Abstract
Introduction
Associations between anxiety symptoms and cannabis use have been previously explored, yet the directionality of these associations remains highly debatable. The present study aims to prospectively examine patterns of cannabis use and anxiety during adolescence focusing on their co-development and bidirectional influences.
Methods
Adolescents (n = 250) of predominantly Hispanic ethnicity, aged 14-17 at baseline, exposed to drugs, alcohol, or cigarettes completed three (bi-annual) assessments across a 1-year period. Latent growth curve modeling (LGCM) and parallel process growth curves were conducted to examine potential associations in the joint development of anxiety and cannabis use.
Results
Our results suggest that, during adolescence, early cannabis use has a greater influence on prospective reports of anxiety, than vice versa. Specifically, adolescents exhibiting higher initial levels of cannabis use displayed more persisting self-reported anxiety across time, as compared to those with less frequent use (b = .28, p = .024). In contrast, early levels of anxiety were not found to influence rates of change in cannabis use. These analyses considered concurrent depression, alcohol, and nicotine use.
Conclusions
Our findings suggest that prevention and targeted intervention programs for cannabis use in adolescence would benefit from anxiety management strategies; in order to reduce subsequent anxiety associated with cannabis use. Future studies should continue to employ longitudinal designs across larger time periods and aim to replicate these findings with more diverse samples.
Keywords: cannabis, anxiety, adolescents, parallel process, LCGM
1.1 Introduction
Cannabis remains among the most abused substances during adolescence, with 35% of high school seniors reporting past year use (Johnston et al., 2016). Reductions in risk perception among youth over the past 5 years (Johnston et al., 2016) and a growing trend toward legalization across numerous states raise concerns about increased cannabis use (CU) among youth and an increase in associated adverse consequences. As of June 2017, 29 states in the U.S. and the District of Columbia either allow medical or recreational cannabis use, with several more considering the legalization of any form of use (Caulkins et al., 2015). Yet, to-date, the consequences of cannabis use on adolescents' health and mental health remain largely unknown. In this study, we aim to address part of this important gap in the existing literature by investigating patterns of cannabis use and anxiety disorder symptoms during adolescence and their associations.
Associations between mental health problems and substance use disorders are commonly documented. Nationally representative epidemiologic studies examining comorbidity patterns in adolescents and adults, such as the National Comorbidity Survey-Adolescent Supplement (NCS-A) and the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), have provided evidence of strong associations between substance use disorders and mental health problems (Conway et al., 2016; Grant et al., 2004). In 2004, 17.71% of individuals who met criteria for a substance use disorder (SUD) had at least one anxiety disorder (Lopez-Quintero et al., 2011; Wu et al., 2010; Grant et al., 2004).
Anxiety disorders are the most common child and adolescent psychiatric conditions (Beesdo et al., 2009; Johnston et al., 2016). It is estimated that approximately 31.9% of adolescents suffer from anxiety disorders (Merikangas et al., 2010). Prior findings from Milich et al. (2000) suggest that frequent cannabis users exhibit higher levels of anxiety when compared to less frequent users and non-users. Associations between post-traumatic stress disorder and CU have been extensively examined in adults, with lifetime post-traumatic stress disorder diagnosis predicting future use (Cougle, Bonn-Miller, Vujanovic, Zvolensky, & Hawkins, 2011). Additional adult studies have found CU to be associated with an increase in current and prospective panic attacks and panic disorder (Zvolesnky, Cougle, Johnson, Bonn-Miller, & Bernstein, 2010; Zvolensky et al., 2008; Zvolensky et al., 2006). Furthermore, adults with past or current panic symptoms were found to show increased anxiety while under acute cannabis intoxication compared to non-anxious users (Szuster, Pntius, & Campos, 1988).
Despite the high prevalence of CU and anxiety disorders in adolescents, surprisingly little is known about their relationship across time, particularly among adolescents in the early stages of use. This is even more striking when considering that one of the primary reasons given for using cannabis is its subjective anxiolytic effects (Green et al., 2003). While tetrahydrocannabinol (THC) can bind to receptors in limbic regions which can lead to self-reported anxiolytic effects (Atakan, 2012), a growing body of literature has also revealed an association between increases in use and anxiety. Notably, a dose-dependent bi-phasic relationship has been observed within the animal literature, with low doses of cannabis exposure appearing to have an anxiolytic effect and higher doses inducing anxiety-like behaviors (Patel & Hillard, 2006). Chronic exposure during adolescence in rodents has also been associated with increases in anxiety-like behaviors (O'Shea et al., 2004) and has been shown to alter CB1 receptor densities, influencing both anxiogenic and anxiolytic effects (Dalton & Zavitsanou, 2010). Similarly, incremental cannabinoid exposure over a three-week period resulted in increased anxiety-like behaviors in perinatal, adolescent, and adult rats, regardless of the age at which cannabis exposure occurred (O'Shea et al., 2006). Thus, the preclinical evidence suggests that cannabis use may prospectively worsen, rather than ameliorate, symptoms of anxiety.
