Abstract
Control strategies to reduce human schistosomiasis have evolved from ‘snail picking’ campaigns, a century ago, to modern wide-scale human treatment campaigns, or preventive chemotherapy. Unfortunately, despite the rise in preventive chemotherapy campaigns, just as many people suffer from schistosomiasis today as did fifty years ago. Snail control can complement preventive chemotherapy by reducing the risk of transmission from snails to humans. Here, we present ideas for modernizing and scaling up snail control, including spatiotemporal targeting, environmental diagnostics, better molluscicides, new technologies (e.g. gene drive), and ‘outside the box’ strategies such as natural enemies, traps, and repellants. We conclude that, to achieve the World Health Assembly’s stated goal to eliminate schistosomiasis, it is time to give snail control another look.
Targeting snails is a key to success for schistosomiasis control
Soon after Japanese researchers resolved the schistosome life cycle and identified its snail hosts in 1913, Japan launched a ‘snail picking’ effort that offered children a 0.5-yen bounty per container of snails they collected and destroyed [1]. After seven years, Japan shifted from this labor-intensive (and ineffective) effort [1], to controlling snails by cementing irrigation canals, draining wetlands, and applying molluscicides. By 1994, this sustained snail control effort plus drug treatment of infected people, led to the eradication of schistosomiasis in Japan [2]. Other countries, such as Guadeloupe, Iran, Iraq, Lebanon, Martinique, Morocco, Oman, Puerto Rico, Saint Lucia, Saudi Arabia, Tunisia, and Venezuela, have also controlled or eliminated schistosomiasis using snail control [3] (Table 1). Brazil, China, Egypt, Indonesia, the Philippines, and Zanzibar have long used snail control alongside preventive chemotherapy and other strategies to suppress schistosomiasis prevalence, whereas countries that have not pursued snail control have been less successful [3]. Snail control appears to be a key intervention needed to achieve the World Health Assembly’s stated goal to eliminate schistosomiasis [3, 4] (Table 1).
Table 1.
Outcomes and control strategies of all national schistosomiasis control programs during the past century.a
| Country/ territory name |
Control outcome |
Successful prevalence reduction (%) |
Successful population- at-risk reduction (%) |
Molluscicides | Snail habitat reduction |
Snail biological control |
Human tx by MDA | Other controls | Details on other control measures | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Iran | E | 100 | 100 | • | • | • | • | land reclamation, draining swamps, providing latrines | [100, 101] | |
| Japan | E | 100 | 100 | • | • | • | human test and tx; cementing irrigation canals | [1, 2, 102, 103] | ||
| Jordan | E | 100 | 100 | • | • | low coverage; human test and tx | http://www.who.int/schistosomiasis/resources/EMRO_report_Schistosomiasis.pdf | |||
| Lebanon | E | 100 | 100 | • | • | • | cementing irrigation canals; improved water and sanitation | [101] | ||
| Martinique | E | 100 | 100 | • | • | improved water and sanitation | [104] | |||
| Mauritius | E | 100 | 100 | • | some enigmatic snail declines | [105] | ||||
| Morocco | E | 100 | 100 | • | • | • | mobile envoys to support human test and tx | [21, 106–112] | ||
| Puerto Rico | E | 100 | 100 | • | • | • | human test and tx; improved water; health education | [113] | ||
| Tunisia | E | 100 | 100 | • | • | human test and tx | [114] | |||
| Saudi Arabia | N | 99.8 | ND | • | • | • | • | little MDA; land reclamation, cementing canals | [23, 115–120] | |
| Indonesia | N | 99.5 | ND | • | • | • | little MDA; agro-engineering; sanitation improvement | [121] | ||
| Iraq | N | 99.5 | ND | • | • | targeted tx of schoolchildren in early years | [120, 122] | |||
| Egypt | N | 99 | ND | • | • | • | • | safe water supply and agricultural drainage projects | [101, 120, 123–128] http://documents.worldbank.org/curated/en/756051468036566715/pdf/444660PPAR0P0051520Box327410B01PUBLIC1.pdf | |
| China | N | 98.9 | 79 | • | • | • | • | tx of cattle and buffalo; agricultural engineering and safe water | [129, 130] | |
| Venezuela | N | 98.6 | ND | • | • | • | human test and tx; invasive competitor snails | [22, 131–133] | ||
| Philippines | N | 98.3 | ND | • | • | • | • | shift from early focus on snails and targeted tx to later MDA | [134, 135] | |
| St. Lucia | N | 98.2 | 84.3 | • | • | • | • | site of early Rockefeller studies on. control of schistosomes | [136] | |
| Guadeloupe | N | 96 | ND | • | • | • | • | bridges; canal engineering to reduce snail habitat | [133, 137] | |
| Zanzibar | N | 84.7 | ND | • | • | • | • | recent initiation of elimination program | [138–140] | |
| Laos | N | 84.6 | ND | • | • | limited health education and improved sanitation | [141–143] | |||
| Cambodia | N | 83 | 90 | • | • | limited health education and improved sanitation | [141, 144] | |||
| Brazil | N | 80 | 69 | • | • | • | early human test and tx; improved water and sanitation | [145–148] | ||
| Burundi | N | 74.4 | ND | • | • | • | focal mollusciciding and sanitation were implemented rarely | [149–151] | ||
| Ghana | N | 73.9 | ND | • | low coverage | [152] | ||||
| Madagascar | N | 73.8 | ND | • | • | • | molluscicides used rarely; improved water and sanitation | [153, 154] http://www.who.int/neglected_diseases/preventive_chemotherapy/PCTNewsletter12_En.pdf?ua=1, http://digitalcollections.sit.edu/isp_collection/1675/, http://apps.who.int/medicinedocs/pdf/whozip48e/whozip48e.pdf | ||
| Rwanda | N | 69.5 | ND | • | • | mapping and training health personnel | [155] | |||
| Libya | N | 66.7 | ND | • | • | • | human test and tx | http://www.who.int/schistosomiasis/resources/EMRO_report_Schistosomiasis.pdf | ||
| Surinam | N | 61.5 | 69.3 | • | • | • | human test and tx; education | [156, 157] http://www.who.int/schistosomiasis/resources/PAHO_report_Schistosomiasis_carribean.pdf | ||
| Burkina Faso | N | 61.2 | ND | • | [29, 158, 159] | |||||
| Tanzania | N | 60 | 0 | • | • | • | limited mollusciciding and human test and tx | [160, 161] | ||
| Uganda | N | 55.4 | ND | • | [162, 163] | |||||
| Mali | N | 51.8 | ND | • | [41, 164] | |||||
| Sierra Leone | N | 51.4 | ND | • | [165, 166] | |||||
| Kenya | N | 51 | ND | • | • | limited biological control in early years with Louisiana Crayfish | [74, 167] | |||
| Niger | N | 44 | ND | • | [168–171] | |||||
| Yemen | N | 44 | ND | • | [172] | |||||
| Malawi | N | 43.3 | ND | • | • | • | • | limited experimental mollusciciding and biological control | [173–176] | |
| Congo | N | 41.7 | ND | • | • | • | limited mollusciciding, education and health training | http://apps.who.int/medicinedocs/pdf/whozip48e/whozip48e.pdf | ||
| Togo | N | 30.9 | ND | • | [162, 177] | |||||
| Mozambique | N | 28.9 | ND | • | http://www3.imperial.ac.uk/schisto/wherewework/mozambique/mozambiquestrategy | |||||
| Zambia | N | 26.6 | ND | • | [178, 179] | |||||
| Cameroon | N | 16.7 | ND | • | [180] | |||||
| Swaziland | N | 9.6 | ND | • | [181] | |||||
| Senegal | N | 1 | ND | • | • | limited trials for biological control with river prawns | [9, 182] http://apps.who.int/iris/bitstream/10665/65978/1/WHO_CDS_CPC_SIP_99.2.pdf | |||
| Oman | N | 0.6 | ND | • | • | low coverage of control; human test and tx; concrete reservoirs | http://www.who.int/schistosomiasis/resources/EMRO_report_Schistosomiasis.pdf | |||
| Benin | N | −2 | ND | • | • | improved water and sanitation (incidental to development) | http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=153562 | |||
| Somalia | N | −24 | NA | • | low coverage of control | http://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/. | ||||
| Sudan | N | −29.7 | 47 | • | low coverage of control | [183–187] | ||||
| Central AfricanRepublic | N | −58 | ND | • | low coverage of control | http://apps.who.int/iris/bitstream/10665/69740/1/WHO_CDS_NTD_2007.4_eng.pdf | ||||
| DominicanRepublic | N | ND | ND | • | • | lacking quantitative data on success | [13, 157, 188] | |||
| Syria | N | ND | ND | • | early human test and tx; cementing canals; conflict hinders effort | http://www.who.int/schistosomiasis/resources/EMRO_report_Schistosomiasis.pdf |
The most successful programs have focused on widespread and early snail control activities. Countries and territories are ordered by most to least successful in terms of reducing schistosomiasis prevalence. More details and data are available at [3], http://schisto.stanford.edu and https://purl.stanford.edu/yt060bn1019. “E” = schistosomiasis eliminated from the country/territory, “N” = schistosomiasis is not yet eliminated, “ND” = no data, “tx” = treatment campaigns.
Despite these many successes, the modern orthodoxy paints snail control as old fashioned, preferring to focus instead on preventive chemotherapy via mass drug administration (MDA) of praziquantel [5–7]. Praziquantel’s introduction in the late 1970s and early 1980s, and the release of its generic form in the 1990s, led the World Health Assembly to adopt, in 2001, preventive chemotherapy as the recommended global strategy for schistosomiasis reduction [7, 8] (http://apps.who.int/gb/archive/pdf_files/WHA54/ea54r19.pdf). This is in line with recent emphasis on integrated preventive chemotherapy (distributing drugs against various preventable diseases). But despite distributing millions of pills in recent decades, sub-Saharan Africa’s schistosomiasis problem is as serious now as it was before praziquantel’s discovery, in part because reinfection after treatment can thwart long-term control [3]. Given this disappointing outcome, the World Health Assembly’s 2012 resolution 65.21 advocates adding modernized snail control and other control methods to preventive chemotherapy in order to achieve schistosomiasis elimination (http://www.who.int/neglected_diseases/mediacentre/WHA_65.21_Eng.pdf).
