Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social deficits and repetitive behaviors that typically emerge by 24 months of age. To develop effective early interventions that can potentially ameliorate the defining deficits of ASD and improve long-term outcomes, early detection is essential. Using prospective neuroimaging of 59 6-month-old infants with a high familial risk for ASD, we show that functional connectivity magnetic resonance imaging correctly identified which individual children would receive a research clinical best-estimate diagnosis of ASD at 24 months of age. Functional brain connections were defined in 6-month-old infants that correlated with 24-month scores on measures of social behavior, language, motor development, and repetitive behavior, which are all features common to the diagnosis of ASD. A fully cross-validated machine learning algorithm applied at age 6 months had a positive predictive value of 100% [95% confidence interval (CI), 62.9 to 100], correctly predicting 9 of 11 infants who received a diagnosis of ASD at 24 months (sensitivity, 81.8%; 95% CI, 47.8 to 96.8). All 48 6-month-old infants who were not diagnosed with ASD were correctly classified [specificity, 100% (95% CI, 90.8 to 100); negative predictive value, 96.0% (95% CI, 85.1 to 99.3)]. These findings have clinical implications for early risk assessment and the feasibility of developing early preventative interventions for ASD.
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by deficits in social behavior and the presence of restrictive and repetitive behaviors (1). It is estimated that 1 in 68 children are affected by the disorder (2), and despite tremendous research efforts, ASD still confers substantial burden to affected individuals, their families, and the community (3, 4). Intervention is critically important, and there is a general consensus that early detection paired with early intervention would have a significant impact on improving outcomes (5–7).
One barrier to early (that is, before 24 months) detection is that the defining behavioral characteristics of ASD generally unfold during the second year of life, typically showing consolidation of the full behavioral syndrome by about 24 months of age or later (8, 9). Behavioral differences in ASD have been observed as early as 6 months of age in characteristics such as gross motor ability, visual reception, and patterns of eye tracking (10–14); however, these associated characteristics have not been able to predict which children will later receive a diagnosis. Given the known plasticity of the brain and behavior during the first year of life, together with the absence of the defining features of the disorder, intervention during this presymptomatic phase, before consolidation of the full syndrome of ASD, is likely to show considerably stronger benefits compared with later treatments (5).
Research on neurodegenerative disorders has shown that changes in the brain are often seen preceding clinical manifestations. For example, in Parkinson's disease, about 50% of the neurons in the substantia nigra are lost before clinical features become apparent (15). This suggests that brain-related changes appear earlier than behavioral changes and may be useful in predicting future behavioral diagnosis. In ASD, a number of selected morphological (16–18) and electrophysiological (19) brain differences have been reported as early as 6 months of age in infants later diagnosed with ASD; however, the reported group differences in these specific brain structures have not yet shown the sensitivity and specificity required to be effective for the early detection of ASD.
Given the complexity and heterogeneity of ASD, methods for the early detection of ASD using brain metrics will likely require information that is multivariate, complex, and developmentally sensitive. Recent research using functional connectivity magnetic resonance imaging (fcMRI) has linked the functional organization of the human brain to individual cognitive profiles (20–22). These measures of brain functional connectivity are reliable (23) and can accommodate participants as young as neonates (24). Furthermore, in conjunction with machine learning approaches, fcMRI data have provided predictions of brain maturation (25, 26) and diagnostic category (27–32) at the single-subject level. By training machine learning algorithms to identify underlying patterns that separate individuals into these different groups, researchers can predict which group a new individual will likely be in (33).
Here, we postulated that brain functional connectivity at 6 months of age would capture the complexity of ASD and provide a robust method for predicting later diagnosis. Our results revealed that machine learning, applied to fcMRI data at 6 months of age in infants at high familial risk for ASD, can accurately predict an ASD diagnosis at 24 months of age.
Results
A cohort of 59 infants with a high familial risk for ASD was included in this study. There were 11 infants diagnosed with ASD at 24 months of age and 48 infants who did not have ASD at 24 months of age. Prospective neuroimaging data were collected from each infant at 6 months of age while they were sleeping naturally. Cognitive, behavioral, and diagnostic assessments were completed at their follow-up visit at 24 months of age.
Brain functional connectivity and infant behavior
A set of 230 regions that were previously defined across the whole brain (see Materials and Methods) was used to create functional connectivity matrices from each participant's functional MRI data at their 6-month visit. This resulted in 26,335 pairs of regions used for further analysis that represented the whole-brain functional organization of an individual infant. From this complete set, two subsets of brain-behavior features were defined separately for visualizing group discrimination and for the classification analysis.