Studies on anxiety and CU among samples consisting exclusively of adolescent human subjects remain scarce, and rarely incorporate longitudinal designs (Degenhardt et al., 2001). Findings from the few longitudinal studies that have assessed the association between CU and anxiety suggest that accelerating patterns of CU throughout adolescence and young adulthood may be contingent on past mood disorder diagnosis at different stages of development (McGee et al., 2000). Alternatively, a three-year prospective study of adults from the NESARC found that CU and cannabis use disorders (CUDs) were not associated with increased likelihood of an anxiety disorder at follow-up (Buckner et. al., 2012). Similarly, adult anxiety disorders (with the exception of panic disorder) were not found to be associated with increased incidence of CU or CUD (Feingold et al., 2016). Although this study suggests that neither CU nor the majority of anxiety sub-types are interrelated, specific symptoms of anxiety (e.g., panic symptoms) may trigger CU (Feingold et al., 2016). It is worth noting that this study failed to account for early levels of use and anxiety, which may have influenced these findings and the generalizability of results.
While associations between CU and anxiety disorders have been previously explored, particularly in adult samples, explanations regarding the directionality of the association remain highly debatable. Prior research has identified theories which include CU promoting increases in anxiety symptoms and disorder development (Arendt et al., 2007), anxiety promoting “self-medicating” through CU (Stewart, Karp, Pihl, & Peterson, 1997), and confounding sources that exert simultaneous influence on both, CU and anxiety (McNally, 1996). A review of 15 studies on CU and anxiety highlighted the divide in the field, with seven studies reporting an anxiogenic effect, five reporting an anxiolytic effect, and two reporting no significant effect of CU on anxiety levels (Crippa et al., 2009). Although these findings highlight conflicting results in the literature, modern data analytic techniques may help to disentangle the nature of this association. Clarifying these associations during adolescence remains particularly important, considering that anxiety and CU are known to undergo dramatic changes during this period as compared to other stages of development (Patton et al., 2002).
Using data from a sample of adolescents participating in an ongoing longitudinal study, the current investigation aimed to examine: (1) whether early symptoms of adolescent anxiety predict subsequent changes in CU over time, (2) whether early adolescent CU predicts subsequent changes in anxiety symptoms over time, and (3) whether initial levels and the developmental course of CU and anxiety symptoms covary across time. To our knowledge, this is the first study to employ latent growth curve models (LGCM) and a parallel process analysis to study longitudinal associations between adolescent CU and anxiety; through the use of continuous measures of anxiety symptoms and CU. We hypothesized that higher levels of initial anxiety would be associated with increases in CU growth over time, whereas higher initial levels of CU would be related to increases in anxiety symptom endorsement over time. In addition, we also anticipated that anxiety and CU would increase concurrently across the study period
1.2 Methods
1.2.1 Partic ipants and Proc edures
Participants were the first 250 adolescents to complete a baseline evaluation and two follow-up visits (each approximately 6 months apart) as part of an ongoing longitudinal investigation examining associations between decision-making and CU trajectories (R01 DA031176). Participants in the parent project were youths between the ages of 14-17 at the time of their baseline evaluation, recruited primarily via informational flyers distributed at local High Schools/Junior Highs, and word-of-mouth in the greater Miami area. Eligibility for inclusion was ascertained via phone screen. To obtain a sample consisting predominantly of youth at risk for CU escalation, inclusion criteria consisted of exposure to either alcohol, cigarettes, cannabis, or other drugs (even if only minimal; >1 time) and ability to read and write in English. Exclusion criteria included self-reported developmental disorders, neurological conditions, birth complications, history of mood or thought disorder, traumatic brain injury or loss of consciousness > 10 minutes, history of significant alcohol use or substance use suggestive of an alcohol use disorder (AUD) or CUD, use of other drugs (besides alcohol, cannabis, and nicotine) more than 10 times, use of any other drugs in the two weeks prior to assessment, and use of any other drug to an extent greater than cannabis (although a small percentage of the sample, ≈10% was allowed to have no history of use).
The sample consisted predominantly of Hispanic youth, reflecting the demographics of the greater Miami metropolitan area, with relatively low levels of nicotine, alcohol, and cannabis use at baseline. Despite overall low levels of cannabis use across the study sample, there was significant variability in reported levels of cannabis use among participants (i.e., range from 0 to 60 joints within the past 30-days). Few participants met criteria for a mental health disorder at baseline based the Computerized Diagnostic and Interview Schedule for Children (Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000). Detailed participant characteristics are presented in Table A.1.
Table A.1. Participant demographics and characteristics (N=250).