Together, preventive chemotherapy and snail control techniques offer our best opportunity for schistosomiasis elimination – and current technology for snail control has come a long way from snail picking. Here we argue it is time to refocus on snail control in the fight against schistosomiasis. We discuss which strategies remain relevant, and propose what future snail control might look like.
Snails and the schistosome life cycle
The schistosome life cycle encompasses two transmission processes: human-to-snail transmission and snail-to-human transmission (Fig 1). Schistosome eggs from human urine or feces reach fresh water, where eggs hatch and release the miracidia larvae that infect freshwater snails. After completing asexual reproduction in the snails, the schistosomes then release free-swimming cercariae that penetrate human skin, eventually migrating to the portal or pelvic veins, depending on the schistosome species.
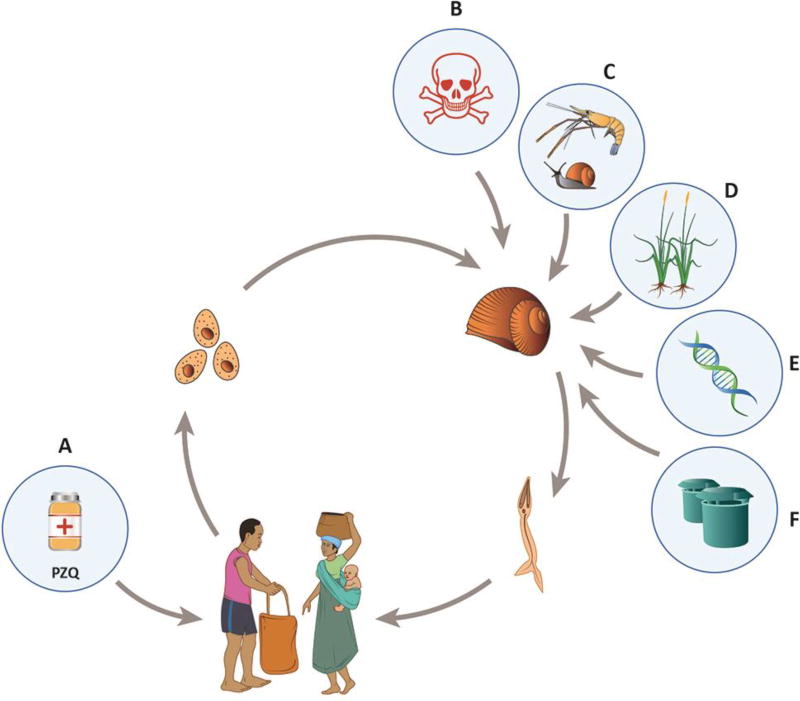
Figure 1. The Schistosoma sp. lifecycle and snail control strategies.
human to snail transmission occurs via free-living miracidia released from eggs in urine and feces; and snail to human transmission occurs through free-living cercariae that exit infected snails into the water, seeking new vertebrate hosts. Control strategies should combine (A) human drug treatment or preventive chemotherapy with praziquantel (PZQ) with (B-F) creative methods to control infected snails such as: (B) chemical molluscicides; (C) natural enemies; (D) habitat modification; (E) creative technologies such as gene drive; (F) traps or repellants and other out of the box strategies.
It is not easy to eradicate snails, but snail eradication is not necessary for the elimination of schistosomes. To break the schistosome life cycle, snail densities must be driven below a threshold where snail infection rates are lower than snail death rates [9]. Schistosome and snail compatibility is complex and there are many strain differences across the world [10, 11], but despite this complexity, the simple fact remains that where schistosome-susceptible snails have been reduced schistosomiasis has often been eliminated from large areas (even whole countries). In Japan, where schistosomiasis has been eliminated since the 1990s, the snail intermediate host, Oncomelania nosophora, persists to this day – although its abundance is low enough to merit a vulnerable ranking on the International Union for Conservation of Nature (IUCN) red list (http://www.iucnredlist.org) [12]. In Guadeloupe, where snail reductions interrupted schistosomiasis transmission (with few to no documented cases during the past several decades [13, 14]), Biomphalaria glabrata intermediate host snails are still present and still susceptible to infection, at least up to 2005 [15]. The recalcitrance of snails to eradication means that snail control must be deployed with other approaches to reduce the chance that infected humans will re-introduce the parasite.
‘Old-fashioned’ snail control has included chemical molluscicides, habitat modification, and biological control, but modern methods could add ‘outside the box’ strategies – including some under development or yet to be devised. Given that snail populations persist, these snail control interventions are best complemented with traditional, human-centric schistosomiasis control strategies, like human drug treatment (such as mass drug administration or targeted testing and treatment), water, sanitation, and hygiene infrastructure (WASH), or behavior modification through education. Snail control – or any environmental intervention that reduces schistosomiasis transmission and slows reinfection after treatment – should decrease the frequency at which preventive chemotherapy is required and thus would spare drugs, increase MDA efficacy, reduce costs, and improve scalability. Simply put, elimination is possible if human infections can be interrupted via preventive chemotherapy, and snail densities can be reduced (Fig 1).
Looking back
Effective ‘old-fashioned’ snail control strategies have included chemical molluscicides, reducing snail habitat, and biological control (i.e., intentional or unintentional introductions of competitor snails or snail predators) and snail control has sometimes been combined with a number of other strategies including human mass drug administration (MDA), human testing and treatment campaigns, and engineering interventions (Table 1).
Success with chemical molluscicides
During the 20th century, molluscicides were among the most commonly used snail control strategies by governments and public health agencies, but molluscicides fell out of favor as costs of the chemicals increased, and concurrently, the cost of praziquantel fell, beginning in the 1990s [3]. Although the environmental impacts associated with chemical applications limit their acceptability in some circumstances, molluscicides have been effective in controlling schistosomiasis [3, 4, 16]. Since the 1960’s, the most-used chemical has been niclosamide, a formulation with lethal effects for snails up to 24 hours after application and low lethal concentration (LC90) for snails, at <1ppm [4]. In theory, these low concentrations are non-toxic to vertebrates including fish and humans, but uneven dispersal can lead to fish kills and health concerns [17]. Some countries have imposed restrictions on the use of niclosamide in the environment due to health concerns and to concerns regarding its non-target effects [18]. However, it is interesting to note that niclosamide has been approved for many decades as an anthelminthic treatment in people and has recently been explored as an anti-cancer therapy and a treatment for Zika virus [19, 20].
Success with snail habitat modification
Snail habitat modification for schistosomiasis control has taken several forms – including vegetation removal, land reclamation (e.g. wetland drainage), cementing canals, and occasionally, hydrological interventions to increase or alter stream flow (Table 1). For example, these strategies have controlled snails in Japan, Morocco, Saudi Arabia and Venezuela [2, 21–23]. In contrast, habitat changes linked to dam construction, irrigation expansion, and other water-related changes have resulted in unintentional and sometimes dramatic schistosomiasis outbreaks [24, 25].
Success with biological control
Schistosome-transmitting snails have various natural enemies. Some crustaceans, birds and fishes eat them. Other snail species compete with them. Non-schistosome trematodes castrate them. Such natural enemies can regulate snail populations, but most enemies have limited natural ranges, and could have non-target effects where they are non-native. Biological control has a bad reputation for non-target effects – but this stems from a few examples where spectacular collateral impacts have accompanied ill-conceived strategies [26]. Biological control can be both safe and effective in a modern context, especially when native species that are natural enemies of pests are used [9, 27].
Many biological control strategies have been researched for schistosomiasis control (for example, introduction of predators, competitors, and parasites of snails), but few strategies have been used widely in practice. One exception is the widespread use of competitor snail species that are not competent hosts for schistosome infection in Caribbean countries such as Antigua, Guadeloupe, Martinique, Montserrat, Puerto Rico, and St. Lucia; non-competent snails were introduced and successfully displaced schistosome-competent intermediate host snails. Schistosomiasis control has been pursued through snail introductions with species such as: Pomacea glauca, Marisa cornuarietis, Melanoides tuberculata, or Tarebia granifera [3, 13]. Displacement can be long-lasting if competitor snail populations are self-sustaining [13, 28].
No one-size-fits-all solution
No single strategy will reduce schistosomiasis transmission everywhere. For example, past attempts at widespread biological control using snail competitors worked to eliminate schistosomiasis on some Caribbean islands but not others [28]; and mass drug administration using praziquantel has durably reduced schistosomiasis in some parts of Burkina Faso but not others [29]. What worked well in one place or time can be ineffective or inappropriate in another. Deploying multiple strategies may help to balance the control portfolio. In particular, snail control is likely to be synergistic with traditional drug distribution campaigns employed in preventive chemotherapy and other well-established interventions like WASH infrastructure improvements, education, and sustainable development.
Looking forward
Future snail control strategies should build on past successes while responding to changing conditions and incorporating modern technologies. History has shown that controlling complex life-cycle parasites, like Schistosoma spp., requires interrupting transmission from humans to intermediate hosts and vice versa. Embracing a synergistic approach might deliver lasting disease reductions beyond those achievable by focusing on any single aspect of transmission [9]. Public health, conservation and sustainable development goals could be aligned if health interventions capitalize on co-benefits – as has been suggested, for example, in recent studies that focus on complementing human drug treatment with species restoration (of snail predators) to reduce snails, control schistosomiasis transmission, alleviate poverty, and restore ecosystems [9, 30–32].
