 54.1% lower (p = 0.0192) OTX2 protein (OTX2-fluorescence intensity) in VTA DA neurons of tau+/−mice and 43.6% lower (p = 0.0249) OTX2 protein in VTA DA neurons of tau−/−mice at 18 months old. Combined with the relevant reports, our results suggested that tau deficiency alone might not be enough to mimic the pathology of Parkinson’s disease. However, OTX2 down-regulation indicates that mDANs of tau-deficient mice will be more sensitive to toxic damage from MPTP.
Keywords: tau deficiency, midbrain, dopaminergic neuron, orthodenticle homeobox 2
INTRODUCTION
Microtubule-associated protein tau, a neuron-specific cytoskeletal protein, participates in the assembly and stabilization of microtubules, and plays an important role in regulating axonal transport and maintaining neuronal morphology (Drubin and Kirschner, 1986; Gendron and Petrucelli, 2009). Furthermore, tau protein has also been reported to be involved in multiple novel functions, including long-term depression (LTD), chromosome stability and DNA protection, adult neurogenesis as well as modulation of enzymatic activity (Perez et al., 2009; Hong et al., 2010; Sultan et al., 2011; Kimura et al., 2014; Wang and Mandelkow, 2016). Structurally, tau is a highly soluble and natively unfolded protein, and the decreased levels of functional soluble tau caused by the aggregation of tau are putatively involved in the pathogenesis of tau-mediated neurodegeneration including Parkinson’s disease (PD) (Zhukareva et al., 2003; Lei et al., 2012; Wang and Mandelkow, 2016).
To date, four independent tau-knock-out (tau−/−) mouse models have been generated (Harada et al., 1994; Dawson et al., 2001; Tucker et al., 2001; Hashimoto et al., 2008) and three of them have been used to study the role of tau deficiency in neurodegeneration by examining the motor and cognitive behaviors of mice (Ikegami and Harada, 2000; Roberson et al., 2007; Dawson et al., 2010; Morris et al., 2011, 2013; Lei et al., 2012, 2014, 2015; Ahmed et al., 2014; Ma et al., 2014). However, most of the findings on the relationship between tau deficiency and the development of overt motor deficits in mice were largely contradictory. For example, Lei and colleagues have observed that aged (12–24 months of age) tau−/−mice (generated by Dawson) (Dawson et al., 2001) presented overt PD-like motor deficits in the open-field, rotarod, gait and pole test (Lei et al., 2012, 2014), which were attributed to the loss of mDANs (Lei et al., 2012, 2015). However, Morris and colleagues found that only middle-aged (12–15 months of age) tau−/−mice (generated by Dawson) (Dawson et al., 2001) showed subtle dopamine-independent motor deficits in the rotarod, pole and balance beam test and these motor deficits were undetectable in older (21–22 months of age) tau−/− mice (Morris et al., 2013).
In order to have a better understanding of the effects of tau deficiency on motor behaviors and the survival of mDANs, we used the adult and aged tau−/− mice generated by Dawson (Dawson et al., 2001) to examine the motor behaviors and key transcription factors implicated in the function maintenance of mDANs, such as OTX2, LIM homeobox transcription factors 1-beta (Lmx1b) and Pituitary homeobox 3 (Pitx3) (Di Salvio et al., 2010a, 2010b; Hegarty et al., 2013). In our research, no overt motor deficits or obvious differences in mDANs expression were present in adult and older aged tau-deficient mice. Unexpectedly, the key transcription factors OTX2 showed a significant decline in 18-month-old tau−/− and tau+/−mice. These results indicate that the absence of tau is not enough to mimic the pathology of Parkinson’s disease.
EXPERIMENTAL PROCEDURES
Generation of tau−/− mice
Tau-deficient mice (Dawson et al., 2001) were supplied by Jackson Laboratory (007251-B6.129X1-Mapttm/Hnd/J). Wild-type (WT, tau+/+) and knock-out (KO, tau−/−) litter-mates were obtained by crossbreeding of heterozygous (HEZ, tau+/−) mice and the series of the mice were maintained on C57BL/6J background. All the mice were housed in a 12 h light/dark cycle and fed on regular diet ad libitum. All animal experimental procedures were approved by the Sun Yat-sun University Animal Care and Use Committee.
Genotyping
Mice genomic DNA was extracted from tail biopsy by Direct PCR Lysis Reagent (Viagen Biotech). In order to identify three genotypes of experimental mice (tau+/+, tau+/−, tau−/−), we performed PCR amplification by using specific primers (Dawson et al., 2001) as follows:
oIMR7415: GCC AGA GGC CAC TTG TGT AG,
oIMR7824: AAT GGA AGA CCA TGC TGG AG,
oIMR7825: ATT CAA CCC CCT CGA ATT TT.
Behavior tests
At least ten male mice were used per genotype in the behavior tests for each age group.