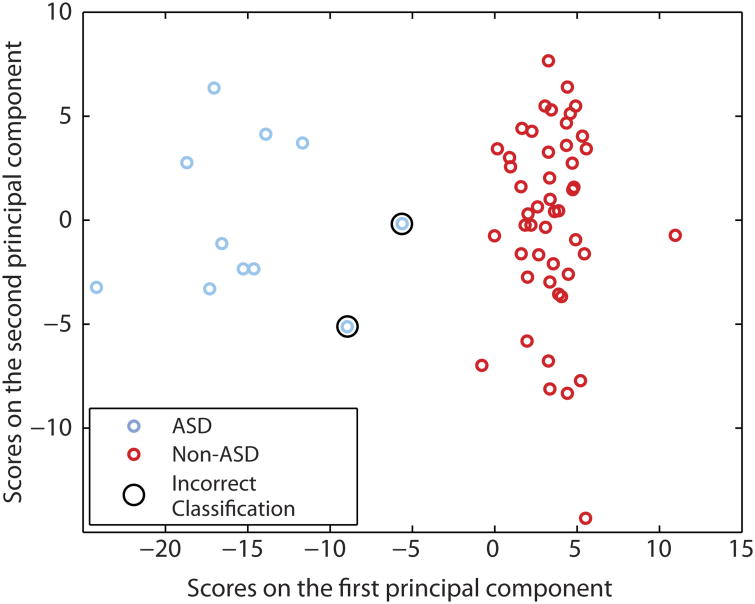
To visualize the ability of early brain features to discriminate between ASD and non-ASD groups of infants, we computed brain-behavior correlations with each participant's 24-month assessment scores of social interactions, communication, motor development, and repetitive behavior. Table 1 shows the average raw scores and SE for each of these behavioral measures by group. In addition, between-groups t tests were calculated across all the functional connections, and the intersection of functional connections that showed both a nominal brain-behavior correlation and a between-groups difference (P < 0.05) was used to define a feature space across all participants. This resulted in a total of 974 functional connections in the 6-month-old brain that showed a relationship with behavior at 24 months and were different between groups. Together, these functional connections constituted <4% of the potential 26,335 total functional connections studied. The participants' scores on the first and second principal components of this feature space were plotted against each other in Fig. 1, revealing an evident linear separation between the ASD and non-ASD groups.
Table 1. Average raw scores for each of the 24-month infant assessments.
SE is shown for both high-risk ASD and non-ASD groups. The number of participants that contributed to each measure is listed in parentheses. The details of the assessments as well as the specific items and subscales are included in Materials and Methods. Behavioral tests included the Repetitive Behaviors Scale–Revised (RBS-R), Mullen Scales of Early Learning (MSEL), and Communication and Symbolic Behavior Scales (CSBS).
| Assessment | n (ASD+/ASD−) | Measure | ASD | Non-ASD |
|---|---|---|---|---|
| CSBS: social communication | 50 (8/42) | Social interaction | 0.75 ± 0.3 | 2.51 ± 0.2 |
| CSBS: social communication | 50 (8/42) | Joint attention | 1.63 ± 0.6 | 3.66 ± 0.3 |
| MSEL: cognitive ability | 57 (10/47) | Expressive language | 18.91 ± 1.4 | 20.82 ± 0.6 |
| MSEL: cognitive ability | 58 (11/47) | Fine motor | 24.08 ± 1.0 | 23.84 ± 0.3 |
| MSEL: cognitive ability | 53 (10/43) | Gross motor | 24.64 ± 0.5 | 25.79 ± 0.4 |
| MSEL: cognitive ability | 57 (10/47) | Visual reception | 20.18 ± 2.0 | 23.58 ± 0.6 |
| MSEL: cognitive ability | 58 (11/47) | Receptive language | 24.42 ± 0.8 | 26.53 ± 0.5 |
| RBS-R: repetitive behavior | 47 (9/38) | Self-injurious | 2.3 ± 1.0 | 0.16 ± 0.1 |
| RBS-R: repetitive behavior | 47 (9/38) | Stereotyped | 3.4 ± 0.8 | 0.31 ± 0.1 |
| RBS-R: repetitive behavior | 47 (9/38) | Sameness | 4.9 ± 0.9 | 0.9 ± 0.2 |
| RBS-R: repetitive behavior | 47 (9/38) | Ritualistic | 2.2 ± 0.5 | 0.5 ± 0.1 |
| RBS-R: repetitive behavior | 47 (9/38) | Compulsive | 3.4 ± 0.4 | 0.4 ± 0.1 |
| RBS-R: repetitive behavior | 47 (9/38) | Repetitive | 1.8 ± 0.9 | 0.41 ± 0.1 |
Fig. 1. Correct classification of 6-month-old infants at high familial risk for ASD using functional connectivity MRI.