Schistosomiasis, today, is linked to poverty [33, 34] and the long time course required to reduce or eliminate schistosomiasis can erode interest by philanthropic organizations and individual donors [3]. Economic sustainability therefore remains a pressing concern for the future of schistosomiasis control.
For snail control, cost-effectiveness could benefit from strategic improvements such as: i) targeting control to where and when most transmission occurs to increase effectiveness while reducing coverage needs (e.g. considering hubs and hotspots of transmission in space and time), ii) using complementary natural enemies (e.g., predator ducks and their echinostome trematodes) that offer affordable win-win solutions that simultaneously reduce schistosomiasis and generate revenue or other co-benefits, iii), applying novel technologies to improve snail management and control (such as gene drive), iv) discovering molluscicide formulations that are less harmful and more sustainable, and finally, v) integrating snail control with other available tools, including preventive chemotherapy, education, and sanitation.
Understanding the landscape of schistosomiasis infection risk: ecological surveillance, network theory, and optimal control
Snail populations and their schistosome parasites can be dynamic and difficult to predict at the spatial and temporal scales relevant to control campaigns. Theory and empirical data from other disease systems indicate that strategic timing and spatial distribution of control effort improves the efficiency of control, but little schistosomiasis-specific research on this topic exists [35–39].
Although there are few empirical data on snails and their schistosome parasites, especially for Sub-Saharan Africa where most human schistosome infections occur today, the existing data suggest that schistosome-infected snails have aggregated distributions, so that infection risk is distributed in hotspots [40, 41]. A hotspot might be a particular water access site or village, with infection risk varying from village to village (across tens to hundreds of meters; e.g., [42–44]). Furthermore, water flow can move cercariae away from high densities of infected snails [45], making it harder to pinpoint the source of infection risk to humans.
Planning and assessing the success of snail control requires mapping and tracking snail abundance and infection prevalence, but the most common traditional snail sampling technique is timed snail counts (Box 1). Although useful for evaluating relative risk among sites or across time within a single study, the relative abundance method does not measure absolute risk, which is best expressed as infected snail density (combined with information on the density of cercariae emitted from snails through time, Box 1). The use of relative abundance snail sampling methods has been rationalized by invoking investigator safety, time constraints, and the need for simple, straightforward sampling designs when working in challenging field conditions. Absolute sampling using quadrats – that is, the kind of quantitative invertebrate sampling used in other aquatic habitats [46]–is time-consuming and logistically challenging, but yields a more useful, quantitative measure of snail abundance.
Box 3. Quantifying snails and their trematode infections.
Researchers define and measure human risk for schistosomiasis transmission in several ways: prevalence or density of infected snails assessed through snail surveys [189, 190], cercarial density measured via cercariometry or molecular detection in water, [191, 192], or density of infective cercariae derived through mouse exposure [193]. Because snail densities vary widely, the prevalence of infected snails is a poor way to estimate infection risk in humans. Infection rates in sentinel mice are the most direct way to measure risk in humans. However, mouse exposures are expensive and pose ethical concerns to some [192]. Next best is cercarial density, but filtering for cercariae is challenging because waters are often turbid, cercariae have short lifespans (hours), are small, and have soft bodies [194]. Newly available environmental DNA sampling still requires ground truthing and cannot distinguish cercariae (infective to humans) from miracidia (infective to snails). Therefore, snail sampling via timed searches (e.g. [9, 52, 59]) has been by far the most common way to measure risk in research studies and monitoring efforts for the last several decades. In a traditional search, trained technicians spend a set time (e.g., 15 minutes) searching for potential snail habitat at water access points, then agitate the substrate or vegetation with fine-mesh scoops (~ 1–2 mm mesh size, pictured) – and retrieve the scoops and pull out the snails [194] (Figure I). Collected snails are put in vials under bright light to shed cercariae, which can then be identified and used to estimate which snails are infected[194]. Such timed searches are quick and inexpensive, but by targeting snail habitat, the actual measure probably reflects snails density within snail habitat rather than overall snail density, perhaps explaining why many past studies conclude that infected snail density at a site does not correlate well with measures human infection rates [195, 196]. On the contrary, studies using systematic or random quadrat sampling (including dissecting snails to examine for trematode infections) have found more robust correlations between infected snail density and human infection rates [197]. Future work should aim to develop cost-effective and accurate ways to assess infection risk.
In recent years, advances in molecular genetic techniques, spatial statistics, and GIS mapping have made it possible to examine schistosomiasis transmission risk at fine-grained spatial scales [198]. These technologies, coupled with more robust and spatially quantitative snail sampling techniques – borrowed from ecological studies on freshwater invertebrates (e.g. [46, 197]) – could improve prediction capabilities for schistosomiasis transmission.
Figure I.
Two different snail scoops designed and deployed to sample snails in schistosomiasis transmission sites in Senegal. Image courtesy of The Upstream Alliance (http://wwwtheupstreamalliance.org), under the terms of the Creative Commons Attribution License CC BY 2.0.
In addition to improved methodologies to assess snail abundance and to sample transmission stages, species distribution models (habitat suitability models), environmental DNA, network models, and optimal control theory might improve current snail sampling efforts. Some indirect sampling methods might become cost-effective with additional refinement. For example, species distribution modeling [47, 48] encompasses various methods to correlate species occurrences to underlying habitat variables, such as temperature, rainfall, vegetation cover, etc. This technique could help generate maps that predict schistosomiasis transmission hotspots using readily available data, like land features and environmental variables [49]. For example, recent reviews [50, 51] concluded that spatial risk profiling for schistosomiasis using remotely sensed data is an under-used strategy in schistosomiasis research and control. Species distribution modeling might be particularly effective where strong seasons lead to dramatic snail-habitat ephemerality that is easily mapped, as in Burkina Faso and Cote D’Ivoire [52, 53]. These models still require ground truthing using environmental data for training and validation. An alternative indirect approach is to use environmental DNA (eDNA) to track snail density or parasite presence by detecting genetic material directly from water, soil, or other environmental samples without evidence of their biological source [54, 55]. The eDNA technique also requires more refinement and validation [54], especially before it can be calibrated for quantitative assessments. Furthermore, because schistosome eDNA might arise from DNA in living or dead miracidia or living or dead infectious cercariae, it might be hard to translate an eDNA signal to infection risk.
Schistosomiasis transmission maps onto where people work, live, and travel. Understanding the spatial and seasonal connectivity among snail and human populations (e.g. through network modeling, which tracks populations and their interconnections) could indicate critical links where control would be most effective. For example, targeting snail control based on identification of villages that are important hubs of transmission could reduce costs and improve scalability [37].
In Senegal, network models including human mobility – tracked through mobile phone records – predicted schistosomiasis prevalence better than models assuming homogenous mixing of people across cities and villages [37]. Ciddio et al. showed how a network model tracking human mobility and water-mediated snail and cercarial dispersal could be used to target environmental interventions to reduce human exposure and contamination risks [56].
In addition to network modeling, there is little published work on how to apply optimal control theory to neglected tropical diseases, including schistosomiasis. Yet, this approach, which is often used in optimization problems from engineering and economics [57] and more recently from biology and epidemiology [58], could provide a platform to tackle schistosomiasis transmission control, considering a complex landscape of competing costs and benefits [52, 59]. By incorporating economic considerations in the form of a cost function and considering control strategies that can vary continuously through time along an optimal path (rather than an “either or” or a “one size fits all” approach), these models could offer insight needed for ecosystem-specific decision-making on complex trade-offs in health, economic, and environmental factors influencing the management and control of schistosomiasis.
Future molluscicide formulations
New molluscicides (or new niclosamide formulations) that are safer, more effective, more specific, or that disperse more evenly would be beneficial in the fight against schistosomiasis. For example, some promising research areas include: slow-release niclosamide formulations [16], extracts from molluscicidal plants such as endod and others [4, 16, 60], and surfactant formulations that help disperse niclosamide or other molluscicides more evenly, delivering snail-killing efficacy with less opportunity for accumulating unsafe concentrations. Although some of these strategies have been investigated at small scales for many decades (e.g. molluscicidal plants), the investment of time, energy, and funding has not yet been sufficient to allow scale-up [61]. Understanding the spatiotemporal heterogeneity in snail and trematode abundance, as discussed above, could contribute to better targeting of molluscicide applications in space and time, and improve safety, efficacy, and cost-effectiveness for this historically successful, chemical-based snail control strategy.
Gene drive technologies for snail control
We might soon engineer snail hosts with new genetic properties similar to gene drive engineered malaria-resistant Anopheles gambiae mosquitos [62]. In 2016, a CRISPR-Cas9-based gene drive was used to insert genes conferring sterility to female A. gambiae mosquitos, revealing the potential for gene drive technologies to reduce malaria transmission [63]. Despite the fitness costs to the mosquitos that result from sterility-inducing genes, the gene drive system successfully increased the allele frequency of these genes in lab-reared populations over six generations.
The CRISPR-cas9 gene drive system deserves to be explored as an avenue to schistosomiasis control. Some barriers to employing this technology have already been surmounted: genes that confer schistosome resistance have been identified in wild snail populations [15]; the Biomphalaria glabrata genome has been sequenced [64] and CRISPR-cas9 gene editing has been carried out in a marine gastropod [65]. However, a caveat is that Biomphalaria and Bulinus spp. snails (but not Onchomelania spp.), are hermaphroditic and can self-fertilize, making gene drive systems for population suppression more challenging, because drives intended to suppress population growth might lead to compensation by the wild-type snails in the form of more asexual reproduction (selfing) [66]. Gene drives that confer schistosome resistance are an alternative strategy, but seem limited in application given that existing resistance genes do not spread to fixation in host snails [67] (presumably due to associated fitness costs of resistance in uninfected snails). Though it is often implied to be highly precise, CRISPR-cas9 gene editing can produce off-target mutations with unpredictable effects so more work is required to ensure safety of releasing gene-edited snails into the wild [68]. Ethical limitations and methodological hurdles notwithstanding [69], the potential for this new gene drive technology to revolutionize control for human disease, including schistosomiasis and other vector-borne and environmentally transmitted diseases, is tantalizing, so long as safety, efficacy, and implementation constraints can be surmounted.