Rotarod test
The motor coordination and balance of mice were assessed using a Rotarod apparatus (RotaRod system, YLS-4C, Yi Yan Technology Co. Ltd, China). First, the male mice were trained to adapt to the rod rotating at constant speed (10 rpm and 15 rpm, respectively) for 10 min. Then, five mice were placed on the rotarod with auto acceleration from 0 rpm to 40 rpm for 2 minutes. The mice were subjected to four trials in a 2 h interval per day for three consecutive days. The length of time the mice stayed on the rotating rod was recorded and the averages of the first day were analyzed.
Open-field test
The spontaneous motor activity of mice was measured by the Flex-Field open fields (San Diego Instruments). The Flex-Field activity system is a 40 * 40 * 38 cm Plexiglas box, containing 2 arrays of 16 *16 photobeams. First, the male mice were placed in the dark test room with a faint light in the corner. The mice remained in this environment for 30 min to allow adaptation to the settings. The mice were then placed into the chamber to explore for 30 min. The mice movements were traced and quantified by the number of beam breaks using Flex-Field software.
Grip strength measurement
The mice were allowed to use their forepaws or hindpaws to pull the metal bar of the Grip Strength Meter (YLS-13A, Yi Yan Technology Co. Ltd, China) which was set up to record the maximal pulling or compressing force. Two criteria were assessed (the force of forelimb and hindlimb) for each mouse.
Gait analysis
Gait movements of the mice were assessed using the TreadScan Gait Analysis System (Clever Sys, Inc) (Lai et al., 2007). Before testing, each male mouse was placed on the treadmill for 15-s training, with the belt speed set at 8 cm/s. After 1 min rest, the mouse was allowed to walk on the treadmill with a constant speed of 8 cm/s for 20 s and the movements of the mouse were recorded at 100 frames per second. After analyzing the frames with the TreadScan system software and excluding all erroneous data, the rest of the data were exported to analyze the gait parameters of the mouse. Eight major criteria were assessed in the test: (1) left forelimb stride, left hindlimb stride, right forelimb stride, and right hindlimb stride (the distance between two consecutive left and right forepaw/hindpaw footprints, respectively); (2) front base and hind base (the separation distance between left and right forepaw/hindpaw footprints of the same strides); (3) left overlap and right overlap (used to measure the uniformity of the left and right strides).
Mouse brain tissues preparation
Mice were anesthetized with 5% chloral hydrate and conducted myocardial perfusion with 1× PBS (pH 7.4). Brain tissues were quickly removed and immediately dissected on an ice-cold plate. The right cerebral hemisphere was fixed by 4% paraformaldehyde (PFA, pH 7.4), and then submerged in 30% sucrose/PBS for 48 h. After total dehydration, brain tissues were cut into 30 mm coronal sections by calibrated microtome (Leica, SM, 2010R). The left cerebral hemisphere was dissected into olfactory bulb, midbrain, striatum, cerebellum, hippocampus and cerebral cortex in cold 1 × PBS (pH 7.4), then they were stored at −80°C immediately before use.
Immunohistochemistry
For TH immunostaining, sections were first incubated with 0.3% H2O2 in PBS for 30 min and then blocked with 3% bovine serum albumin (BSA, containing 0.3% tritonX-100) for 1hour. After that, sections were incubated with primary rabbit anti-TH (1:1000, PelFreez) at 4°C overnight, secondary biotinylated goat anti-rabbit IgG (1:1000, Sigma) at room temperature for 2 h. For visualization, the ABC-Elite kit (Vector Laboratories) with diaminobenzidine (DAB) (Sigma) as a final reaction product was used.
For immunofluorescence, sections were preprocessed with 10% donkey serum albumin (DSA) blocking solution (0.3% Triton X-100) for 1 h, then incubated with primary antibodies specific to TH (1:500, Millipore) and OTX2 (1:50, R&D Systems) at 4°C overnight, and followed by the incubation with secondary donkey anti-goat Alexa Fluor 488 and donkey anti-mouse Alexa Fluor 555 (1:500, Invitrogen) for 1 h.
Images of staining slices (n = 5 per group) were captured by Nikon Eclipse Ni-U microscope and laser-scanning confocal microscope (LSM 710; Zeiss). The paired images in all figures were collected at the same setting. The number of TH-positive neurons was assessed by the Optical Fractionator function of Stereo Investigator 8 (Micro Bright Field), an unbiased stereological program (Micro Bright Field) as described previously (Lin et al., 2012). When we performed stereology, we referred to the stereotaxic coordinates in the Mouse Brain (Franklin and Paxinos, 2008) and the above article (Nelson et al., 1996) to define the boundary between SN and VTA. The fluorescence intensity and area of OTX2 staining were measured by Image J to calculate the average optical density to quantify the expression level of OTX2. At least 20 neurons were measured per section.