Functional connections were selected as those that showed a correlation with at least one of the 24-month ASD-related behaviors, which included measures of social behavior, language, motor development, and repetitive behavior. The top two principal components of the functional connections that showed a correlation with these behaviors are shown for both ASD (blue) and non-ASD (red) 6-month-old infants. The two participants that were incorrectly classified in the leave-one-out nested cross-validation analysis are circled; these two participants were diagnosed with ASD but were classified as non-ASD. Classification was correct for 96.6% of 6-month-old high-risk infants.
Predicting individual 24-month clinical diagnoses
To determine whether 6-month-old functional connectivity features were capable of predicting the clinical diagnostic outcome of an individual infant, we used a fully cross-validated approach with a “nested” leave-one-out procedure. In this procedure, the diagnostic outcome of each infant was predicted from an independent training sample, without being used to define features or build the classifier. The features were chosen within each training sample as showing a brain-behavior correlation, creating a feature space that reflected functional connections in 6-month-old infants that showed a relationship with 24-month-old behaviors. This process created 59 sets of features that were used to train individual classifiers. This procedure (detailed in Materials and Methods) allowed each test infant to be predicted individually, requiring only information from their 6-month-old functional MRI scan.
The classification accuracy using functional connectivity data in 6-month-old infants was 96.6% [95% confidence interval (CI), 87.3 to 99.4; P < 0.001]. Figure S2 marks the observed accuracy of this classification analysis against the null distribution generated using randomized diagnosis labels (see Materials and Methods). Sensitivity of this approach was 81.8% (95% CI, 47.8 to 96.8), and specificity was 100% (95% CI, 90.8 to 100). The probability that infants with a positive classification truly had ASD (positive predictive value) at 24 months was 100% (95% CI, 62.9 to 100). The probability that infants with a negative classification did not have ASD (negative predictive value) at 24 months was 96.0% (95% CI, 85.1 to 99.3). Infants who were incorrectly classified are circled in Fig. 1, within the feature space defined across the whole group. Using the classification feature sets, Fig. 2 presents a subset of the connections that show reduced or increased functional connectivity in 6-month-old infants who developed ASD. These features appear in each of the 59 independent classifiers built during the nested cross-validation procedure (see Materials and Methods) but do not represent the full feature set of any individual classifier.
Fig. 2. Differences in functional connectivity in ASD infants versus non-ASD infants.
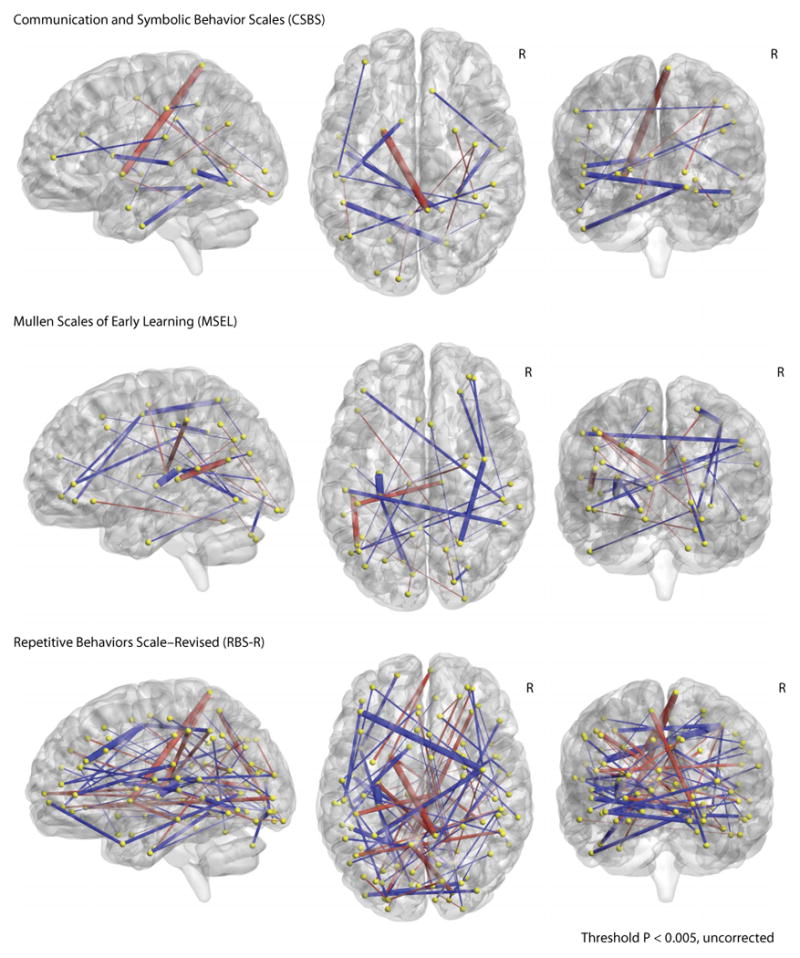
Each panel represents the functional connections that show a relationship to scores on each of the behavioral assessments (see Table 1): CSBS (top), MSEL (middle), and RBS-R (bottom). For each assessment, the functional connections associated with individual measures were combined and projected onto a Talairach brain, with the right hemisphere marked (R). The color and thickness of each connection signify the sign and strength of the t value it represents. Unpaired two-sample t tests were used to test the difference between group means (ASD versus non-ASD) for each functional connection. Red signifies a connection that shows more negative connectivity in the ASD infant group on average, whereas blue signifies more positive connectivity. t values were set to a threshold of P < 0.005 (uncorrected), and the thickness of each bar represents its strength. Coordinates for each sphere are listed in table S1. These calculations are only for visualization and should not be interpreted as differences directly contributing to any individual's classification.