Thinking outside the box: traps, repellants, and natural enemies
Attempts to trap and kill snails or schistosomes emitted from snails, or repel them from humans, have not yet been applied widely in practice, but such ‘outside the box’ strategies could prove useful if new technologies make them more effective, feasible, or scalable. For instance, snails are attracted to lettuce homogenates (specifically, the amino acids glutamate and proline [70]) and wheat germ cereal [71] which could be used to bait traps. Snails can be repelled by molluscicides [71], artificial shade [72], and topical lipid formulations of N,N-Diethyl-meta-toluamide (DEET) applied to exposed skin [73].
Snail predators – particularly crustaceans, fish and birds – have been effective at reducing snail populations in the past, warranting more research to develop and scale-up the use of snail predators for disease control. For example, Louisiana crayfish (Procambarus clarkii) introduced to Kenya and Egypt can reduce snail abundance and therefore human schistosomiasis transmission [3, 74, 75]. More recently, native river prawns have been proposed as snail control agents in their native coastal ranges, where human-driven environmental change (e.g. dam building) has reduced prawn numbers [24, 32]. Dams are associated with greater increases in human schistosomiasis risk within river prawn native ranges than outside them, suggesting that prawns might have once controlled snail populations [24]. Indeed, reintroducing native river prawns (Macrobrachium volenhovenii) into Senegalese water access points – where they had been present before the nearby Diama Dam was built [32] – resulted in a reduction in snail density and human schistosomiasis re-infection rates [9]. In theory, prawn ladders designed to help juvenile prawns surmount dams could help restore river prawn migration pathways [76]. Other crustaceans might suppress snails and thus schistosomiasis transmission. For example, the Malaysian river prawn, Macrobrachium rosenbergii – in the same genus as the African river prawn – also eats schistosome-hosting snails [77]; unlike the African-native, M. rosenbergii is domesticated, and could therefore be deployed as a biological control agent in managed landscapes [9, 31, 77].
Some fish eat snails [78, 79]. The observation that fish might control snails has inspired efforts to use fish as a biological control tool, with mixed results [80]. However, one snail-eating cichlid, Trematocranus placodon, has shown promise [78], as has the African catfish, Clarias gariepinus [81].
With respect to birds, non-native, domestic ducks reduce snail density in Zimbabwean ponds, but present many logistical challenges – including high costs for duck breeding, maintenance, and protection against poaching [82]. Another role for birds might be in the trematodes they carry. Non-schistosome trematodes that use birds as final hosts, such as Ribeiroia guadeloupensis, castrate host snails and outcompete schistosomes inside infected snails [83], and other similar trematode species have been investigated for similar applications [84].
Competition with other species can suppress snails or schistosomes. Past schistosomiasis control strategies have been successful in using competitor snails and this strategy could be revisited for deployment in modern schistosomiasis hotspots (see Looking Back section). In addition, schistosome species are outcompeted in their snail hosts by other trematode species that produce rediae – jawed reproductive structures that can kill sporocysts [85]. Indeed, many echinostome species including Echinostoma spp.[86, 87] as well as Exorchis sp. [88], Cotylurus lutzi [89], paramphistomoids [90] and others have been investigated for this purpose. However, other trematode species might facilitate schistosome infection, possibly by reducing the host’s immune defenses; evidence for this comes from Calicophoron microbothrium [91] and Zygocotyle lunata [92]. Such differences must be well understood before deploying trematodes as natural enemies. ‘Decoy hosts’ – non-competent snails and other aquatic organisms, such as fish and amphibians – absorb schistosome miracidia without becoming infected, potentially diverting miracidia from competent snail hosts and reducing infected snail prevalence. Though this effect has been observed in laboratory [93] and meso-cosm experiments [94] its success in scaled up control programs has not yet been demonstrated. The parasites’ free living stages also have predators that consume them directly (such as Chaetogaster spp., filter feeders, and small fish [95]); the use of trematode predators in schistosomiasis biological control is beyond the scope of this paper but remains an interesting and relatively unexplored alternative strategy that may – in some instances – complement snail control for schistosomiasis reduction.
Concluding Remarks
“Without snails, there can be no schistosomiasis.” This quote, from the World Health Organization Working Group on Schistosomiasis in 2005 (http://apps.who.int/iris/bitstream/10665/69482/1/TDR_SWG_07_eng.pdf) represents a necessary but insufficient assessment. Indeed, where the snail intermediate hosts for schistosome parasites cannot persist, there is no opportunity for schistosomiasis transmission, but even where snails and schistosomes co-exist, schistosome transmission might not be successful. Therefore more ecological research on schistosome-hosting snails and the conditions permissive to schistosome transmission seems warranted.
For the past century, snail control has been successful in reducing schistosomiasis transmission in many countries, but has fallen out of favor in the last few decades. Here, we have discussed how both new and old fashioned snail control technologies can be used to reduce the risk of schistosome transmission from snails to humans, but many questions remain unanswered (see Outstanding Questions box). We presented some ideas for modernizing, improving, and scaling up snail control, such as spatial targeting, temporal targeting, gene drive technologies, affordable environmental diagnostics, and outside the box strategies such as traps, repellants, natural enemies, and decoys. The goal of snail control is to reduce transmission. This can be maximized by better synergy between mass drug administration and environmental interventions that affordably slow human reinfection after treatment. A synergistic approach spares drugs and likely improves efficacy, cost effectiveness, and scalability.
Most of the two and a half billion dollars disbursed each year to treat and control neglected tropical diseases [96, 97] is directed toward mass drug administration. Although treatment has been effective, control has not, because there is not enough praziquantel to reach all 800 million people at risk today and drugs, alone, cannot address the environmental components of transmission [98, 99]. Coupling drug delivery with snail control has proven effective in the past, and seems the most cost effective option for the future global fight against schistosomiasis.
Trends Box.
Despite a rise in the global effort towards preventive chemotherapy, just as many people suffer from schistosomiasis today as did fifty years ago
Snail control can complement medical treatment, especially where transmission is endemic and reinfection after treatment is a common occurrence
It is time to give snail control another look
Modernizing snail control is a priority and might benefit from more research on spatiotemporal targeting, environmental diagnostics, better molluscicides, new technologies, and ‘outside the box’ strategies such as natural enemies, traps, and repellants
Outstanding questions.
Can network analysis of schistosomiasis transmission reveal hotspots and hubs to target for more efficient snail control?
At what spatial scale does schistosomiasis transmission occur? Can understanding transmission improve control (i.e., the spatial extent that must be treated to protect humans using a given water body).
Might CRISPR-cas9 gene editing and gene drive technologies be a safe and effective way to reduce schistosomiasis-infected snails?
Are molluscicides outdated or are there future formulations that could deliver successful snail control with fewer non-target effects?
How can natural enemies, repellants, traps, and decoys be used for snail control?
What is the most efficient and synergistic use of preventive chemotherapy and environmental controls, including snail control, in the global fight against schistosomiasis?
Can environmental DNA (eDNA) technology be used to indirectly track snail or schistosome presence and distribution in the environment?
Can optimal control theory contribute to an improved strategy for schistosomiasis elimination?