Western blot analysis
The brain tissues were homogenized in RIPA buffer (pH 8.0) (50 mM Tris–HCl, 150 mM Nacl, 1 mM EDTA, 1% Tritonx-100, 1% Sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitors (Roche Applied). After ultrasonic fragmentation for 9 s, which was performed 3 times each time, the protein extracts were centrifuged at 15,000 × g for 30 min at 4°C. The supernatants were quantified using an assay kit based on bicinchoninic acid (Thermo Fisher) and separated by 8–12% Tris–HCl SDS–PAGE gel in an electrophoresis system (Bio-Rad Laboratories). The membranes were transferred to polyvinylidene fluoride (PVDF) (Millipore) and then they were blocked with 5% not-fat milk for 1 h. The membranes were then incubated with the following primary antibodies: Pitx3 (1:1000, abcam), TH (1:500, Santa Cruz), OTX2 (1:1000, Sigma), Lmx1b (1:500, Sigma), DAT (1:1000, Millipore), Girk2 (1:1000, Alomone labs), and β-actin (1:5000; Sigma). The signals were visualized using the ECL Western Blotting Substrate (Thermo Fisher) and quantified by Image J.
Statistical analysis
All data in the figures were presented as the mean ± S EM. Statistical analysis was performed by Graphpad Prism 5 (GraphPad Software). The following statistical methods were applied in this project: one-way ANOVA and two-way ANOVA followed by the post hoc test (*P < 0.05, **P < 0.01).
RESULTS
Tau deficiency adult mice did not present obvious motor deficits
In order to determine whether the tau-deficient mice presented with motor deficits, we recorded motor behavior in tau+/+, tau+/−and tau−/− mice when the mice reached 6, 12, 18, and 24 months of age. In the open-field test, even the tau−/− mice showed more activity in horizontal, vertical movement and total movement (Fig. 1A–C), and there were no significant differences among tau+/+, tau+/−and tau−/−mice at the different time intervals. In the rotarod test, the tau−/− mice appeared to spend less time on the rod, but there was no statistically different when compared with tau+/+ and tau+/− mice (Fig. 1D). Similarly, there were no significant statistical differences in the grasping force among the three genotypes of mice, although tau−/− mice showed a weaker forelimb grip strength from 12 months of age onwards (Fig. 1E). There were no differences among the three genotypes of mice in the analysis of gait when looking at the following parameters stride length, paws overlap and base width (Fig. 1G). In addition, the weight of the tau−/− mice showed no significant differences in comparison with tau+/+, tau+/− mice at different age stages (Fig. 1F). All these results suggested that tau deficiency could not affect mice motor behavior.
Fig. 1.
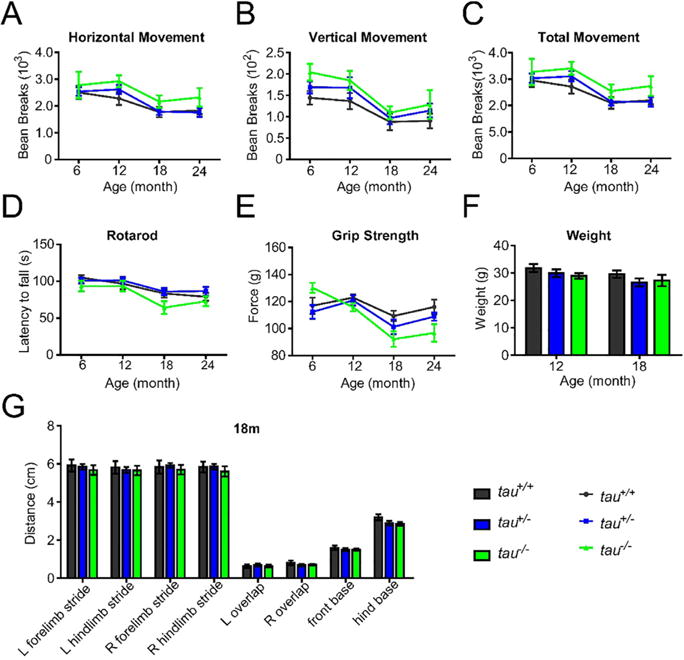
Tau-deficient mice did not display obvious motor deficits. The tau+/+, tau+/−, and tau−/− mice were measured in different motor tests at 6, 12, 18, and 24 months of age (n = 10–20 per genotype and age). (A–C) The horizontal movement (A), vertical movement (B), and total movement (C) in the open-field test. (D) The latency to fall in the Rotarod test. (E) The grip strength of forelimb in Grip Strength analysis. (F) The weight of 12- and 18-month-old mice. (G) The stride length, paw overlap and base width of right (R) and left (L) forelimb/hindlimb of 18-month-old mice in the Gait analysis. All data were presented as mean ± SEM, *p < 0.05.
mDANs number had no significant variation in the midbrain of tau deficiency mice
To detect the variation of mDANs number in the midbrain, we counted the numbers of TH-positive neurons in SN, ventral tegmental area (VTA), and retro-rubral field (RRF) of tau+/+, tau+/−, tau−/− mice at 18 months of age. We found no significant difference in the numbers of TH-positive neurons in the midbrain among the three genotypes of mice (Fig. 2A, B). The TH protein level in these mice was consistent with their number of TH-positive neurons (Fig. 2C, D). These findings further proved that tau deficiency did not affect either TH expression level or the number of mDANs.
Fig. 2.