| Demographics | T1 | T2 | T3 |
|---|---|---|---|
| Sex (% male) | 56.4 | ||
| Age (years), mean (SD) | 15.41 (.72) | ||
| Ethnicity/Race (%) | |||
| White (%) | 4.4 | ||
| Black(%) | |||
| Hispanic (%) | |||
| Other (%) | 1.2 | ||
| Years of education, mean (SD) | 9.27 (.87) | ||
| Years of maternal education, mean (SD) | 15.27 (2.25) | ||
| WRAT-4 Reading Standard Score | 108.88 | ||
|
| |||
| Substance Use | |||
|
| |||
| Use in the last 30 days [Md, Range] | |||
| Cannabis (days) | 1 (30) | 1 (30) | 1 (30) |
| Cannabis (joints) | 3 (60) | 1 (65.35) | 2 (60) |
| Alcohol (days) | 0 (20) | 0 (12) | 0 (15) |
| Alcohol (drinks) | 1 (60) | 0 (80) | 1 (68) |
| Tobacco (days) | 0 (30) | 0 (30) | 0 (30) |
| Tobacco (cigarettes) | 0 (90) | 0 (275) | 0 (360) |
| Current cannabis use disorder (%) | 5.6% | 8.8.% | 14.0% |
|
| |||
| Mental Health | |||
|
| |||
| DASS-21 Depression Raw (Md, range) | 1 (17) | 1 (15) | 1(20) |
| DASS-21 Anxiety Raw (Md, range) | 2 (21) | 2 (17) | 1 (15) |
| Current panic disorder diagnosis (%) | 2.8 | - | 3.3 |
| Current GAD diagnosis (%) | 2.8 | - | 1.7 |
| Current OCD diagnosis (%) | 14.4 | - | 5.8 |
| Current major depression diagnosis (%) | 4 | - | 4.1 |
| Current mania diagnosis (%) | 0 | - | 3.3 |
| Current ADHD diagnosis (%) | 1.6 | - | 1.2 |
| Current ODD diagnosis (%) | 3.6 | - | 2.1 |
| Current conduct disorder diagnosis (%) | 9.2 | - | 7.1 |
Note: WRAT-4, Wide Range Achievement Test; DASS-21, De Anxietess, Scale, 21-item version; GAD, Generalized Anxiety Disorder; OCD, Obsessive Compulsive Disorder; ADHD, Attention-Deficit/Hyperactivity Disorder; ODD, Oppositional Defiant Disorder; Mental health diagnoses presented were determined with the Computerized Diagnostic Interview Schedule for Children at baseline and T3.
Participants completed a detailed assessment by trained examiners. The baseline and 1-year follow-up assessments were conducted at the laboratory during a visit lasting approximately 3 hours. The 6-month follow-up was conducted through a telephone call (approximately 30 minutes) which focused on past 6-month substance use and mood. The Institutional Review Board at the Florida International University approved the study, and both written adolescent assent and parental consent were obtained before baseline assessment. Participants were compensated with monetary incentives for each respective assessment (i.e., Baseline through 1-year follow-up).
1.2.2 Measures
Substance use
Detailed substance use history was obtained using the Drug-Use History Questionnaire (DUHQ; Gonzalez et al., 2012; Rippeth et al., 2004), a semi-structured interview assessing self-reported frequency and quantity of use of 15 different classes of substances during a participant's lifetime, the past 6 months, and the past 30 days. CU frequency (i.e., number of days used) in the past 30 days was the primary CU measure in our analyses.
Anxiety and Mental Health
Anxiety symptoms were assessed using the short version of the Depression, Anxiety, Stress Scale (DASS-21; Henry & Crawford, 2005), a 21-item self-report questionnaire used to assess symptoms of depression, anxiety, and stress during the past week. Items are rated on a 4-point Likert-type scale, ranging from 0 (“Did not apply to me at all”) to 3 (“Applied to me very much, or most of the time”). Evidence of reliability and validity of the DASS-21 (α=.81; 95% CI:.79-.84) among adolescents has been well established (Osman et al., 2012; Szabo, 2010). Seven statements assessing anxiety included awareness of dryness of mouth, breathing problems, action of heart, trembling, feeling worried, close to panic, and scared. The current study used this measure's 7-item anxiety subscale total raw scores as our primary measure of anxiety and the depression subscale total raw score as a covariate for all subsequent analyses (Willemsen et al., 2011).
1.2.3 Data Analytic Plan
Latent growth curve modeling was used to identify trajectories of anxiety symptoms (i.e., DASS-21 anxiety subscale) and CU (i.e., past 30-day frequency) across three bi-annual assessments. Total raw scores from the DASS-21 anxiety subscale and past 30-day CU frequency were used as the dependent variables. First, unconditional (univariate) linear growth curve models were fit separately for anxiety symptoms and CU. Subsequent to establishing appropriate model fit and examining each of these growth curves individually, we then combined these two processes within a single multivariate (i.e., parallel process) LGCM for simultaneous estimation. Theoretically relevant confounds (i.e., sex, depression, alcohol use, and nicotine use) that exhibited significant associations with anxiety and CU were also included as covariates in these analyses (Cummings et al., 2014; Farrell et al., 2001; Brady & Kendall, 1992). Finally, sex and time-varying depression, alcohol use, and nicotine use were added as covariates to the parallel process model to examine their influence on the joint associations between the anxiety symptoms and CU.
All models used maximum likelihood estimation with standard errors and a chi-square statistic which are robust to non-normality (MLR) in Mplus 7.2 (Muthen & Muthen, 1998–2012). Model fit was assessed using both absolute and relative fit indices. Absolute fit indices used to examine model fit included the Comparative Fit Index (CFI) and the root mean square error of approximation (RMSEA). Cutoff values of .90 or greater were used to indicate acceptable fit, and .95 or greater to indicate excellent fit, for CFI (Hu & Bentler, 1999; McDonald & Ho, 2002). RMSEA values between .05 and .10 were considered to represent an acceptable fit, whereas values less than .05 were considered to indicate excellent fit (McDonald & Ho, 2002). Relative fit indices including sample-size adjusted Bayesian Information Criterion (BIC), and the Akaike Information Criterion (AIC) were also used to investigate model fit.