Acknowledgments
The authors thank Lee Marom, Greg Galin and Elsevier Workshop services for artwork, and John McLaughlin and Andy Chamberlin for useful comments and discussions. SHS, IJJ, and GADL were funded by NSF CNH grant # 1414102, NIH Grant 1R01TW010286-01, a GDP SEED grant from the Freeman Spogli Institute at Stanford University, and a grant from the Bill and Melinda Gates Foundation. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kajihara N, Hirayama K. The War against a Regional Disease in Japan A History of the Eradication of Schistosomiasis japonica. Trop Med Health. 2011;39:3–44. doi: 10.2149/tmh.39-1-suppl_1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka H, Tsuji M. From discovery to eradication of schistosomiasis in Japan: 1847–1996. Int J Parasitol. 1997;27:1465–1480. doi: 10.1016/s0020-7519(97)00183-5. [DOI] [PubMed] [Google Scholar]
- 3.Sokolow SH, et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. Plos Neglected Tropical Diseases. 2016;10:e0004794. doi: 10.1371/journal.pntd.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King CH, Bertsch D. Historical perspective: snail control to prevent schistosomiasis. PLoS Negl Trop Dis. 2015;9:e0003657. doi: 10.1371/journal.pntd.0003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray DJ, et al. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 6.Fenwick A, Savioli L. Schistosomiasis elimination. Lancet Infect Dis. 2011;11:346. doi: 10.1016/S1473-3099(11)70110-4. author reply 346–347. [DOI] [PubMed] [Google Scholar]
- 7.Bergquist R, et al. Controlling schistosomiasis with praziquantel: How much longer without a viable alternative? Infect Dis Poverty. 2017;6:74. doi: 10.1186/s40249-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engels D, et al. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokolow SH, et al. Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proc Natl Acad Sci U S A. 2015;112:9650–9655. doi: 10.1073/pnas.1502651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollinson D, et al. Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group. Parasitology. 2001;123(Suppl):S245–260. doi: 10.1017/s0031182001008046. [DOI] [PubMed] [Google Scholar]
- 11.Galinier R, et al. A multistrain approach to studying the mechanisms underlying compatibility in the interaction between Biomphalaria glabrata and Schistosoma mansoni. PLoS Negl Trop Dis. 2017;11:e0005398. doi: 10.1371/journal.pntd.0005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.IUCN. The IUCN Red List of Threatened Species 2017 [Google Scholar]
- 13.Pointier J, et al. The biological control of the snail hosts of schistosomes: the role of competitor snails and biological invasions. In: Toledo R, editor. Biomphalaria snails and larval trematodes. Springer: Science+Business Media, LLC; 2011. [Google Scholar]
- 14.Rollinson D, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–440. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Allan ERO, et al. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Negl Trop Dis. 2017;11:e0005362. doi: 10.1371/journal.pntd.0005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCullough FS, et al. Molluscicides in schistosomiasis control. Bull World Health Organ. 1980;58:681–689. [PMC free article] [PubMed] [Google Scholar]
- 17.Dai JR, et al. A novel molluscicidal formulation of niclosamide. Parasitol Res. 2008;103:405–412. doi: 10.1007/s00436-008-0988-2. [DOI] [PubMed] [Google Scholar]
- 18.Coelho P, Caldeira RL. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect Dis Poverty. 2016;5:57. doi: 10.1186/s40249-016-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, et al. Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Lett. 2014;349:8–14. doi: 10.1016/j.canlet.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laamrani H, et al. Evaluation of environmental methods to control snails in an irrigation system in Central Morocco. Trop Med Int Health. 2000;5:545–552. doi: 10.1046/j.1365-3156.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 22.Incani RN. The Venezuelan experience in the control of schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 1987;82(Suppl 4):89–93. doi: 10.1590/s0074-02761987000800014. [DOI] [PubMed] [Google Scholar]
- 23.al-Madani AA. Schistosomiasis control in Saudi Arabia with special reference to the period 1983–1988. Public Health. 1990;104:261–266. doi: 10.1016/s0033-3506(05)80475-5. [DOI] [PubMed] [Google Scholar]
- 24.Sokolow SH, et al. Nearly 400 million people are at higher risk of schistosomiasis because dams block the migration of snail-eating river prawns. Philosophical Transactions of the Royal Society B-Biological Sciences. 2017;372:20160127. doi: 10.1098/rstb.2016.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinmann P, et al. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 26.Howarth FG. Environmental impacts of classical biological control. Annual Reviews of Entomology. 1991;36:485–501. [Google Scholar]
- 27.Bale JS, et al. Biological control and sustainable food production. Philos Trans R Soc Lond B Biol Sci. 2008;363:761–776. doi: 10.1098/rstb.2007.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pointier JP, Jourdane J. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Tropica. 2000;77:53–60. doi: 10.1016/s0001-706x(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 29.Ouedraogo H, et al. Schistosomiasis in school-age children in Burkina Faso after a decade of preventive chemotherapy. Bull World Health Organ. 2016;94:37–45. doi: 10.2471/BLT.15.161885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartz SJ, et al. Infection with schistosome parasite in snails leads to increased predation by prawns: implications for human schistosomiasis control. J Exp Biol. 2015;218:3962–3967. doi: 10.1242/jeb.129221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sokolow SH, et al. Regulation of laboratory populations of snails (Biomphalaria and Bulinus spp.) by river prawns, Macrobrachium spp. (Decapoda, Palaemonidae): implications for control of schistosomiasis. Acta Trop. 2014;132C:64–74. doi: 10.1016/j.actatropica.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkalay AS, et al. The prawn Macrobrachium vollenhovenii in the Senegal River basin: towards sustainable restocking of all-male populations for biological control of schistosomiasis. PLoS Negl Trop Dis. 2014;8:e3060. doi: 10.1371/journal.pntd.0003060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garchitorena A, et al. Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philosophical Transactions of the Royal Society B-Biological Sciences. 2017;372:20160128. doi: 10.1098/rstb.2016.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chades I, et al. General rules for managing and surveying networks of pests, diseases, and endangered species. Proc Natl Acad Sci U S A. 2011;108:8323–8328. doi: 10.1073/pnas.1016846108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McVinish R, et al. Limiting the spread of disease through altered migration patterns. J Theor Biol. 2016;393:60–66. doi: 10.1016/j.jtbi.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Mari L, et al. Big-data-driven modeling unveils country-wide drivers of endemic schistosomiasis. Sci Rep. 2017;7:489. doi: 10.1038/s41598-017-00493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King CH. Toward the elimination of schistosomiasis. N Engl J Med. 2009;360:106–109. doi: 10.1056/NEJMp0808041. [DOI] [PubMed] [Google Scholar]
- 39.Gurarie D, King CH. Heterogeneous model of schistosomiasis transmission and long-term control: the combined influence of spatial variation and age-dependent factors on optimal allocation of drug therapy. Parasitology. 2005;130:49–65. doi: 10.1017/s0031182004006341. [DOI] [PubMed] [Google Scholar]
- 40.Brown DS. Freshwater snails of Africa and their medical importance. Taylor and Francis: 1994. [Google Scholar]
- 41.Clements A, et al. A comparative study of the spatial distribution of schistosomiasis in Mali in 1984–1989 and 2004–2006. Plos Neglected Tropical Diseases. 2009;3:e431. doi: 10.1371/journal.pntd.0000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloos H, et al. Water contact behavior and schistosomiasis in an upper Egyptian village. Soc Sci Med. 1983;17:545–562. doi: 10.1016/0277-9536(83)90297-6. [DOI] [PubMed] [Google Scholar]
- 43.Babiker A, et al. Focality and seasonality of Schistosoma mansoni transmission in the Gezira Irrigated Area, Sudan. J Trop Med Hyg. 1985;88:57–63. [PubMed] [Google Scholar]
- 44.Woolhouse ME, Chandiwana SK. Spatial and temporal heterogeneity in the population dynamics of Bulinus globosus and Biomphalaria pfeifferi and in the epidemiology of their infection with schistosomes. Parasitology. 1989;98(Pt 1):21–34. doi: 10.1017/s0031182000059655. [DOI] [PubMed] [Google Scholar]
- 45.Muhoho ND, et al. Cercarial density in the river of an endemic area of schistosomiasis haematobia in Kenya. Am J Trop Med Hyg. 1997;57:162–167. doi: 10.4269/ajtmh.1997.57.162. [DOI] [PubMed] [Google Scholar]
- 46.Kuris AM, et al. Ecosystem energetic implications of parasite and freeliving biomass in three estuaries. Nature. 2008;454:515–518. doi: 10.1038/nature06970. [DOI] [PubMed] [Google Scholar]
- 47.Beale CM, Lennon JJ. Incorporating uncertainty in predictive species distribution modelling. Philos Trans R Soc Lond B Biol Sci. 2012;367:247–258. doi: 10.1098/rstb.2011.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wardrop NA, et al. Interpreting predictive maps of disease: highlighting the pitfalls of distribution models in epidemiology. Geospat Health. 2014;9:237–246. doi: 10.4081/gh.2014.397. [DOI] [PubMed] [Google Scholar]
- 49.Stensgaard AS, et al. Modeling freshwater snail habitat suitability and areas of potential snail-borne disease transmission in Uganda. Geospat Health. 2006;1:93–104. doi: 10.4081/gh.2006.284. [DOI] [PubMed] [Google Scholar]
- 50.Walz Y, et al. Use of an ecologically relevant modelling approach to improve remote sensing-based schistosomiasis risk profiling. Geospat Health. 2015;10:398. doi: 10.4081/gh.2015.398. [DOI] [PubMed] [Google Scholar]