Leave-10-out classification analysis
To test the generalizability and validity of our results, we used a similar classification analysis with a greater number of participants held independent (leave-10-out) to show that our results were fairly robust. On average, the leave-10-out analysis performed with 92.7 ± 0.7% accuracy, indicating that it correctly predicted between 9 and 10 of the 10 independent participants for most of the 1000 iterations and was nearly as accurate as the nested leave-one-out analysis. This result suggests that the classifier may be able to generalize to new samples of infants and is fairly robust.
Discussion
The public health importance of ASD has been increasingly recognized over the last 15 to 20 years (2, 3). Treatment studies have shown modest effects in improving the core characteristics of ASD (34, 35). Research on infants at high familial risk for ASD has revealed a seemingly narrow window of opportunity, before the age of 24 months, when intervention may have the potential to ameliorate the unfolding of the core features of this disorder (5, 6). Intervention studies with infants at high familial risk for ASD (6, 7) suggest that behavioral intervention in the latter part of the first, and early second, year may be more effective than later (post-diagnosis) intervention. Unfortunately, early behavioral markers have not had sufficient power as predictors of later diagnosis to be clinically useful, and so, to date, methods for presymptomatic detection have not been available.
Our results suggest that early brain metrics, identified on the basis of their association with later ASD-related behaviors, are able to accurately predict an individual infant's 24-month diagnosis of ASD, by 6 months of age. We focused on predicting diagnostic outcome at 24 months of age, a time when the full syndrome of ASD begins to consolidate and can be reliably diagnosed (8, 9). These findings converge with another MRI study showing that structural information at 6 and 12 months of age can accurately predict anASD diagnosis (36). Using functional neuroimaging, the current study extends these previous findings by using brain data from a single time point (6 months of age) to accurately predict an ASD diagnosis in 9 of 11 infants at high familial risk for ASD. These findings demonstrate the potential for early detection of autism in infants at high familial risk and serve as a proof of concept that patterns of infant brain measures precede the defining behavioral characteristics of ASD.
Infants with high familial risk for ASD begin life with about a 20% chance of developing ASD (37) compared to ∼1.5% in infants with low or unknown risk (2). Because of the 1 in 68 prevalence of ASD in the population, the clinical application of functional neuroimaging is likely to be the most valuable in evaluating infants at high familial risk. Current intervention research that has focused on the first year of life has been limited to studying entire cohorts of infants at high familial risk, with little to no ability to assess a specific individual's likelihood of receiving a diagnosis beyond the expected recurrence risk. In our sample, even in the lower bound of the CI, the positive predictive value of this classifier shows a higher ability to correctly detect ASD in 6-month-old high-risk infants than has been possible with behavioral screening alone in this age range (38). If these results are replicated in a new high-risk infant cohort, functional neuroimaging at 6 months of age could provide a clinically valuable tool for the detection of ASD in high-risk infants before the development of the full syndrome. This would open the door to randomized controlled trials aimed at identifying effective interventions by recruiting high-risk infants who have been identified as having an even greater risk based on their 6-month neuroimaging assessment.