Retention rates remained high across each of the assessment waves in the current study (> 97%). Full-information maximum likelihood (FIML) estimates were used to handle missing data, as this procedure uses all available data points to construct parameter estimates under the assumption that the data are missing at random. Even when data are not missing at random, FIML estimation tends to produce less biased estimates than more traditional techniques for handling missing data (Enders & Bandalos, 2001).
1.3 Results
1.3.1 Descriptive analyses
Descriptive information for primary study variables is presented in Table A.1. ANOVA and chi-square analysis was used to examine baseline sex differences on all characteristics presented in Table A.1. Males and females showed no significant differences on any of the variables (p > .05).
1.3.2 Developmental trajectories of anxiety symptoms and CU
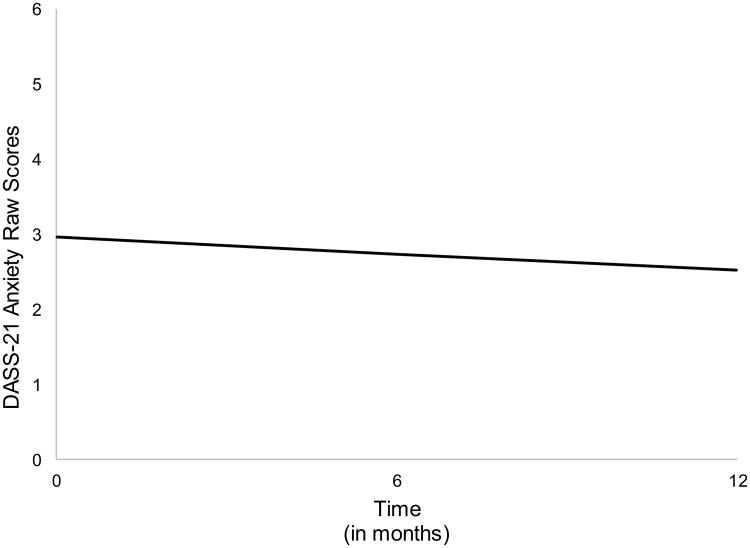
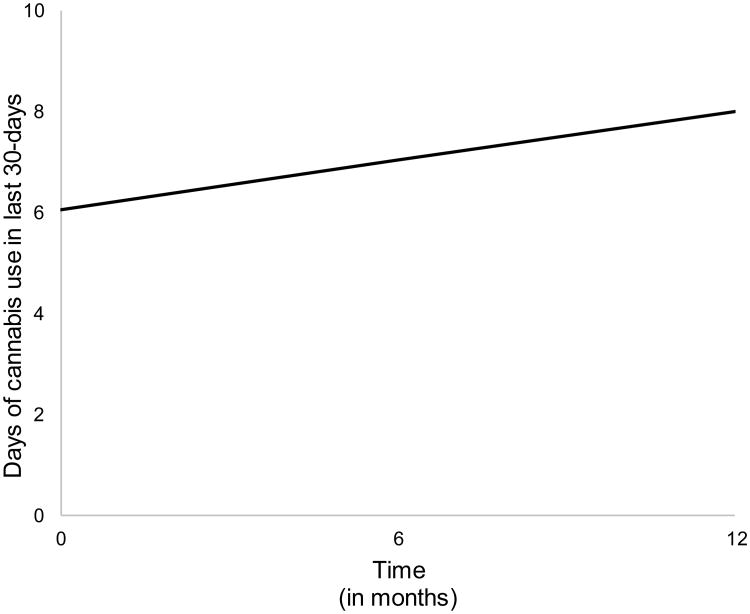
An examination of the separate unconditional LGCMs for anxiety and CU shows both models to provide an acceptable fit to the data (Table A.2). Further inspection of these model parameter estimates reveals a small systematic decrease in mean-levels of anxiety across time (p =.051). However, there was no evidence of within-individual variability in participants' rates of change (b=.201, p=.837). Taken together, this indicates that on average, participants' anxiety symptoms decreased slightly during the study period and that this pattern was relatively consistent across participants. In contrast to anxiety symptoms, there was a systematic increase in the mean level of CU over the study period (b=.97, p=.021. However, as with the anxiety symptom LGCM, there was no evidence of significant within-individual variability in growth rates of CU. That is, participants tended to exhibit a similar increasing trend of CU throughout the study window (see Table A.2).