- 51.Walz Y, et al. Risk profiling of schistosomiasis using remote sensing: approaches, challenges and outlook. Parasit Vectors. 2015;8:163. doi: 10.1186/s13071-015-0732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez-Saez J, et al. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proc Natl Acad Sci U S A. 2016;113:6427–6432. doi: 10.1073/pnas.1602251113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walz Y, et al. Modeling and Validation of Environmental Suitability for Schistosomiasis Transmission Using Remote Sensing. PLoS Negl Trop Dis. 2015;9:e0004217. doi: 10.1371/journal.pntd.0004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bass D, et al. Diverse Applications of Environmental DNA Methods in Parasitology. Trends Parasitol. 2015;31:499–513. doi: 10.1016/j.pt.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 55.Thomsen PF, Willerslev E. Environmental DNA - An emerging tool in conservation for monitoring past and present biodiversity. Biol Conserv. 2015;183:4–18. [Google Scholar]
- 56.Ciddio M, et al. The spatial spread of schistosomiasis: A multidemensional network model applied to Saint-Louis region, Senegal. Adv Water Resour. 108:406–415. doi: 10.1016/j.advwatres.2016.10.012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee EB, Markus L. Foundations of Optimal Control Theory. John Wiley & Sons, Inc.; 1967. [Google Scholar]
- 58.Lenhart S, Workman JT. Optimal control applied to biological models. Chapman & Hall/CRC; 2007. [Google Scholar]
- 59.Sturrock RF, et al. Seasonality in the transmission of schistosomiasis and in populations of its snail intermediate hosts in and around a sugar irrigation scheme at Richard Toll, Senegal. Parasitology. 2001;123:S77–S89. doi: 10.1017/s0031182001008125. [DOI] [PubMed] [Google Scholar]
- 60.Kloos H, McCullough F. Molluscicidal effects of eucalyptus. Vet Rec. 1982;111:148. doi: 10.1136/vr.111.7.148-a. [DOI] [PubMed] [Google Scholar]
- 61.Chimbari MJ. Enhancing schistosomiasis control strategy for zimbabwe: building on past experiences. J Parasitol Res. 2012;2012:353768. doi: 10.1155/2012/353768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Windbichler N, et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature. 2011;473:212–215. doi: 10.1038/nature09937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hammond A, et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol. 2016;34:78–83. doi: 10.1038/nbt.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adema CM, et al. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nat Commun. 2017;8:15451. doi: 10.1038/ncomms15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perry KJ, Henry JQ. CRISPR/Cas9-mediated genome modification in the mollusc, Crepidula fornicata. Genesis. 2015;53:237–244. doi: 10.1002/dvg.22843. [DOI] [PubMed] [Google Scholar]
- 66.Esvelt KM, et al. Concerning RNA-guided gene drives for the alteration of wild populations. Elife. 2014;3 doi: 10.7554/eLife.03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allan ER, et al. Schistosome infectivity in the snail, Biomphalaria glabrata, is partially dependent on the expression of Grctm6, a Guadeloupe Resistance Complex protein. PLoS Negl Trop Dis. 2017;11:e0005362. doi: 10.1371/journal.pntd.0005362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaefer KA, et al. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat Methods. 2017;14:547–548. doi: 10.1038/nmeth.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baltimore D, et al. Biotechnology. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;348:36–38. doi: 10.1126/science.aab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uhazy LS, et al. Schistosoma mansoni: identification of chemicals that attract or trap its snail vector, Biomphalaria glabrata. Science. 1978;201:924–926. doi: 10.1126/science.684418. [DOI] [PubMed] [Google Scholar]
- 71.Etges FJ, Gilbertson DE. Repellent action of some chemical molluscicides on schistosome vector snails. Am J Trop Med Hyg. 1966;15:618–624. doi: 10.4269/ajtmh.1966.15.618. [DOI] [PubMed] [Google Scholar]
- 72.Loreau M, Baluku B. Shade as a means of ecological control of Biomphalaria pfeifferi. Ann Trop Med Parasitol. 1991;85:443–446. doi: 10.1080/00034983.1991.11812590. [DOI] [PubMed] [Google Scholar]
- 73.Ramaswamy K, et al. Topical application of DEET for schistosomiasis. Trends Parasitol. 2003;19:551–555. doi: 10.1016/j.pt.2003.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mkoji GM, et al. Impact of the crayfish Procambarus clarkii on Schistosoma haematobium transmission in Kenya. American Journal of Tropical Medicine and Hygiene. 1999;61:751–759. doi: 10.4269/ajtmh.1999.61.751. [DOI] [PubMed] [Google Scholar]
- 75.Khalil M, Sleem SH. Can the freshwater crayfish eradicate schistosomiasis in Egypt and Africa? Journal of American Science. 2011;7:457–462. [Google Scholar]
- 76.Benstead J. Effects of a low-head dam and water abstraction on migratory tropical stream biota. Ecological Applications. 1999;9:656–668. [Google Scholar]
- 77.Roberts JK, Kuris AM. Predation and control of laboratory populations of the snail Biomphalaria glabrata by the freshwater prawn Macrobrachium rosenbergii. Ann Trop Med Parasitol. 1990;84:401–412. doi: 10.1080/00034983.1990.11812486. [DOI] [PubMed] [Google Scholar]
- 78.Evers BN, et al. The schistosome intermediate host, Bulinus nyassanus, is a 'preferred' food for the cichlid fish, Trematocranus placodon, at Cape Maclear, Lake Malawi. Ann Trop Med Parasitol. 2006;100:75–85. doi: 10.1179/136485906X78553. [DOI] [PubMed] [Google Scholar]
- 79.Madsen H, Stauffer JR. Density of Trematocranus placodon (Pisces: Cichlidae): a predictor of density of the schistosome intermediate host, Bulinus nyassanus (Gastropoda: Planorbidae), in Lake Malawi. Ecohealth. 2011;8:177–189. doi: 10.1007/s10393-011-0737-3. [DOI] [PubMed] [Google Scholar]
- 80.Slootweg R, et al. The biological control of intermediate hosts of schistosomiasis by fish. Rev Fish Biol Fish. 1994;4:67–90. [Google Scholar]
- 81.Gashaw F, et al. Assessment of the potential of competitor snails and African catfish (Clarias gariepinus) as biocontrol agents against snail hosts transmitting schistosomiasis. Trans R Soc Trop Med Hyg. 2008;102:774–779. doi: 10.1016/j.trstmh.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 82.Ndlela B, Chimbari MJ. A preliminary assessment of the potential of the Muschovy duck (Cairina maschata) as a biocontrol agent of schistosomiasis intermediate host snails. Cent Afr J Med. 2000;46:271–275. doi: 10.4314/cajm.v46i10.8568. [DOI] [PubMed] [Google Scholar]
- 83.Nassi H, et al. [Evaluation of a trial to control Biomphalaria glabrata in Guadeloupe by using a sterilizing trematode (author's transl)] Ann Parasitol Hum Comp. 1979;54:185–192. [PubMed] [Google Scholar]
- 84.Pointier JP, Jourdane J. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Trop. 2000;77:53–60. doi: 10.1016/s0001-706x(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 85.Hechinger RF, et al. Social organization in a flatworm: trematode parasites form soldier and reproductive castes. Proc Biol Sci. 2011;278:656–665. doi: 10.1098/rspb.2010.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jourdane J, et al. Influence of intramolluscan larval stages of Echinostoma liei on the infectivity of Schistosoma mansoni cercariae. J Helminthol. 1990;64:71–74. doi: 10.1017/s0022149x00011925. [DOI] [PubMed] [Google Scholar]
- 87.Jourdane J, Mounkassa JB. Topographic shifting of primary sporocysts of Schistosoma mansoni in Biomphalaria pfeifferi as a result of coinfection with Echinostoma caproni. J Invertebr Pathol. 1986;48:269–274. doi: 10.1016/0022-2011(86)90054-6. [DOI] [PubMed] [Google Scholar]
- 88.Tang CT, et al. Development of larval Schistosoma japonicum blocked in Oncomelania hupensis by pre-infection with larval Exorchis sp. J Parasitol. 2009;95:1321–1325. doi: 10.1645/GE-2055.1. [DOI] [PubMed] [Google Scholar]
- 89.Basch PF. Cotylurus lutzi sp. n. (Trematoda: Strigeidae) and its life cycle. J Parasitol. 1969;55:527–539. [PubMed] [Google Scholar]
- 90.Laidemitt MR, et al. Loads of trematodes: discovering hidden diversity of paramphistomoids in Kenyan ruminants. Parasitology. 2017;144:131–147. doi: 10.1017/S0031182016001827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Southgate VR, et al. The influence of Calicophoron microbothrium on the susceptibility of Bulinus tropicus to Schistosoma bovis. Parasitol Res. 1989;75:381–391. doi: 10.1007/BF00931134. [DOI] [PubMed] [Google Scholar]
- 92.Spatz L, et al. Susceptibility of wild populations of Biomphalaria spp. from neotropical South America to Schistosoma mansoni and interference of Zygocotyle lunata. J Parasitol. 2012;98:1291–1295. doi: 10.1645/GE-3002.1. [DOI] [PubMed] [Google Scholar]
- 93.Johnson PT, et al. Community diversity reduces Schistosoma mansoni transmission, host pathology and human infection risk. Proc Biol Sci. 2009;276:1657–1663. doi: 10.1098/rspb.2008.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Upatham ES. Interference by unsusceptible aquatic animals with the capacity of the miracidia of Schistosoma mansoni Sambon to infect Biomphalaria glabrata (Say) under field-simulated conditions in St. Lucia, West Indies. J Helminthol. 1972;46:277–283. doi: 10.1017/s0022149x00024421. [DOI] [PubMed] [Google Scholar]
- 95.Hopkins SR, et al. Parasite predators exhibit a rapid numerical response to increased parasite abundance and reduce transmission to hosts. Ecol Evol. 2013;3:4427–4438. doi: 10.1002/ece3.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molyneux DH. The 'Neglected Tropical Diseases': now a brand identity; responsibilities, context and promise. Parasit Vectors. 2012;5:23. doi: 10.1186/1756-3305-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergquist R, et al. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Remais JV, Eisenberg JN. Balance between clinical and environmental responses to infectious diseases. Lancet. 2012;379:1457–1459. doi: 10.1016/S0140-6736(11)61227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Secor WE, Montgomery SP. Something old, something new: is praziquantel enough for schistosomiasis control? Future Med Chem. 2015;7:681–684. doi: 10.4155/fmc.15.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arfaa F, et al. Progress towards the control of bilharziasis in Iran. Trans R Soc Trop Med Hyg. 1970;64:912–917. doi: 10.1016/0035-9203(70)90111-2. [DOI] [PubMed] [Google Scholar]
- 101.El-Halawani A. Evaluation of molluscicidal control of schistosomiasis in the Middle East. In: Abdallah A, editor. Proceedings of the International Conference on Schistosomiasis. Egypt Ministry of Health; 1978. pp. 349–357. 1978. [Google Scholar]