Although we have taken many precautions to test the internal validity of our classification analysis, there are several limitations that will need to be addressed by future research before the clinical utility of this method can be fully realized. Although our results are strong within this sample of high-risk infants, these findings need to be replicated and extended to an independent high-risk sample of infants. In addition, there is uncertainty associated with a 24-month diagnosis of ASD. Future research will have to address the meaning of this uncertainty with regard to the negative predictive value of the classifier. An effective classifier in the general population would likely require a much larger sample to demonstrate its ability to capture the full breadth of the heterogeneity in ASD. Finally, MRI is likely too expensive to be feasible as a general screening tool; however, as genetic information or more advanced screening techniques become available, neuroimaging may be useful as a secondary confirmation of enhanced risk. If these findings could be generalized to more cost-effective and mobile neuroimaging technologies, it would greatly increase the accessibility of early screening. Even with the limited sample size of the present study, the ability of the classifier to predict an individual infant's later diagnosis is substantial. This high accuracy was maintained when the classifier was trained on a smaller subsample and was used to predict 10 independent infants, suggesting that these results are fairly robust. Therefore, despite the noted limitations, our results suggest that early differences in the brain's functional connections are useful in predicting a later diagnosis of ASD as early as 6 months of age, well before the onset of the defining behavioral characteristics of ASD. As the field begins to incorporate other risk markers (for example, parental or genetic factors, multimodal imaging, and other measures of infant behavior), we will improve our ability to determine which infants may benefit from early functional neuroimaging.
Our results show that functional neuroimaging with 6-month-old infants at high familial risk for ASD can accurately predict which individuals receive a clinical diagnosis of ASD at 24 months of age. Ultimately, this study represents an initial first step toward developing the earliest diagnostic methods available and may yield the clues necessary to build efficacious early interventions based on individual risk profiles.
Materials And Methods
Study design
Participants were part of the Infant Brain Imaging Study (IBIS), an ongoing longitudinal study of infants at low and high familial risk for ASD. Infants were recruited, screened, and assessed at one of four clinical sites: University of North Carolina, University of Washington, The Children's Hospital of Philadelphia, and Washington University in St. Louis. The research protocol was approved by the institutional review board at all clinical sites, and parents provided written informed consent after receiving a detailed description of the study. Data were used for research purposes only.
A cohort of 59 (18/41, female/male) infants at high familial risk for ASD was included in this study: 11 infants diagnosed with ASD at 24 months (11 male) and 48 non-ASD infants at 24 months (30 male). High-risk infants were defined as having at least one sibling with an ASD diagnosis. Participants were excluded for comorbid medical or neurological diagnoses influencing growth, development, or cognition; previous genetic conditions; premature birth or low birth weight; maternal substance abuse during pregnancy; contraindication for MRI; or family history of psychosis, schizophrenia, or bipolar disorder.
Diagnostic testing
All infants included in these analyses participated in a comprehensive battery of behavioral assessments including the Autism Diagnostic Observation Schedule (ADOS) (39) and Autism Diagnostic Interview– Revised (40) at 24 months. The ADOS and all other testing and interview data were independently reviewed by experienced clinicians for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (41) criteria for autistic disorder or pervasive developmental disorder not otherwise specified. All ASD-positive infants were assigned a diagnosis according to clinical best estimate using DSM-IV-TR at 24 months of age.
Cognitive and behavioral assessments
The RBS-R (42, 43) is a parent/caregiver-rated measure covering a broad range of repetitive behaviors. The RBS-R is a questionnaire that focuses exclusively on restricted/repetitive behaviors. It includes 43 items rated on a four-point scale: 0, behavior does not occur; 1, behavior occurs and is a mild problem; 2, behavior occurs and is a moderate problem; 3, behavior occurs and is a severe problem. Items are grouped into six conceptually derived subscales: stereotyped behavior, self-injurious behavior, compulsive behavior, ritualistic behavior, sameness behavior, and restricted behavior. Scores on these subscales were used to determine brain-behavior features used in the analysis.
The MSEL (44) is a standardized, normed, developmental assessment that provides an overall index of cognitive ability and delay. Children were assessed at 24 months of age, and their scores on the receptive language, expressive language, visual reception, fine motor, and gross motor subscales were used to determine brain-behavior features used in the analysis.