Table A.2. Unconditional Anxiety Symptomology and Cannabis Frequency Growth Models.
| X2 | df | CFI | RMSEA | AIC | ΔBIC | Intercept x | Slope x | Intercept σ2 | Slope σ2 | Cov (I/S) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AS | 3.64 | 1 | .947 | .103 | 3645.91 | 3674.08 | 2.97** | -0.23* | 5.82* | 0.21 | 4.24** |
| CU | 1.743 | 1 | .995 | .055 | 4813.52 | 4841.63 | 6.07** | 0.97* | 45.70* | 4.53 | 82.51* |
Note:
= p-value < 0.05,
= p-value < 0.01
1.3.3 Joint development of anxiety symptoms and CU
Subsequent to inspecting the univariate anxiety symptom and CU growth curves separately, these processes were modeled simultaneously within a parallel process (i.e., multivariate) LGCM framework to investigate their joint development (see Figure A.1). Results from the parallel process revealed no significant association between initial levels of anxiety and CU (b=.01, p=.978), i.e., levels of anxiety and CU were not significantly associated at baseline. In addition, there was no evidence that initial levels of anxiety influenced change in CU over time (b=-.15, p=.661). However, findings did reveal a significant positive association between initial levels of CU and subsequent changes in anxiety (b=.28, p=.024), such that those reporting greater initial levels of CU at baseline displayed a more gradual rate of decline in their anxiety symptoms over time. Importantly, this association remained significant even when accounting for participants' sex, depression and use of alcohol and nicotine. The lack of significant variability of within-individual anxiety symptom growth over time, precluded us from conducting bidirectional slope growth analysis. Thus, examining the association between rate of change in CU and rate of change in anxiety symptoms (Aim 3) was not possible.
Figure A.1.
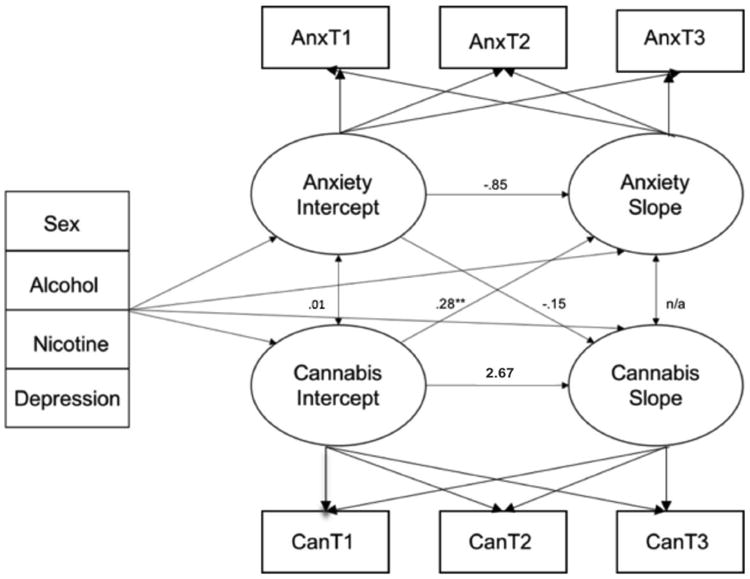
Parallel-process latent growth model of anxiety symptoms and CU frequency with arrows representing directional paths between latent variables and covariates sex, alcohol, nicotine, and depression (N=250). Displayed estimates are specific aims tested.
1.4 Discussion
This study examined the co-development of anxiety symptoms and CU among participants in mid-adolescence. Findings revealed that higher levels of CU assessed at baseline were associated with slower rates of decline in anxiety symptoms across the study window. This association was not accounted for by the influence of alcohol, nicotine, depression, or sex. In contrast, no association was observed between initial levels of anxiety and CU growth over time. This suggests that CU may have a greater prospective influence on anxiety symptoms, than initial levels of anxiety have on prospective cannabis use. Our analyses extend extant research by adding a dual process analysis within a singular model to examine potential directionality of associations.
More specifically, these findings suggest that adolescent CU may influence developmental patterns of anxiety. Amount of initial CU affected the rate of this change, with those reporting higher levels of CU at baseline showing less decrease in anxiety symptomatology over time. These findings are consistent with prior research indicating that adolescents with high rates of anxiety-related symptoms, daily CU, and prior psychopathology are more likely to have more symptoms of anxiety over time (Patton et. al, 2002; Comeau et al., 2001). A cross-sectional study of over 10,000 Australian adults found associations between CU and increased mental health problems. However, despite these associations, when confounding variables were introduced into their statistical models, the associations between cannabis use and anxiety waned or dissipated (Degenhardt et al., 2001); unlike our results which were maintained with the addition of potentially confounding variables including other substance use and depression.
Additionally, evidence found within animal literature and human PET studies suggest chronic cannabinoid exposure during adolescence and adulthood is associated with downregulation of CB1 receptors in the CNS (Hirvonen et al., 2012; Dalton & Zavitsanou, 2010). However, dose dependent downregulation of CB1 receptors has been correlated with both anxiogenic and anxiolytic effects (Witkin et al., 2005).
Our sample replicates earlier findings of decreases in anxiety symptomology across adolescence (Hale et al., 2008). Although the present study found support for the influence of initial levels of CU on rate of change in anxiety, anxiety was not found to significantly predict subsequent CU. Nevertheless, the absence of a bidirectional association provides useful insights. The self-medicating hypothesis, which indicates a person's use escalates due to preexisting psychopathology (in this case anxiety), was not supported among our participants. Additionally, it is worth noting that our sample consisted of adolescents who were not specifically selected for high levels of anxiety symptoms, and prevalence of anxiety disorder diagnoses were low. Consequently, our results may not generalize to older, more anxious individuals, with greater and lengthier histories of CU.
