Targeted therapies for cancer have changed treatment approaches for several solid tumor malignancies. Analyses of cell-free circulating tumor DNA (ctDNA) enable noninvasive cancer detection and characterization, prediction of treatment response, monitoring of disease relapse, and identification of mechanisms of resistance to targeted therapies. With newer technologies, the sensitivity and specificity of ctDNA detection assays have improved and facilitate a greater role for ctDNA diagnostics in clinical practice (Figure).
Figure. Analysis of ctDNA and Clinical Applications.
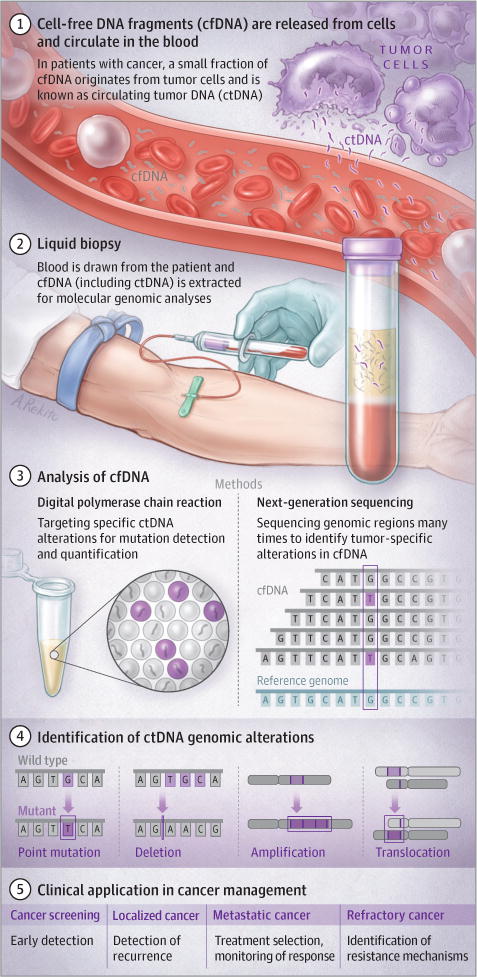
A variety of tumor-derived genomic alterations may be detected in cfDNA (see text for description of methods for cfDNA analysis). Current clinical applications include treatment selection and identification of resistance mutations; future applications include monitoring of treatment response, detection of recurrence, and screening.
How Liquid Biopsies Work
Circulating cell-free DNA (cfDNA) was discovered in plasma more than 60 years ago.1 Several decades later, abnormalities in cfDNA obtained from cancer patients were described and are now known as ctDNA.2 Most cfDNA in the circulation is derived from ruptured nonmalignant cells and is of germline origin. ctDNA is thought to be a result of tumor cell apoptosis and necrosis. Analysis of ctDNA can identify tumor-specific DNA abnormalities that can be used as a basis for highly specific disease testing strategies. Detecting the small amount of tumor-derived mutations in cfDNA has been a major limitation in the clinical application of ctDNA testing. Recently developed novel genomic and bioinformatic approaches have facilitated highly sensitive molecular assays that can detect tumor-specific aberrations from ctDNA.3–5
Large-Scale Sequencing Approaches
Next-generation sequencing (NGS) provides high-throughput analysis of a large number of DNA molecules in a single reaction.6 NGS can typically detect tumor-specific mutations in the whole genome, exome, or a panel of genes in tissue specimens where mutations are present in at least 5% of the cells analyzed. Because mutations in ctDNA are difficult to detect and may occur in less than 1% of the cfDNA molecules analyzed, new approaches have been developed that use much deeper sequencing (each base is analyzed redundantly >20 000 times) to detect alterations present at frequencies aslowas0.05%.3–5 These methods have the advantage of being able to test for a variety of aberrations in key genes through sequencing of DNA that has been amplified or captured through hybridization from regions of the genome. Targeted deep sequencing combined with analyses that remove sequencing errors allow for accurate detection of a broad range of sequence alterations. New NGS methods may also be used for detection of DNA rearrangements or copy number changes in ctDNA,7 because these alterations represent important therapeutic targets in many cancers. Extension of these targeted approaches to whole-exome or genome strategies in plasma have identified novel genomic aberrations in tumor DNA that may have been selected for cell survival during cancer therapy—similar to the selection of resistant microorganisms resulting from antibiotic use.
Digital Polymerase Chain Reaction
Digital polymerase chain reaction (PCR) is an approach to quantify specific alterations in ctDNA by separating one PCR reaction into multiple reactions, each with a single or small number of DNA molecules.8 In this process, PCR amplifies cfDNA fragments, and fluorescent probes are used to differentially bind to mutant and wild-type sequences. Each reaction is scored based on the presence or absence of mutant or wild-type signal, and the proportion of different signals represents the prevalence of the mutant and wild-type alleles. Recent refinements of digital PCR technology have allowed higher accuracy and throughput. These methods provide high sensitivity but are limited by the requirement of prior knowledge of specific point mutations or copy number variants to be analyzed. In a study of patients with advanced lung cancer, the detection limit by digital PCR of EGFR, KRAS, or BRAF mutations was approximately 0.01% of mutant to wild-type DNA molecules.9 The relatively low cost and rapid turnaround time of a few days make digital PCR a useful platform for clinical applications in lung and other cancers.