- 102.Jordan P. From katayama to the Dakhla Oasis: the beginning of epidemiology and control of bilharzia. Acta Trop. 2000;77:9–40. doi: 10.1016/s0001-706x(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 103.Ebisawa I. Epidemiology and eradication of Schistosomiasis japonica in Japan. J Travel Med. 1998;5:33–35. doi: 10.1111/j.1708-8305.1998.tb00454.x. [DOI] [PubMed] [Google Scholar]
- 104.Pointier J-p. Invading freshwater snails and biological bontrol in Martinique Island, French West Indies. Memorias do Instituto Oswaldo Cruz. 2001;96:67–74. doi: 10.1590/s0074-02762001000900009. [DOI] [PubMed] [Google Scholar]
- 105.Dhunputh J. Progress in the control of schistosomiasis in Mauritius. Trans R Soc Trop Med Hyg. 1994;88:507–509. doi: 10.1016/0035-9203(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 106.Amarir F, et al. National serologic survey of Haematobium schistosomiasis in Morocco: evidence for elimination. Am J Trop Med Hyg. 2011;84:15–19. doi: 10.4269/ajtmh.2011.10-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Barkia H, et al. Contribution of mobile teams to efforts to eliminate schistosomiasis at Schistosoma haematobium in Morocco - narrative review article. Iranian Journal of Public Health. 2014;43:1167–1175. [PMC free article] [PubMed] [Google Scholar]
- 108.Boelee E, Laamrani H. Environmental control of schistosomiasis through community participation in a Moroccan oasis. Trop Med Int Health. 2004;9:997–1004. doi: 10.1111/j.1365-3156.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- 109.Khallaayoune K, Laamrani H. Seasonal patterns in the transmission of Schistosoma haematobium in Attaouia, Morocco. J Helminthol. 1992;66:89–95. doi: 10.1017/s0022149x00012645. [DOI] [PubMed] [Google Scholar]
- 110.Laamrani H, et al. New challenges in schistosomiasis control in Morocco. Acta Trop. 2000;77:61–67. doi: 10.1016/s0001-706x(00)00114-5. [DOI] [PubMed] [Google Scholar]
- 111.Laamrani H, et al. Schistosoma haematobium in Morocco: moving from control to elimination. Parasitol Today. 2000;16:257–260. doi: 10.1016/s0169-4758(00)01665-3. [DOI] [PubMed] [Google Scholar]
- 112.Nuttall I, et al. GIS Management Tools for the Control of Tropical Diseases: Applications in Botswana, Senegal, and Morocco. In. In: De Savigny D, Wijeyaratne P, editors. GIS for Health and the Environment. International Development Research Center; 1994. [Google Scholar]
- 113.Haddock KC. Control of schistosomiasis: the Puerto Rican experience. Soc Sci Med D. 1981;15:501–514. doi: 10.1016/0160-8002(81)90045-9. [DOI] [PubMed] [Google Scholar]
- 114.Rey L, et al. Schistosomiasis in Tunisia. Results after 10 years of the endemics control. Bulletin de la Societe de Pathologie Exotique et de Ses Filiales. 1982;75:505–522. [PubMed] [Google Scholar]
- 115.Al-Madani AA. Schistosomiasis control in Saudi Arabia with special reference to the period 1983–1988. Public Health. 1990;104:261–266. doi: 10.1016/s0033-3506(05)80475-5. [DOI] [PubMed] [Google Scholar]
- 116.Barakat R, et al. Human Schistosomiasis in the Middle East and North Africa Region. In: McDowell MA, Rafati S, editors. Neglected Tropical Diseases - Middle East and North Africa. Springer; Vienna: 2014. [Google Scholar]
- 117.Hotez PJ, et al. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS neglected tropical diseases. 2012;6:e1475. doi: 10.1371/journal.pntd.0001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lotfy WM, Alsaqabi SM. Human schistosomiasis in the Kingdom of Saudi Arabia: A review. Journal of the Medical Research Institute. 2010;31:1–6. [Google Scholar]
- 119.WHO. World Health Organization of the EMR. World Health Organization; 2007. Inter-country meeting on strategies to eliminate schistosomiasis from the Eastern Mediterranean Region. [Google Scholar]
- 120.Youssef AR, et al. Schistosomiasis in Saudi Arabia, Egypt, and Iraq. Urology. 1998;51:170–174. doi: 10.1016/s0090-4295(98)00061-2. [DOI] [PubMed] [Google Scholar]
- 121.Izhar A, et al. Recent situation of schistosomiasis in Indonesia. Acta Tropica. 2002;82:283–288. doi: 10.1016/s0001-706x(02)00020-7. [DOI] [PubMed] [Google Scholar]
- 122.Baquir H. Letter: Present status of Hor Rajab bilharziasis control project Iraq 15, WHO-TA. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1974;68:345. doi: 10.1016/0035-9203(74)90053-4. [DOI] [PubMed] [Google Scholar]
- 123.Lotfy WM. Human schistosomiasis in Egypt: Historical review, assessment of the current picture and prediction of the future trends. Journal of the Medical Research Institute. 2009;30:1–7. [Google Scholar]
- 124.Khalil M, Sleem SH. Can the freshwater crayfish eradicate schistosomiasis in Egypt and Africa? Journal of American Science. 2011;7 [Google Scholar]
- 125.Farooq M, et al. The effect of area-wide snail control on the endemicity of bilharziasis in Egypt. Bulletin of the World Health Organization. 1966;35:369–375. [PMC free article] [PubMed] [Google Scholar]
- 126.El-Khoby T, et al. The epidemiology of schistosomiasis in Egypt: Summary findings in nine governorates. American Journal of Tropical Medicine and Hygiene. 2000;62:88–99. doi: 10.4269/ajtmh.2000.62.88. [DOI] [PubMed] [Google Scholar]
- 127.El Khoby T, et al. The USAID/Government of Egypt's Schistosomiasis Research Project (SRP) Parasitology Today. 1998;14:92–96. doi: 10.1016/s0169-4758(97)01206-4. [DOI] [PubMed] [Google Scholar]
- 128.Barakat RMRR. Epidemiology of schistosomiasis in Egypt: Travel through time: Review. Cairo University Journal of Advanced Research. 2013;4:425–432. doi: 10.1016/j.jare.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhou X-N, et al. The public health significance and control of schistosomiasis in China--then and now. Acta tropica. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Xianyi C, et al. Policy and practice schistosomiasis control in China: The impact of a 10-year World Bank Loan Project (1992 – 2001) Bulletin of the World Health Organization. 2005;83:43–48. [PMC free article] [PubMed] [Google Scholar]
- 131.de Noya BA, et al. New approaches for the control and eradication of schistosomiasis in Venezuela. Memorias do Instituto Oswaldo Cruz. 1992;87:227–231. doi: 10.1590/s0074-02761992000800035. [DOI] [PubMed] [Google Scholar]
- 132.De Noya BA, et al. The last fifteen years of schistosomiasis in Venezuela: Features and evolution. Memorias do Instituto Oswaldo Cruz. 1999;94:139–146. doi: 10.1590/s0074-02761999000200002. [DOI] [PubMed] [Google Scholar]
- 133.Pointier JPP, Jourdane J. Biological control of the snail hosts of schistosomiasis in areas of low transmission: the example of the Caribbean area. Acta Tropica. 2000;77:53–60. doi: 10.1016/s0001-706x(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 134.Bergquist R, Tanner M. Controlling schistosomiasis in Southeast Asia: a tale of two countries. Advances in parasitology. 2010;72:109–144. doi: 10.1016/S0065-308X(10)72005-4. [DOI] [PubMed] [Google Scholar]
- 135.Blas BL, et al. The schistosomiasis problem in the Philippines: a review. Parasitology international. 2004;53:127–134. doi: 10.1016/j.parint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 136.Jordan P. Schistosomiasis: The St Lucia Project. Cambridge University Press; 1985. [Google Scholar]
- 137.Pointier JP, Théron A. Ecology and control of the snail intermediate hosts of trematodes in an heterogenous environment: the Biomphalaria glabrata model in the insular focus of. Research and Reviews in Parasitology. 1995;55:121–133. [Google Scholar]
- 138.Stothard JR, et al. The epidemiology and control of urinary schistosomiasis and soil-transmitted helminthiasis in schoolchildren on Unguja Island, Zanzibar. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2009;103:1031–1044. doi: 10.1016/j.trstmh.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 139.Knopp S, et al. From morbidity control to transmission control: time to change tactics against helminths on Unguja Island, Zanzibar. Acta tropica. 2013;128:412–422. doi: 10.1016/j.actatropica.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 140.Knopp S, et al. Study and implementation of urogenital schistosomiasis elimination in Zanzibar (Unguja and Pemba islands) using an integrated multidisciplinary approach. BMC public health. 2012;12:930. doi: 10.1186/1471-2458-12-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Urbani C, et al. Epidemiology and control of mekongi schistosomiasis. Acta Tropica. 2002:157–168. doi: 10.1016/s0001-706x(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 142.Sornmani S. Current status of schistosomiasis in Laos, Thailand and Malaysia. The Southeast Asian Journal of Tropical Medicine and Public Health. 1976;7:149–154. [PubMed] [Google Scholar]
- 143.Ohmae H, et al. Schistosomiasis mekongi: From discovery to control. Parasitology International. 2004:135–142. doi: 10.1016/j.parint.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 144.Sinuon M, et al. Control of Schistosoma mekongi in Cambodia: results of eight years of control activities in the two endemic provinces. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:34–39. doi: 10.1016/j.trstmh.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Coura JR, Amaral RS. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Memorias do Instituto Oswaldo Cruz. 2004:13–19. doi: 10.1590/s0074-02762004000900003. [DOI] [PubMed] [Google Scholar]
- 146.Barbosa FS, et al. Control of schistosomiasis mansoni in a small Northeast Brazilian community. Transactions of the Royal Society of Tropical Mediciine Hygiene. 1971;65:206–213. doi: 10.1016/0035-9203(71)90220-3. [DOI] [PubMed] [Google Scholar]
- 147.Amaral RSd, et al. An analysis of the impact of the Schistosomiasis Control Programme in Brazil. Memórias do Instituto Oswaldo Cruz. 2006:79–85. doi: 10.1590/s0074-02762006000900012. [DOI] [PubMed] [Google Scholar]
- 148.Almeida Machado P. The Brazilian program for schistosomiasis control, 1975–1979. American Journal of Tropical Medicine and Hygiene. 1982;31:76–86. doi: 10.4269/ajtmh.1982.31.76. [DOI] [PubMed] [Google Scholar]