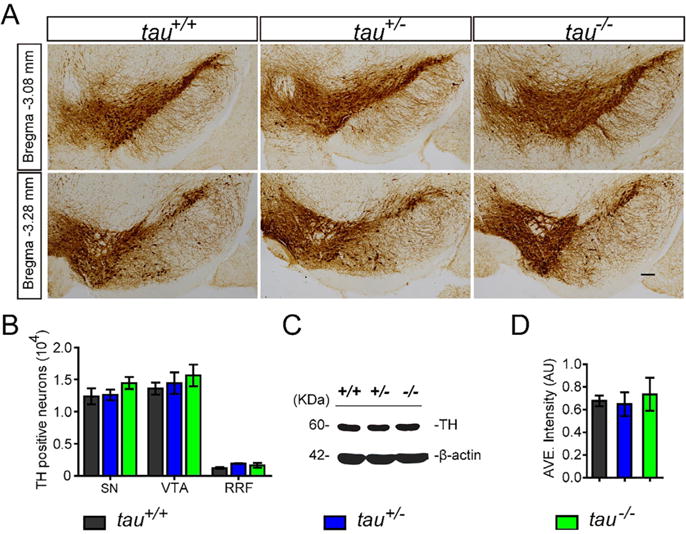
Tau deficiency did not affect the mDANs or TH protein expression in the midbrain. (A) TH staining (brown puncta) of midbrain coronal sections of tau+/+, tau+/−, and tau−/− at 18 months of age. Scale bar = 100 lm. (B) The numbers of TH-positive neurons in SNC, VTA and RRF (n = 5 per genotype, P > 0.05). (C) Western blot showed the expression level of TH in the midbrain homogenates of tau+/+, tau+/−, and tau−/− at 18 months of age. (D) Quantitation of midbrain TH protein level by Western blot analyses, normalized with β-actin (n = 5 per genotype, P > 0.05).
Down-Regulation of OTX2 in the VTA of tau deficiency mice
Previous studies have found that the transcription factors, including Lmx1b, Pitx3 and OTX2, play a pivotal role in the functional maintenance and survival of mDANs (Di Salvio et al., 2010a, 2010b; Hegarty et al., 2013). Although tau deficiency did not affect TH expression level or the number of mDANs, we decided to further look at whether tau deficiency would have an impact on the factors related to the maintenance of function of mDANs. There were no significant differences in the expression levels of Lmx1b and Pitx3 among tau+/+, tau+/−and tau−/− mice at 18 months of age (Fig. 3A, B). However, there was a trend toward reduced OTX2-expression in tau+/−and tau−/− mice. Consequently, we examined the expression of OTX2 in the mDA neurons of tau-deficient mice and found down-regulated OTX2 expression in the DA neurons of VTA in tau−/− (43.6% lower, p = 0.0249) and tau+/− (54.1% lower, p = 0.0192) mice at 18 months of age (Fig. 3C, D). We also detected the OTX2 signals in other brain regions such as superficial subdivision of superior colliculus (SC), deep subdivision of SC (Zhao et al., 2013) and visual cortex. Similarly, we found significantly diminished OTX2 staining signals in the superficial subdivision of SC (tau+/−, 38% lower, p = 0.0071; tau−/−, 51.1% lower, p = 0.0034) (Fig. 4A, B) and a trend toward reduced OTX2 in the deep subdivision of SC and visual cortex (Fig. 4A, B). Together, these observations indicated that tau loss would lower the expression level of OTX2.
Fig. 3.
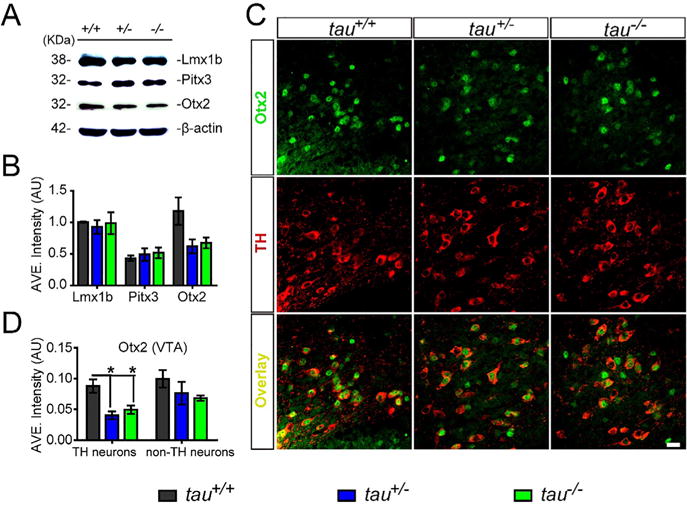
Tau deficiency reduced the expression of transcription factor OTX2 in mDANs of the VTA. (A) Western blot showed the expression level of Lmx1b, Pitx3, and OTX2 in the midbrain homogenates of tau+/+, tau+/−, and tau−/− mice at 18 months of age. (B) Densitometry analyses depicted the Lmx1b, Pitx3, and OTX2 expression level, normalized with β-actin (n = 5 per genotype, P > 0.05). (C) OTX2 (green) and TH (red) co-staining in the VTA coronal sections of tau+/+, tau+/−, and tau−/− mice at 18 months of age. Scale bar = 20 lm. (D) Densitometry analyses of (C) showed OTX2 signals intensity in TH-positive neurons and TH-negative neurons (n = 5 per genotype). All data were presented as mean ± SEM, *P < 0.05, **P < 0.01.