The Communication and Symbolic Behavior Scales Developmental Profile (45) is designed to elicit social and communicative behaviors in infants and was administered at each participant's 24-month visit. Specifically, their scores on items measuring initiation of joint attention and social interaction were used to determine brain-behavior features in the primary analysis. These items were chosen to reflect specific aspects of behavior that we reasoned to be particularly relevant to social development at about 2 years of age.
Image acquisition
All scans were acquired at IBIS Network clinical sites using cross-site calibrated 3T Siemens TIM Trio scanners (Siemens Medical Solutions) equipped with standard 12-channel head coils. Images were acquired during natural infant sleep without sedation. The IBIS imaging protocol included anatomical images (T1- and T2-weighted), diffusion tensor images [25-direction and 65-direction HARDI DWI (high–angular resolution diffusion imaging/diffusion-weighted imaging)], and resting-state fcMRI. This study used a three-dimensional T2-weighted sequence [echo time (TE), 497 ms; repetition time (TR), 3200 ms; matrix, 256 × 256 × 160; voxel size, 1 mm3; sagittal acquisition] and a gradient-echo echo planar image functional sequence (TE, 27 ms; TR, 2500 ms; field of view, 256 mm; matrix, 64 × 64; voxel size, 4 × 4 × 4 mm3; flip angle, 90°; bandwidth, 1906 Hz). All included infants provided data collected during at least two fMRI runs, each run comprising 130 temporally contiguous frames (5.4 min).
fMRI preprocessing
Initial fMRI data preprocessing followed previously described procedures (25, 46, 47) including (i) compensation for slice-dependent time shifts using sinc interpolation, (ii) correction of systematic odd-even slice intensity differences caused by interleaved acquisition, and (iii) spatial realignment to compensate for head motion within and across fMRI runs. Atlas registration of the functional data was achieved by a sequence of affine transforms (individual fMRI average volume → individual T2-weighted → atlas-representative target). All data were registered to an age-specific (6-month) target atlas to handle shape differences across developmental age categories (48). The volumetric time series were resampled in atlas space (3-mm3 voxels) using a resampling procedure that applied all affine registration transform and correction for head movement in a single step. Each atlas-transformed functional data set was visually inspected in sagittal, transverse, and coronal views to exclude potential errors not otherwise identified.
Frame censoring
Head motion, even of submillimeter magnitude, has been identified as a nonphysiological source of spurious variance in resting-state fMRI data (49–51). Data were subjected to rigorous frame censoring based on the frame-to-frame displacement (FD) measure 12, which quantifies movement as the sum of the magnitudes of translational movement (X, Y, and Z) and rotational movement (Pitch, Yaw, and Roll) evaluated at a radius of 50 mm. Frames with FD >0.2 mm were marked for subsequent censoring. Temporally isolated frames, where there were fewer than six contiguous frames of FD <0.2 mm, were also censored. Each of the fMRI runs with fewer than 30 uncensored frames was discarded. To control for potential biases attributable to the amount of data per cohort, exactly 150 noncensored frames were used for correlation analysis in each participant, where runs with the largest number of usable frames were prioritized. There was no between-groups difference in the FD [t(57) = 0.43; P = 0.79] or the number of total frames censored [t(57) = 0.45; P = 0.75].
fcMRI preprocessing
In addition to the previously published procedures (52), further preprocessing was conducted before the computation of region-of-interest (ROI) pair time series correlations. Using only the noncensored frames, the data were voxel-wise demeaned and detrended within runs, and nuisance waveforms were regressed out. Nuisance regressors included (i) the time series of three translation (X, Y, and Z) and three rotation (Pitch, Yaw, and Roll) estimates derived by retrospective head motion correction and Volterra expansion derivatives to comprise 24 total motion regressors (53), and (ii) time series derived from the regions of noninterest (whole brain, white matter, and cerebrospinal fluid) and their first derivatives. After nuisance regression, data in frames marked for censoring were replaced by interpolated values computed by least-squares spectral analysis (52, 54). The fMRI data were then temporally filtered to retain frequencies in the 0.009 Hz < f < 0.08 Hz band. As a last step, the data were spatially smoothed using a Gaussian kernel (6 mm full width at half maximum isotropic).
Definition of ROI and correlation computation
Following Pruett et al. (25), candidate ROIs (n = 280) were adopted from a combination of meta-analyses of ASD studies (46) and of task data and cortical functional areal parcellations obtained in healthy adults (47). Three viewers inspected ROI placements in age-specific atlas templates. Of the 280 ROIs, 50 were partially outside the whole-brain mask and were removed, leaving 230 usable ROIs (25). ROI representative time series were calculated as the average of the time series of each voxel intersecting the 10-mm-diameter sphere located at a given ROI center. Pairwise Pearson correlation values were generated from each of the 26,335 possible pairs of ROIs and then Fisher z–transformed to improve normality.