- 149.Ndayishimiye O, et al. Control of neglected tropical diseases in Burundi: partnerships, achievements, challenges, and lessons learned after four years of programme implementation. PLoS Neglected Tropical Diseases. 2014;8:e2684. doi: 10.1371/journal.pntd.0002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gryseels B. The epidemiology of schistosomiasis in Burundi and its consequences for control. Trans R Soc Trop Med Hyg. 1991;85:626–633. doi: 10.1016/0035-9203(91)90371-5. [DOI] [PubMed] [Google Scholar]
- 151.Engels D, et al. Schistosomiasis mansoni in Burundi: Progress in its control since 1985. Bulletin of the World Health Organization. 1993;71:207–214. [PMC free article] [PubMed] [Google Scholar]
- 152.Linehan M, et al. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: proving the feasibility at national scale. The American Journal of Tropical Medicine and Hygiene. 2011;84:5–14. doi: 10.4269/ajtmh.2011.10-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ollivier G, et al. La schistosomose intestinale à Schistosoma mansoni à Madagascar: extension et focalisation de l’endémie. Parasitologie. 1998;1966:1–5. [PubMed] [Google Scholar]
- 154.Mccullough FS, et al. Molluscicides in schistosomiasis control. Bulletin of the World Health Organization. 1980;58:681–689. [PMC free article] [PubMed] [Google Scholar]
- 155.Ruxin J, Negin J. Removing the neglect from neglected tropical diseases: the Rwandan experience 2008–2010. Glob Public Health. 2012;7:812–822. doi: 10.1080/17441692.2012.699535. [DOI] [PubMed] [Google Scholar]
- 156.Locketz L. Health education in rural Surinam: use of videotape in a national campaign against schistosomiasis. Bulletin of the Pan American Health Organization. 1976;10:219–226. [PubMed] [Google Scholar]
- 157.Hotez PJ, et al. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008;2:e300. doi: 10.1371/journal.pntd.0000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Touré S, et al. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bulletin of the World Health Organization. 2008;86:780–788. doi: 10.2471/BLT.07.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Poda JN, et al. Schistosomiasis endemic in Burkina Faso. Bulletin de la Societe de pathologie exotique (1990) 2004;97:47–52. [PubMed] [Google Scholar]
- 160.Fenwick A, et al. Implementation of human schistosomiasis control: Challenges and prospects. Advances in parasitology. 2006;61:567–622. doi: 10.1016/S0065-308X(05)61013-5. [DOI] [PubMed] [Google Scholar]
- 161.Mazigo HD, et al. Epidemiology and control of human schistosomiasis in Tanzania. Parasit Vectors. 2012;5:274. doi: 10.1186/1756-3305-5-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Moné H, et al. Human Schistosomiasis in the Economic Community of West African States. Advances in Parasitology. 2010:33–91. [Google Scholar]
- 163.Kabatereine NB, et al. Epidemiology and geography of Schistosoma mansoni in Uganda: Implications for planning control. Tropical Medicine and International Health. 2004;9:372–380. doi: 10.1046/j.1365-3156.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 164.Landoure A, et al. Significantly reduced intensity of infection but persistent prevalence of schistosomiasis in a highly endemic region in Mali after repeated treatment. PLoS Negl Trop Dis. 2012;6:e1774. doi: 10.1371/journal.pntd.0001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sesay S, et al. Schistosoma mansoni infection after three years of mass drug administration in Sierra Leone. Parasites & Vectors. 2014;7:14. doi: 10.1186/1756-3305-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hodges M, et al. Improved mapping strategy to better inform policy on the control of schistosomiasis and soil-transmitted helminthiasis in Sierra Leone. Parasites & vectors. 2011;4:97. doi: 10.1186/1756-3305-4-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Samuels AM, et al. Schistosoma mansoni morbidity among school-aged children: A SCORE Project in Kenya. American Journal of Tropical Medicine and Hygiene. 2012;87:874–882. doi: 10.4269/ajtmh.2012.12-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dabo A, et al. Reinfection with Schistosoma haematobium and mansoni despite repeated praziquantel office treatment in Niger, Mali. Med.Trop.(Mars.) 2000;60:351–355. [PubMed] [Google Scholar]
- 169.Fenwick A, et al. The Schistosomiasis Control Initiative (SCI): rationale, development and implementation from 2002–2008. Parasitology. 2009;136:1719–1730. doi: 10.1017/S0031182009990400. [DOI] [PubMed] [Google Scholar]
- 170.Garba A, et al. Implementation of national schistosomiasis control programmes in West Africa. Trends in Parasitology. 2006;22:322–326. doi: 10.1016/j.pt.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 171.Leslie J, et al. Schistosomiasis and soil-transmitted helminth control in Niger: cost effectiveness of school based and community distributed mass drug administration [corrected] PLoS Neglected Tropical Diseases. 2011;5:e1326. doi: 10.1371/journal.pntd.0001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Oshish A, et al. Towards nationwide control of schistosomiasis in Yemen: a pilot project to expand treatment to the whole community. Trans R Soc Trop Med Hyg. 2011;105:617–627. doi: 10.1016/j.trstmh.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 173.Madsen H, et al. Schistosomiasis in Lake Malaŵi villages. EcoHealth. 2011;8:163–176. doi: 10.1007/s10393-011-0687-9. [DOI] [PubMed] [Google Scholar]
- 174.Stauffer JR, et al. Controlling vectors and hosts of parasitic diseases using fishes. Bioscience. 1997;47:41–49. [Google Scholar]
- 175.Stauffer JR, et al. Schistosomiasis in Lake Malawi: Relationship of fish and intermediate host density to prevalence of human infection. EcoHealth. 2006;3:22–27. [Google Scholar]
- 176.Wolff T, Malewezi JG. Organization and decentralization of the Malawi National Bilharzia Control Programme. Tropical Medicine and Parasitology. 1989;40:201–204. [PubMed] [Google Scholar]
- 177.Agbo K, et al. Prevalence des schistosomoses au Togo etude transversale realisee en milieu scolaire. Medecine Tropicale. 1999;59:51–54. [PubMed] [Google Scholar]
- 178.Kabatereine NB, et al. The control of schistosomiasis and soil-transmitted helminths in East Africa. Trends in parasitology. 2006;22:332–339. doi: 10.1016/j.pt.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 179.Fenwick A. The control of schistosomiasis in Africa and the evaluation of integrated control of neglected tropical diseases in Africa. (Grant ID# 13122 and 36202 edn), Bill & Melinda Gates Foundation 2011 [Google Scholar]
- 180.Tchuente LA, et al. Mapping of schistosomiasis and soil-transmitted helminthiasis in the regions of Centre, East and West Cameroon. PLoS Neglected Tropical Diseases. 2013;6:e1553. doi: 10.1371/journal.pntd.0001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maseko TS, et al. Schistosomiasis knowledge, attitude, practices, and associated factors among primary school children in the Siphofaneni area in the Lowveld of Swaziland. J Microbiol Immunol Infect. 2016 doi: 10.1016/j.jmii.2015.12.003. https://doi.org/10.1016/j.jmii.2015.12.003. [DOI] [PubMed]
- 182.Huyse T, et al. Regular treatments of praziquantel do not impact on the genetic make-up of Schistosoma mansoni in Northern Senegal. Infection, Genetics and Evolution. 2013;18:100–105. doi: 10.1016/j.meegid.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 183.el Gaddal AA. Control of schistosomiasis in the Gezira. Mem Inst Oswaldo Cruz. 1989;84(Suppl 1):117–123. doi: 10.1590/s0074-02761989000500012. [DOI] [PubMed] [Google Scholar]
- 184.el Gaddal AA. The Blue Nile Health Project: a comprehensive approach to the prevention and control of water-associated diseases in irrigated schemes of the Sudan. J Trop Med Hyg. 1985;88:47–56. [PubMed] [Google Scholar]
- 185.El-Nagar H. Control of schistosomiasis in the Gezira, Sudan. J Trop Med Hyg. 1958;61:231–235. [PubMed] [Google Scholar]
- 186.Finn TP, et al. Integrated rapid mapping of neglected tropical diseases in three States of South Sudan: survey findings and treatment needs. PLoS One. 2012;7:e52789. doi: 10.1371/journal.pone.0052789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Humaida S, et al. Schistosomiasis: epidemiology and burden of disease in the Sudan. Sudan Medical Journal. 2011;47:63–68. [Google Scholar]
- 188.Ault SK. Environmental management: a re-emerging vector control strategy. Am J Trop Med Hyg. 1994;50:35–49. doi: 10.4269/ajtmh.1994.50.35. [DOI] [PubMed] [Google Scholar]
- 189.Olivier L, Schneidermann M. A method for estimating the density of aquatic snail populations. Exp Parasitol. 1956;5:109–117. doi: 10.1016/0014-4894(56)90008-x. [DOI] [PubMed] [Google Scholar]
- 190.Hairston NG. Suggestions regarding some problems in the evaluation of molluscicides in the field. Bull World Health Organ. 1961;25:731–737. [PMC free article] [PubMed] [Google Scholar]
- 191.Theron A. Cercariometry and the epidemiology of schistosomiasis. Parasitology Today. 1986;2:61–63. doi: 10.1016/0169-4758(86)90156-0. [DOI] [PubMed] [Google Scholar]
- 192.Hung YW, Remais J. Quantitative detection of Schistosoma japonicum cercariae in water by real-time PCR. PLoS Negl Trop Dis. 2008;2:e337. doi: 10.1371/journal.pntd.0000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang K, et al. Spatio-temporal analysis to identify determinants of Oncomelania hupensis infection with Schistosoma japonicum in Jiangsu province, China. Parasit Vectors. 2013;6:138. doi: 10.1186/1756-3305-6-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sturrock RF. Snail collection to detect schistosome transmission sites. Parasitol Today. 1986;2:59–61. doi: 10.1016/0169-4758(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 195.Aoki Y, et al. Cercariometry for detection of transmission sites for schistosomiasis. Parasitology International. 2003;52:403–408. doi: 10.1016/s1383-5769(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 196.Gryseels B, Nkulikyinka L. The distribution of Schistosoma mansoni in the Rusizi plain (Burundi) Ann Trop Med Parasitol. 1988;82:581–590. doi: 10.1080/00034983.1988.11812294. [DOI] [PubMed] [Google Scholar]
- 197.Zhou YB, et al. Multi-host model and threshold of intermediate host Oncomelania snail density for eliminating schistosomiasis transmission in China. Sci Rep. 2016;6:31089. doi: 10.1038/srep31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rollinson D, et al. Genetic diversity of schistosomes and snails: implications for control. Parasitology. 2009;136:1801–1811. doi: 10.1017/S0031182009990412. [DOI] [PubMed] [Google Scholar]