Fig. 4.
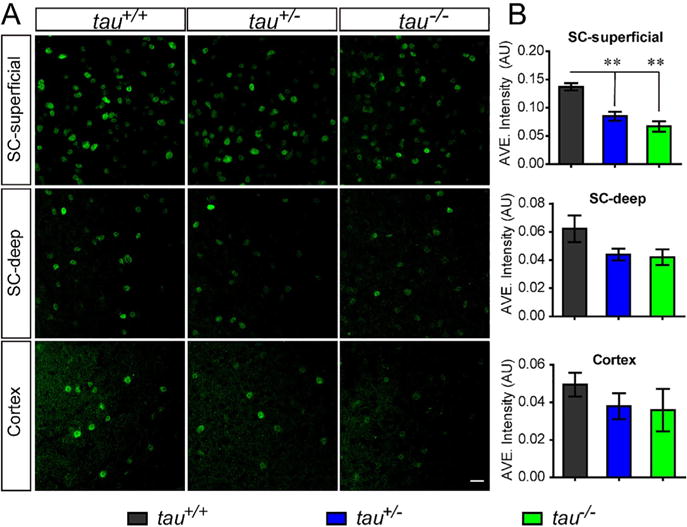
Tau deficiency influenced the expression level of OTX2 in some brain regions. (A) OTX2 staining (green) in the superficial subdivision of SC, deep subdivision of SC and visual cortex of 18-month-old tau+/+, tau+/−and tau−/− mice. Scale bar = 20 lm. (B) Densitometry analyses of A, showed OTX2 signals intensity in the neurons from superficial subdivision of SC, deep subdivision of SC and visual cortex (n = 5 per genotype). All data were presented as mean ± SEM, *P < 0.05, **P < 0.01.
Down-regulated OTX2 did not alter the expression level of DAT and Girk2
Given that Girk2 and DAT are the downstream targets of OTX2, and these two proteins are also involved in the maintenance of function and survival of mDANs (Di Salvio et al., 2010a, 2010b), we further examined DAT expression in the midbrain and striatum, and Girk2 expression in the midbrain by Western blot (Fig. 5A, B). We found that there were no significant differences in the expression levels of DAT and Girk2 among tau+/+, tau+/−and tau−/− mice at 18 months of age (Fig. 5C, D). These findings indicate that the mild-to-moderate reduction of OTX2 expression was insufficient to change the expression levels of DAT and Girk2.
Fig. 5.
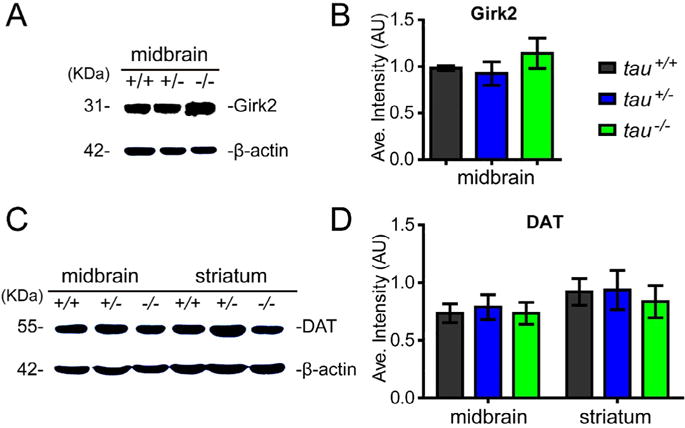
Tau deficiency did not impact the expression of DAT and Girk2. (A, B) Western blot showing the expression level of DAT in the midbrain and striatum (A), and Girk2 in the midbrain (B) of tau+/+, tau+/−, and tau−/−at 18 months of age. (C) Quantitation of DAT expression level by Western blot analyses, normalized with β-actin (n = 5 per genotype, P > 0.05). (D) Quantitation of Girk2 expression level by Western blot analyses, normalized with β-actin (n = 5 per genotype, P > 0.05). All data were presented as mean ± SEM.
DISCUSSION
In the present study, we used a series of motor behavior tests including open-field test, rotarod test, grip strength measurement, and gait analysis to evaluate the influence of tau deficiency on C57BL6/J mice and found that tau deficiency did not significantly impair motor behaviors of adult and older aged C57BL6/J mice. Furthermore, the expression and distribution of mDANs in older tau-deficient and wild-type mice were indistinguishable.
Unexpectedly, we found that OTX2 expression significantly reduced in the aged tau-deficient mice when compared to the age-matched wild-type mice.