Examining the associations between adolescent anxiety and CU is essential in prevention and treatment efforts. Prior research suggests that adolescents with higher levels of substance use are at increased risk of developing a mental disorder such as an anxiety disorder (Grant et al., 2004). Overall, our findings suggest that CU levels influence the risk for higher levels of adolescent anxiety over time. Prevention efforts targeted at identifying anxious youths should consider screening for CU amount and frequency. In addition, the present findings suggest that prevention and targeted intervention programs for problematic CU in adolescence would do well to incorporate anxiety management strategies in order to reduce subsequent anxiety associated with CU. Such efforts will be critical to aid in reducing the persistence of anxiety in adolescent cannabis users.
Further consideration of the limitations of our study should be carefully reviewed. First symptoms of anxiety were assessed using the anxiety subscale of the DASS-21. Although, this measure is routinely used in the assessment of anxiety and anxiety related symptoms, it may not sufficiently capture the complexity of the anxiety disorder continuum, particularly facets of social anxiety which have generally demonstrated the strongest correlations with adolescent CU. Youths in this study were assessed at three bi-annual waves, which may be too small a window to assess more substantive changes in anxiety symptomology stemming from prolonged CU. In addition, CU was assessed based on the frequency of use during the past 30 days, which may not fully encompass other important aspects of use such as amount, potency, and type of cannabis. Additionally, while confounds included in our statistical models were carefully guided by prior research, we acknowledge that this list is not exhaustive and it is possible that other third variable associations (e.g., other drug use [stimulant, sedative, or opiate use], genetic vulnerability, neuromaturation, environmental influences, etc.) may influence the relationship between CU and anxiety and should be explored in future investigations.
Our recruitment strategy for study participants relied heavily on the distribution of flyers at local Middle and High Schools throughout Miami-Dade County and via word-of mouth. Although successful in obtaining our target sample, it is important to consider that this is not a representative nor random sample of the population. Finally, our sample consisted predominantly of Hispanic/Latino adolescents who had already experimented with alcohol, cigarettes, cannabis, and/or other drugs, which may limit the generalizability of our findings to other populations, such as those yet to initiate substance use. Future studies that replicate these findings across diverse samples, larger follow-up windows, and using measures that tap into other facets of anxiety and CU are essential. Furthermore, future investigations examining these associations among CU subgroups may aid in identifying those most at-risk.
1.5 Conclusions
Overall, our results suggest that higher levels of CU among adolescents place them at greater risk for maintaining higher levels of anxiety over time. Specifically, the relationship identified between initial levels of CU and change over time in anxiety symptomology lends further support to theoretical models that suggest that substance use may have long-term impacts on mental health. The results presented here help further inform clinical decision making for mental health professionals treating adolescents with anxiety disorder symptoms, in regards to course and expected outcomes when cannabis use may occur. Furthermore, prevention of problematic CU in early adolescence may have important secondary effects on preventing the onset of anxiety disorders. Future studies should continue to employ longitudinal protocols that examine CU and anxiety across longer assessment windows and samples that are more diverse.
Figure A.2.
Growth model trajectory of the DASS-21 anxiety subscale raw scores over the three study time points (i.e., baseline, 6-month, and 1-year follow-up).
Figure A.3.
Growth model trajectory of frequency of cannabis use within the last 30-days across the three study time points (i.e., baseline, 6-month, and 1-year follow-up). Overall, adolescents within our sample showed increases in cannabis use across a one-year period.
Highlights.
Joint development of adolescent anxiety and cannabis use are examined
Greater baseline cannabis use associated with slower decreases in anxiety
No evidence to suggest baseline anxiety influenced subsequent cannabis use
Findings persisted when accounting for participant sex and other confounders
Acknowledgments
This work was supported by grants R01 DA031176, R01 DA033156 (PI:Gonzalez) from the National Institute of Drug Abuse. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of Funding: This work was supported by grants R01 DA031176, R01 DA033156, and CNS-1532061 (PI: Gonzalez) from the National Institute of Drug Abuse. This funding agency had no role in study design or data collection.
Footnotes
Contributors: JCD, SH, CQ, IPC, JC, and RG were involved in the development of the research questions, study design, statistical analysis, interpretation of findings, and manuscript writing. All authors contributed to and have approved the final manuscript.