Important Care Considerations
ctDNA analyses have been approved by the US Food and Drug Administration (FDA) for use in lung cancer patients for the initial genotyping of tumors when not enough tissue is available to characterize the molecular composition of the tumor. These analyses may be more cost-effective than multiple tissue biopsies and may greatly reduce risks associated with biopsies. The Cobas EGFR test (Roche)was approved by the FDA in 2016 as the first diagnostic plasma test for detecting multiple EGFR mutations for identifying patients with non–small-cell lung cancer eligible for treatment with EFGR inhibitors.10 In 2015, the Therascreen EGFR kit (Qiagen) received Conformité Européene in vitro diagnostic approval for an in vitro diagnostic medical device to include the liquid biopsy companion diagnostic for detecting EGFR mutations in patients from whom tumor tissue cannot be obtained to facilitate targeted treatments. While these tests are limited to a specific gene, expanded ctDNA analyses using NGS gene panels for broader mutation profiling and therapeutic target selection are now available for clinical use as laboratory-developed tests, and some are likely to become FDA approved.
The emergence of genomic alterations that confer resistance to targeted cancer therapies often results in treatment failure for late-stage cancers. In a prospective trial of patients with treated EGFR-mutant non–small-cell lung cancer, the serial assessment of ctDNA levels correlated with treatment response and detected early emergence of the resistance EGFRT790M mutation.9 Strategies are being evaluated to consider plasma testing as an initial strategy for detecting resistance mutations in clinically resistant lung cancer patients, followed by tissue-based testing for when assessment of ctDNA is unrevealing.
Value and Evidence Base
National Comprehensive Cancer Network guidelines currently recommend plasma testing for non–small-cell lung cancer patients in certain circumstances, and other cancers may soon follow. Initial applications include cancer genotyping and early resistance monitoring for patients who cannot have biopsies performed because of safety concerns or who have insufficient tissue for genomic characterization. Detection of ctDNA in patients with recurrence may precede radiographic detection of cancers by more than 6 months, facilitating earlier treatment. Research is under way to identify early indicators of treatment response, evaluate economic cost-benefit analyses of these approaches, and uncover methods for early detection of cancer in high-risk populations.5
Bottom Line
ctDNA analyses offer a promising approach for noninvasive detection of tumor DNA variants in human plasma. It is likely that ctDNA analyses will see increasing use in diagnosis, treatment selection, and treatment resistance monitoring for a variety of cancers.
Acknowledgments
Funding/Support: This work was supported by the following grants (Velculescu): National Institutes of Health (CA121113, CA006973, CA180950), Commonwealth Foundation, Dr Miriam and Sheldon G. Adelson Medical Research Foundation, and SU2C-DCS International Translational Cancer Research Dream Team (Stand Up To Cancer, a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research) (SU2C-AACR-DT1415).
Footnotes
CME Quiz at jamanetwork.com/learning
Section Editor: W. Gregory Feero, MD, PhD, Associate Editor, JAMA.
Conflict of Interest Diclosures: Both authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Husain reports participation on clinical advisory boards for Foundation Medicine, Abbvie, and AstraZeneca; a grant from Pfizer; and lecture honoraria from Bristol-Myers Squibb, Merck, and AstraZeneca. Dr Velculescu reports scientific advisory board and board of directors membership, stock ownership (subject to certain restrictions under university policy), and being a founder of Personal Genome Diagnostics; and being a member of the Ignyta scientific advisory board. The terms of these arrangements are managed by the Johns Hopkins University in accordance with its conflict of interest policies.
Contributor Information
Hatim Husain, Division of Hematology and Oncology, Moores Cancer Center, University of California, San Diego, La Jolla.
Victor E. Velculescu, The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, Maryland.
References
- 1.Mandel P, Metais P. Les acides nucléiques du plasma sanguin chez l’homme. C R Seances Soc Biol Fil. 1948;142(3–4):241–243. [PubMed] [Google Scholar]
- 2.Sidransky D. Nucleic acid-based methods for the detection of cancer. Science. 1997;278(5340):1054–1059. doi: 10.1126/science.278.5340.1054. [DOI] [PubMed] [Google Scholar]
- 3.Kinde I, Wu J, Papadopoulos N, Kinzler KW, Vogelstein B. Detection and quantification of rare mutations with massively parallel sequencing. Proc Natl Acad Sci U S A. 2011;108(23):9530–9535. doi: 10.1073/pnas.1105422108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phallen J, Sausen M, Adleff V, et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med. 2017;9(403):eaan2415. doi: 10.1126/scitranslmed.aan2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans JP, Powell BC, Berg JS. Finding the rare pathogenic variants in a human genome. JAMA. 2017;317(18):1904–1905. doi: 10.1001/jama.2017.0432. [DOI] [PubMed] [Google Scholar]
- 7.Leary RJ, Sausen M, Kinde I, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4(162):162ra154. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci U S A. 1999;96(16):9236–9241. doi: 10.1073/pnas.96.16.9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. Cobas EGFR mutation test v2-P150047. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm504540.htm. Accessed September 17, 2017.