To date, four different tau−/− mouse models have been reported (Ke et al., 2012), and three of them have been used in previous studies to explore the pathomechanisms of tau-mediated neurodegeneration using assessments of cognitive and motor functions of mice (Ikegami et al., 2000; Roberson et al., 2007; Dawson et al., 2010; Morris et al., 2011; Lei et al., 2012; Morris et al., 2013; Ahmed et al., 2014; Lei et al., 2014, 2015; Ma et al., 2014). However, the conclusions about whether tau deficiency affects motor behaviors of adult and aged mice are still a matter of debate. For example, Ikegami and colleagues have reported that young (10–11 weeks of age) tau−/− mice (generated by Harada) (Harada et al., 1994) showed hyperactivity in the open-field test, muscle weakness in the wire-hanging test and reduced balance in the rod-walking test (Ikegami et al., 2000). But other groups have reported that adult (3–7 months of age) tau−/− mice (two different strains generated by Dawson and Tucker, respectively) (Dawson et al., 2001; Tucker et al., 2001) showed no differences in gross motor functions (Roberson et al., 2007; Lei et al., 2012; Ahmed et al., 2014) or only developed minor motor deficits in the open-field test and balance beam test (Morris et al., 2011) when compared to age-matched wild-type mice. Besides, Lei and colleagues have observed that aged (12–24 months of age) tau−/− mice (generated by Dawson) (Dawson et al., 2001) showed reduced coordination and balance in the rotarod and pole test, decreased locomotion in the open-field test, and abnormal gait pattern in the gait analysis (Lei et al., 2012, 2014). However, Morris and colleagues reported that only middle-aged (12–15 months of age) tau −/−mice (generated by Dawson) (Dawson et al., 2001) showed subtle motor deficits in the rotarod test, pole test and balance beam test (Morris et al., 2013). These subtle motor deficits were undetectable in the older (21–22 months of age) tau−/−mice and were strongly correlated with an increase in body weight (Morris et al., 2013) in these mice. In the present study, we found that both young and old tau-deficient mice had no overt motor deficits in the open-field, rotarod, gait and grip strength test. These findings are in line with the findings of other research groups (Roberson et al., 2007; Ahmed et al., 2014; van Hummel et al., 2016), and partly consistent with the results of Morris’s group (Morris et al., 2013), but completely different from the reports of Lei’s group (Lei et al., 2012).
In summary, the effects of tau deficiency reported by different groups varied. We purpose that the mice line (different gene targeting approach), genetic background, age, and gender, might influence motor behaviors of tau−/−mice (Crawley, 1999; Homanics et al., 1999). In addition, other variables such as environment, instruments, procedures, parameters of the experiments as well as the diet the mice fed during the study could have influenced the behavioral results and account for the variability of finding detailed above. Furthermore, the diet of tau−/− mice was previously shown to reverse the motor phenotype (Ma et al., 2014; Lei et al., 2015). In the present study, the mice were given a basic sustained mouse chow without extra omega-3 fatty acid (Ma et al., 2014) and clioquinol (Lei et al., 2015). Actually, clioquinol is a meta-binding compound, which might show efficacy in combining Cu/Zn and discharges them out of the body (Nguyen et al., 2005).
In order to test the effect of tau deficiency on midbrain, on the other hand, we further detected the number of mDANs and their regulative factors, and discovered that the knock-out of tau did not affect the number of mDANs and expression level of TH, Pitx3 and Lmx1b. The results were consistent with the behavior manifestations of mice in our previous experiment and Hummel’s research group’s experiment. van Hummel et al. (2016), and somewhat consistent with the results described by Morris et al. (2013). Thus, we considered that mere tau deficiency is not enough to contribute to the PD-like movement disorder or a decline in the number of mDANs.
Interestingly, we found that the expression of OTX2 was reduced in mDANs of VTA and superficial subdivision of SC in aged tau-deficient mice. OTX2 is a homeodomain transcription factor, which is expressed in the forebrain and midbrain during nervous system development and determines the proliferation and differentiation of midbrain dopaminergic neuronal progenitors (Omodei et al., 2008) and retina (Bovolenta et al., 1997; Baas et al., 2000). In the superficial subdivision of SC, OTX2 is thought to be implicated in the formation and maintenance of accurate retinotopic neuronal circuits (Drager and Hubel, 1975; Zhao et al., 2013), neural plasticity (Sugiyama et al., 2008), axonal navigation (Prochiantz and Joliot, 2003; Chung et al., 2010) and visual information processing (Nishida et al., 2003; Sakami et al., 2005). Therefore, the reduction of OTX2 in the SC might affect the formation and function of the subcortical visual pathway. On the other hand, OTX2 protein is restricted to the neurons of the central and medial-ventral VTA of the ventral midbrain (Di Salvio et al., 2010a, 2010b), and could protect VTA DA neurons against MPTP-induced neurodegeneration by down-regulating the expression of Girk2 and DAT (Di Salvio et al., 2010a, 2010b) and up-regulating the expression of neuropeptides (Chung et al., 2010). Furthermore, in retinal bipolar cells, OTX2 was reported to resist glutamate excitotoxicity (Kim et al., 2015). These results indicate that OTX2 might be an effective protective factor for neurons against the toxic effect of MPTP or other toxins, and could be used as a therapeutic target in Parkinson’s disease. Hence, the reduction of OTX2 in the VTA might render the mDANs of tau-deficient mice more susceptible to other insults (Morris et al., 2011) or induce other detrimental effects through its downstream targets.