Conflict of Interests: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42-item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychological Assessment. 1998;10(2):176. [Google Scholar]
- Arendt M, Rosenberg R, Fjordback L, Brandholdt J, Foldager L, Sher L, Munk-Jørgensen P. Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychological Medicine. 2007;37(07):935–945. doi: 10.1017/S0033291706009688. [DOI] [PubMed] [Google Scholar]
- Atakan Z. Cannabis, a complex plant: different compounds and different effects on individuals. Therapeutic Advances in Psychopharmacology. 2012;2(6):241–254. doi: 10.1177/2045125312457586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and Anxiety Disorders in Children and Adolescents: Developmental Issues and Implications for DSM-V. The Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady EU, Kendall PC. Comorbidity of anxiety and depression in children and adolescents. Psychological bulletin. 1992;111(2):244. doi: 10.1037/0033-2909.111.2.244. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. In: Alternative Ways of Assessing Model Fit in Testing Structural Equation Models. Bollen KA, Long JS, editors. 1993. pp. 136–162. [Google Scholar]
- Buckner JD, Mallott MA, Schmidt NB, Taylor J. Peer influence and gender differences in problematic cannabis use among individuals with social anxiety. Journal of Anxiety Disorders. 2006;20(8):1087–1102. doi: 10.1016/j.janxdis.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Bobadilla L, Taylor J. Social anxiety and problematic cannabis use: evaluating the moderating role of stress reactivity and perceived coping. Behaviour Research and Therapy. 2006;44(7):1007–1015. doi: 10.1016/j.brat.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Bonn-Miller MO, Zvolensky MJ, Schmidt NB. Marijuana use motives and social anxiety among marijuana-using young adults. Addictive behaviors. 2007;32(10):2238–2252. doi: 10.1016/j.addbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. Journal of Psychiatric Research. 2008;42(3):230–239. doi: 10.1016/j.jpsychires.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Heimberg RG, Schneier FR, Liu SM, Wang S, Blanco C. The relationship between cannabis use disorders and social anxiety disorder in the National Epidemiological Study of Alcohol and Related Conditions (NESARC) Drug and Alcohol Dependence. 2012;124(1):128–134. doi: 10.1016/j.drugalcdep.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulkins JP, Kilmer B, Kleiman MA, MacCoun RJ, Midgette G, Oglesby P, Reuter PH. Considering marijuana legalization. [Retrieved April, 15, 2016];Santa Monica, CA: RAND. 2015 [Google Scholar]
- Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling. 2003;10(2):238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeau N, Stewart SH, Loba P. The relations of trait anxiety, anxiety sensitivity, and sensation seeking to adolescents' motivations for alcohol, cigarette, and marijuana use. Addictive Behaviors. 2001;26(6):803–825. doi: 10.1016/s0306-4603(01)00238-6. [DOI] [PubMed] [Google Scholar]
- Conway KP, Swendsen J, Husky MM, He JP, Merikangas KR. Association of lifetime mental disorders and subsequent alcohol and illicit drug use: results from the National Comorbidity Survey–Adolescent Supplement. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(4):280–288. doi: 10.1016/j.jaac.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychology of Addictive Behaviors. 2011;25(3):554. doi: 10.1037/a0023076. [DOI] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin. 2014;140(3):816–845. doi: 10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton VS, Zavitsanou K. Cannabinoid effects on CB1 receptor density in the adolescent brain: an autoradiographic study using the synthetic cannabinoid HU210. Synapse. 2010;64(11):845–854. doi: 10.1002/syn.20801. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M. Alcohol, cannabis and tobacco use among Australians: a comparison of their associations with other drug use and use disorders, affective and anxiety disorders, and psychosis. Addiction. 2001;96(11):1603–1614. doi: 10.1046/j.1360-0443.2001.961116037.x. [DOI] [PubMed] [Google Scholar]
- de Lijster JM, Dierckx B, Utens EM, Verhulst FC, Zieldorff C, Dieleman GC, Legerstee JS. The Age of Onset of Anxiety Disorders A Meta-analysis. The Canadian Journal of Psychiatry. 2016 doi: 10.1177/0706743716640757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8(3):430–457. [Google Scholar]
- Farrell M, Howes S, Bebbington P, Brugha T, Jenkins R, Lewis G, Meltzer H. Nicotine, alcohol and drug dependence and psychiatric comorbidity. The British Journal of Psychiatry. 2001;179(5):432–437. doi: 10.1192/bjp.179.5.432. [DOI] [PubMed] [Google Scholar]
- Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and mood disorders: a longitudinal study. Journal of Affective Disorders. 2016;172:211–218. doi: 10.1016/j.jad.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Fleming CB, Mason WA, Mazza JJ, Abbott RD, Catalano RF. Latent growth modeling of the relationship between depressive symptoms and substance use during adolescence. Psychology of Addictive Behaviors. 2008;22(2):186–197. doi: 10.1037/0893-164X.22.2.186. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Schuster RM, Mermelstein RJ, Vassileva J, Martin EM, Diviak KR. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. Journal of Clinical and Experimental Neuropsychology. 2012;34(9):962–976. doi: 10.1080/13803395.2012.703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Archives of general psychiatry. 2004;61(8):807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- Green BOB, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug and Alcohol Review. 2003;22(4):453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Hale WW, Raaijmakers Q, Muris P, Meeus W. Developmental trajectories of adolescent anxiety disorder symptoms: A 5-year prospective community study. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(5):556–564. doi: 10.1097/CHI.0b013e3181676583. [DOI] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. The Lancet. 1998;352(9140):1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, Van Os J. Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people. Bmj. 2004;330(7481):11. doi: 10.1136/bmj.38267.664086.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2005;44(2):227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, Innis RB. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cut-off criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. Publisher Full Text Educational Psychologist, 27(1), 65-90. [Google Scholar]
- Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975-2015: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, The University of Michigan; 2016. [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addiction Biology. 2008;13(2):264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Quintero C, de los Cobos JP, Hasin DS, Okuda M, Wang S, Grant BF, Blanco C. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Drug and alcohol dependence. 2011 doi: 10.1016/j.drugalcdep.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey MT, Fergusson DM, Horwood LJ. The origins of the correlations between tobacco, alcohol, and cannabis use during adolescence. Journal of Child Psychology and Psychiatry. 1998;39(07):995–1005. [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- McGee R, Williams S, Poulton R, Moffitt T. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction. 2000;95(4):491–503. doi: 10.1046/j.1360-0443.2000.9544912.x. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity is distinguishable from trait anxiety. In: Rapee RM, editor. Current Controversies in the Anxiety Disorders. 1996. pp. 214–227. [Google Scholar]
- Milich R, Lynam D, Zimmerman R, Logan TK, Martin C, Leukefeld C, Clayton R. Differences in young adult psychopathology among drug abstainers, experimenters, and frequent users. Journal of Substance Abuse. 2000;11(1):69–88. doi: 10.1016/s0899-3289(99)00021-8. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BOBO. Mplus User's Guide. 1998-2012;7 [Google Scholar]
- National Institute on Drug Abuse. High School and Youth Trends. Retrieved from https://www.drugabuse.gov/publications/drugfacts/high-school-youth-trends on August 26, 2016.