Visualization features and group discrimination
To create the visualizations in Fig. 1, a leave-one-out cross-validation analysis was performed within the entire group of 59 infants to identify a set of functional connections that were both related to behavior and showed differences between groups. We generated 59 sets of features by iteratively removing one participant from the analysis. For each iteration, the remaining 58 participants were used to define region pairs whose connectivity showed both a nominal Pearson correlation with behavior (P < 0.05) and difference between groups (t test, P < 0.05). The final visualization feature space was then defined as the intersection (100% consensus) of these sets. To demonstrate that these features can discriminate between the infant groups, a principal component analysis was used to define the top two dimensions of variance across all participants. Participant's scores on the first and second principal components of this feature space were plotted against each other in Fig. 1. Information from the classification analysis (see below) was included to visualize which of the infants were incorrectly classified; however, the set of visualization features is used only in Fig. 1 to demonstrate that there is variability in the functional connectivity at the group level that can discriminate between infants who receive a diagnosis at 24 months from those who do not.
Classification features and prediction of individual infants
For the classification analysis, one infant was removed from the group to serve as a test case, whereas the remaining 58 infants were used a training set. For each test case in the classification analysis, starting from the full set of 26,335, features were determined within the training set as the functional connections between ROI pairs that show a nominally significant (P < 0.05) behavioral correlation, and a leave-one-out cross-validation analysis was performed within this training set. To be included in the final set of features for the independent test case, a functional connection had to show a correlation with one of the behavioral measures in all the cross-validation sets of training data. This final set of features provided an independently defined set of features that was used to train a classifier with a linear kernel to discriminate between infants who are and are not diagnosed with ASD at 24 months. Finally, the classifier was used to predict the independent test case. This strategy was repeated using each participant as the test case, creating a fully cross-validated approach with a nested leave-one-out procedure to identify features. As a result, the estimation of accuracy was relatively unbiased in the sense that the training features were selected independently of each test case (55). Similar methods are discussed in detail by Pereira et al. (33), with many of the applications reviewed by Gabrieli et al. (29). In the case where a participant did not have behavioral scores, they did not contribute information to the feature selection step (see Table 1). However, their functional connectivity measures were still used to train the classifier to predict the independent infant.
Finally, after completing the classification analysis, we used the intersection of the 59 independent classification feature sets to visualize the functional connections that most likely contributed to the classification accuracy (Fig. 2). Although these features were initially defined by their relationship with behavior only, we performed a between-groups t test and projected the t values onto a Talairach brain with a threshold of P < 0.005 (uncorrected). Our logic was that these regions would be the most likely to contribute to the discrimination between groups in the 59 separate support vector machine (SVM) models. This set of classification features represents only a subset of the features used in the individual classifiers. Because each classifier contains a slightly different set of features and is weighted differently, the calculations of group differences should not be interpreted as directly contributing to any individual's classification. The Talairach coordinates, average connectivity values, t values, and P values by group are listed in table S1.
Predicting individual 24-month clinical diagnoses
To determine whether brain features were capable of predicting the clinical diagnostic outcome of an individual infant, a classification model, built from an independent group of infants (see above), was applied to their 6-month functional connectivity data. When this process was repeated for all 59 infants, we were able to calculate measures of classification performance. Sensitivity was calculated as the proportion of infants with ASD that were correctly identified, whereas specificity was calculated as the proportion of infants that did not have ASD that were correctly identified. The positive predictive value was calculated as the proportion of positive predictions that were truly infants with ASD. Conversely, negative predictive value was calculated as the proportion of negative predictions that were truly infants without ASD. Finally, 95% CIs for the reported proportions (sensitivity, specificity, positive predictive value, and negative predictive value) are calculated according to the efficient score method and corrected for continuity (56).
The significance of the classification accuracy was determined by repeating the entire classification analysis (including feature selection and fitting of the SVM classifier) using randomly shuffled group labels (within the training sets) to predict each test case. This procedure estimated the null distribution of classification accuracy and was repeated 10,000 times to determine what proportion of times a randomly constructed classifier would perform as well as the classifier trained with the correct group labels. This distribution and the observed accuracy of the correct labels are shown in fig. S2.