Nevertheless, the expression of the downstream proteins of OTX2, Girk2 and DAT, the downstream proteins in the OTX2 pathway, were not impacted by a reduced expression of OTX2.
All these results indicate that tau deficiency alone is not sufficient to induce Parkinson’s disease in C57BL/6J mice. However, it may render mDANs more sensitive to insults since it is responsible for decreased expression of OTX2. Further studies are needed to explore the mechanism by which tau deficiency reduces the expression of OTX2 and how it may at least in part contribute to neurodegeneration in Parkinson’s disease.
Acknowledgments
We thank Dr. Raluca Pana (Department of Neurology, Cleverland Clinic, U.S.A.) for her helpful editing this manuscript in english grammar and words.
FUNDING
This work was supported by the National Natural Science Foundation of China (Grant No. 31271555 and No. 81171211); Guangdong Province Special Project of Collaborative Innovation and Platform Environment Construction (2016A050502009); the Scientific Research Foundation for the Returned Overseas Chinese Scholar, Ministry of Education (2012–1707); China’s National Key R&D Program (2016YFC1306600); the Science and Technology Project of Guangdong Province (2015A030311021); the Science and Technology Planning Project of Guangzhou (2018–1202-SF-0019); the intramural research program of National Institute on Aging, and National Institutes of Health, USA (HC, AG000928).
Footnotes
The authors declare no conflicts of interest.
References
- Ahmed T, Van der Jeugd A, Blum D, Galas MD, Hooge R, Buee R, Balschun D. Cognition and hippocampal synaptic plasticity in mice with a homozygous tau deletion. Neurobiol Aging. 2014;35:2474–2478. doi: 10.1016/j.neurobiolaging.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Baas D, Bumsted KM, Martinez JA, Vaccarino FM, Wikler KC, Barnstable CJ. The subcellular localization of Otx2 is cell-type specific and developmentally regulated in the mouse retina. Brain Res Mol Brain Res. 2000;78:26–37. doi: 10.1016/s0169-328x(00)00060-7. [DOI] [PubMed] [Google Scholar]
- Bovolenta P, Mallamaci A, Briata P, Corte G, Boncinelli E. Implication of OTX2 in pigment epithelium determination and neural retina differentiation. J Neurosci. 1997;17:4243–4252. doi: 10.1523/JNEUROSCI.17-11-04243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Licznerski P, Alavian KN, Simeone A, Lin Z, Martin E, Vance J, Isacson O. The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons. Brain. 2010;133:2022–2031. doi: 10.1093/brain/awq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Cantillana V, Jansen M, Wang H, Vitek MP, Wilcock DM, Lynch JR, Laskowitz DT. Loss of tau elicits axonal degeneration in a mouse model of Alzheimer’s disease. Neuroscience. 2010;169:516–531. doi: 10.1016/j.neuroscience.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Di Salvio M, Di Giovannantonio LG, Acampora D, Prosperi R, Omodei D, Prakash N, Wurst W, Simeone A. Otx2 controls neuron subtype identity in ventral tegmental area and antagonizes vulnerability to MPTP. Nat Neurosci. 2010a;13:1481–1488. doi: 10.1038/nn.2661. [DOI] [PubMed] [Google Scholar]
- Di Salvio M, Di Giovannantonio LG, Omodei D, Acampora D, Simeone A. Otx2 expression is restricted to dopaminergic neurons of the ventral tegmental area in the adult brain. Intl J Dev Biol. 2010b;54:939–945. doi: 10.1387/ijdb.092974ms. [DOI] [PubMed] [Google Scholar]
- Drager UC, Hubel DH. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975;38:690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. London: Elsevier Academic Press; 2008. [Google Scholar]
- Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, Sato-Yoshitake R, Takei Y, Noda T, Hirokawa N. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Muramatsu K, Uemura T, Harada R, Sato T, Okamoto K, Harada A. Neuron-specific and inducible recombination by Cre recombinase in the mouse. Neuroreport. 2008;19:621–624. doi: 10.1097/WNR.0b013e3282fb7d99. [DOI] [PubMed] [Google Scholar]
- Hegarty SV, Sullivan AM, O’Keeffe GW. Midbrain dopaminergic neurons: a review of the molecular circuitry that regulates their development. Dev Biol. 2013;379:123–138. doi: 10.1016/j.ydbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Hong XP, Peng CX, Wei W, Tian Q, Liu YH, Yao XQ, Zhang Y, Cao FY, Wang Q, Wang JZ. Essential role of tau phosphorylation in adult hippocampal neurogenesis. Hippocampus. 2010;20:1339–1349. doi: 10.1002/hipo.20712. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Harada A, Hirokawa N. Muscle weakness, hyperactivity, and impairment in fear conditioning in tau-deficient mice. Neurosci Lett. 2000;279:129–132. doi: 10.1016/s0304-3940(99)00964-7. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim SJ, Sohn Y, Paik S, Caplette R, Simonutti M, Moon KH, Lee EJ, Min KW, Kim MJ, Lee D, Simeone A, Lamonerie T, Furukawa T, Choi J, Kweon H, Picaud S, Kim I, Shong M, Kim JW. Mitochondrial protection by exogenous Otx2 in mouse retinal neurons. Cell Rep. 2015;13:990–1002. doi: 10.1016/j.celrep.2015.09.075. [DOI] [PubMed] [Google Scholar]