- O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. Journal of Psychopharmacology. 2004;18(4):502–508. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- O'Shea M, McGregor IS, Mallet PE. Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. Journal of Psychopharmacology. 2006;20(5):611–621. doi: 10.1177/0269881106065188. [DOI] [PubMed] [Google Scholar]
- Osman A, Wong JL, Bagge CL, Freedenthal S, Gutierrez PM, Lozano G. The depression anxiety stress Scales—21 (DASS-21): further examination of dimensions, scale reliability, and correlates. Journal of Clinical Psychology. 2012;68(12):1322–1338. doi: 10.1002/jclp.21908. [DOI] [PubMed] [Google Scholar]
- Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. Bmj. 2002;325(7374):1195–1198. doi: 10.1136/bmj.325.7374.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JM, Graziano P, Pacheco-Colón I, Coxe S, Gonzalez R. Journal of the International Neuropsychological Society: J INS. 2016. Decision-Making Does not Moderate the Association between Cannabis Use and Body Mass Index among Adolescent Cannabis Users; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Desai RA, Cavallo DA, Smith AE, McFetridge A, Liss TB, Krishnan-Sarin S. Gender differences in adolescent marijuana use and associated psychosocial characteristics. Journal of Addiction Medicine. 2011;5(1):65. doi: 10.1097/ADM.0b013e3181d8dc62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Stein MB, Stein DJ. Social anxiety disorder. The Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. Journal of substance abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Szabó M. The short version of the Depression Anxiety Stress Scales (DASS-21): Factor structure in a young adolescent sample. Journal of Adolescence. 2010;33(1):1–8. doi: 10.1016/j.adolescence.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Szuster RR, Pontius EB, Campos PE. Marijuana sensitivity and panic anxiety. Journal of Clinical Psychiatry. 1988 [PubMed] [Google Scholar]
- Tournier M, Sorbara F, Gindre C, Swendsen JD, Verdoux H. Cannabis use and anxiety in daily life: a naturalistic investigation in a non-clinical population. Psychiatry Research. 2003;118(1):1–8. doi: 10.1016/s0165-1781(03)00052-0. [DOI] [PubMed] [Google Scholar]
- Van Oort FVA, Greaves-Lord K, Verhulst FC, Ormel J, Huizink AC. The developmental course of anxiety symptoms during adolescence: the TRAILS study. Journal of Child Psychology and Psychiatry. 2009;50(10):1209–1217. doi: 10.1111/j.1469-7610.2009.02092.x. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacology Biochemistry and Behavior. 2005;81(2):331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Fröhlich C, Behrendt S, Günther A, Rehm J, Zimmermann P, Perkonigg A. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug and alcohol dependence. 2007;88:S60–S70. doi: 10.1016/j.drugalcdep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behavioural Pharmacology. 2005;16(5-6):315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- Wu P, Goodwin R, Comer JS, Hoven C, Cohen P. The relationship between anxiety disorders and substance use among adolescents in the community: Specificity and gender differences. Journal of Youth and Adolescence. 2010;39:177–188. doi: 10.1007/s10964-008-9385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen J, Markey S, Declercq F, Vanheule S. Negative emotionality in a large community sample of adolescents: the factor structure and measurement invariance of the short version of the depression anxiety stress scales (DASS-21) Stress and Health. 2011;27(3):e120–e128. [Google Scholar]
- Zvolensky MJ, Bernstein A, Sachs-Ericsson N, Schmidt NB, Buckner JD, Bonn-Miller MO. Lifetime associations between cannabis, use, abuse, and dependence and panic attacks in a representative sample. Journal of Psychiatric Research. 2006;40(6):477–486. doi: 10.1016/j.jpsychires.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Lewinsohn P, Bernstein A, Schmidt NB, Buckner JD, Seeley J, Bonn-Miller MO. Prospective associations between cannabis use, abuse, and dependence and panic attacks and disorder. Journal of Psychiatric Research. 2008;42(12):1017–1023. doi: 10.1016/j.jpsychires.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Cougle JR, Johnson KA, Bonn-Miller MO, Bernstein A. Marijuana use and panic psychopathology among a representative sample of adults. Experimental and Clinical Psychopharmacology. 2010;18(2):129. doi: 10.1037/a0019022. [DOI] [PMC free article] [PubMed] [Google Scholar]