- Kimura T, Whitcomb DJ, Jo J, Regan P, Piers T, Heo S, Brown C, Hashikawa T, Murayama M, Seok H, Sotiropoulos I, Kim E, Collingridge GL, Takashima A, Takashima KCho. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130144. doi: 10.1098/rstb.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27:13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Ayton S, Appukuttan AT, Volitakis I, Adlard PA, Finkelstein DI, Bush AI. Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis. 2015;81:168–175. doi: 10.1016/j.nbd.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BXW, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI. Tau deficiency induces Parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Moon S, Zhang Q, Volitakis I, Finkelstein DI, Bush AI. Motor and cognitive deficits in aged tau knockout mice in two background strains. Mol Neurodegener. 2014;9:29. doi: 10.1186/1750-1326-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, Ding J, Mateo Y, Sullivan PH, Wu LG, Goldstein DS, Lovinger D, Cai H. Conditional expression of Parkinson’s disease-related mutant a-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Kiosea NC, Nazari S, Chen PP, Nothias F, Chan P, Teng E, Frautschy SA, Cole GM. Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging. J Neurosci. 2014;34:7124–7136. doi: 10.1523/JNEUROSCI.3439-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Hamto P, Adame A, Devidze N, Masliah E, Mucke L. Age-appropriate cognition and subtle dopamine-independent motor deficits in aged tau knockout mice. Neurobiol Aging. 2013;34:1523–1529. doi: 10.1016/j.neurobiolaging.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Koyama A, Masliah E, Mucke L. Tau reduction does not prevent motor deficits in two mouse models of Parkinson’s disease. PLoS One. 2011;6:e29257. doi: 10.1371/journal.pone.0029257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EL, Liang CL, Sinton CM, German DC. Midbrain dopaminergic neurons in the mouse: computer-assisted mapping. J Comp Neurol. 1996;369:361–371. doi: 10.1002/(SICI)1096-9861(19960603)369:3<361::AID-CNE3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Hamby A, Masa SM. Clioquinol down-regulates mutant Huntingtin expression in vitro and mitigates pathology in a Huntington’s disease mouse model. PNAS. 2005;102(33):11840–11845. doi: 10.1073/pnas.0502177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omodei D, Acampora D, Mancuso P, Prakash N, Di Giovannantonio LG, Wurst W, Simeone A. Anterior-posterior graded response to Otx2 controls proliferation and differentiation of dopaminergic progenitors in the ventral mesencephalon. Development. 2008;135:3459–3470. doi: 10.1242/dev.027003. [DOI] [PubMed] [Google Scholar]
- Perez M, Santa-Maria I, Gomez DBE, Zhu X, Cuadros R, Cabrero JR, Sanchez-Madrid F, Dawson HN, Vitek MP, Perry G, Smith MA, Avila J. Tau–an inhibitor of deacetylase HDAC6 function. J Neurochem. 2009;109:1756–1766. doi: 10.1111/j.1471-4159.2009.06102.x. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, Gerstein H, Yu GQ, Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buee L, Galas MC. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem. 2011;286:4566–4575. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- van Hummel A, Bi M, Ippati S, van der Hoven J, Volkerling A, Lee WS, Tan DCS, Bongers A, Ittner A, Ke YD, Ittner LM. No overt deficits in aged tau-deficient C57Bl/6.Mapttm1(EGFP)kit GFP knockin mice. PLoS One. 2016;11:e163236. doi: 10.1371/journal.pone.0163236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Zhao J, Urakawa S, Matsumoto J, Li R, Ishii Y, Sasahara M, Peng Y, Ono T, Nishijo H. Changes in Otx2 and parvalbumin immunoreactivity in the superior colliculus in the platelet-derived growth factor receptor-β knockout mice. Biomed Res Int. 2013;2013:1–17. doi: 10.1155/2013/848265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhukareva V, Sundarraj S, Mann D, Sjogren M, Blenow K, Clark CM, McKeel DW, Goate A, Lippa CF, Vonsattel JP, Growdon JH, Trojanowski JQ, Lee VM. Selective reduction of soluble tau proteins in sporadic and familial frontotemporal dementias: an international follow-up study. Acta Neuropathol. 2003;105:469–476. doi: 10.1007/s00401-002-0668-8. [DOI] [PubMed] [Google Scholar]