Leave-10-out
To complete the leave-10-out analysis, the nested cross-validation procedure was repeated with a random set of 10 infants initially removed as the independent test set. To maintain the general population frequency distribution, each setof10 consisted of two randomly selected ASD-positive and eight randomly selected ASD-negative children. This analysis was run with 1000 random sets, allowing us to assess the distribution of classifier accuracies when more participants were kept independent. This represents a very small sample of the full set of randomized permutations; however, this analysis is meant to serve only as a demonstration of the robustness of the classification analysis.
Statistical analysis
All classification analyses were completed using MATLAB's Statistics and Machine Learning Toolbox (Mathworks Inc.). SVMs were trained using a linear kernel using the default setting of the fitcsvm function, and individual participants were predicted using the predict function. The default setting of this algorithm accounts for imbalances in the groups by setting the class prior probabilities to the relative frequencies of each class and then normalizes the weights to sum to the value of the prior probability in the respective class. Scripts were designed in-house, and their workflow is detailed above. Principal components were calculated using the default settings of the pca function to create a linear combination of the features space, which was then used for the visualization in Fig. 1. As described above, CIs for the reported proportions (sensitivity, specificity, positive predictive values, and negative predictive values) are calculated according to the efficient score method and corrected for continuity (56).
Supplementary Material
Fig. S1. Individual classification accuracies.
Fig. S2. Null distribution of classification accuracy.
Table S1. The Talairach coordinates for each of the ROIs.
Table S2. Comparison to independent high-risk sample.
Acknowledgments
The IBIS Network is an NIH-funded Autism Center of Excellence project and consists of a consortium of eight universities in the United States and Canada. Clinical sites include the University of North Carolina [J. Piven (IBIS Network principal investigator), H. C. Hazlett, and C. Chappell], the University of Washington (S. Dager, A. Estes, and D. Shaw), Washington University (K. Botteron, R. McKinstry, J. Constantino, and J. Pruett), Children's Hospital of Philadelphia (R. Schultz and S. Paterson), the University of Alberta (L. Zwaigenbaum), the University of Minnesota (J. Elison), Data Coordinating Center of Montreal Neurological Institute (A. C. Evans, D. L. Collins, G. B. Pike, V. Fonov, P. Kostopoulos, and S. Das), Image Processing Core of New York University (G. Gerig), the University of North Carolina (M. Styner), and Statistical Analysis Core of the University of North Carolina (H. Gu).
Funding: NIMH R01-MH093510 was awarded to J.R.P. NICHD R01-HD055741 was awarded to J.P., and T32-HD40127 was awarded to R.W.E. The funding sources had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
Footnotes
Author contributions: R.W.E., J.P., J.R.P., H.C.H., L.Z., A.M.E., K.N.B., S.R.D., A.C.E., G.G., R.C.M., R.T.S., and M.S. contributed to the concept and design of the study. R.W.E., C.A., T.N., J.P., J.R.P., H.C.H., L.Z., S.K., L.C., H.G., S.P., J.T.E., J.J.W., A.M.E., K.N.B., S.R.D., A.C.E., G.G., R.C.M., R.T.S., L.Z., J.N.C., M.D.S., M.R.S., and M.S. were involved in the acquisition of data. C.A., T.N., and J.R.P. processed and cleaned the functional connectivity data. R.W.E. performed all analyses with the processed and cleaned data as well as all of the machine learning analyses. R.W.E., J.P., J.R.P., C.A., B.L.S., and T.N. interpreted the results. R.W.E., C.A., T.N., J.P., J.R.P., H.C.H., L.Z., S.K., L.C., H.G., S.P., J.T.E., J.J.W., A.M.E., K.N.B., S.R.D., A.C.E., G.G., R.C.M., R.T.S., L.Z., J.N.C., M.D.S., M.R.S., and M.S. reviewed the manuscript. R.W.E., J.P., and J.R.P. contributed to the drafting and revising of the article.
Competing interests: J.N.C. receives royalties from Western Psychological Services for commercial sales and distribution of the Social Responsiveness Scale 2. A.C.E. holds shares in Biospective Inc. R.C.M. is a paid consultant for Siemens Healthcare. The University of North Carolina has filed a provisional patent related to this work. All the other authors declare that they have no competing interests.
Data and materials availability: The clinical and imaging data from this study will be deposited into the National Database for Autism Research (NDAR) according to the timelines requested by NIH and NDAR.
References and Notes
- 1.American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Association; 2013. [Google Scholar]
- 2.Developmental, Disabilities Monitoring Network Surveillance Year, and 2010 Principal Investigators, Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network. Morbidity and Mortality Weekly Report, Surveillance Summaries. 2002 [PubMed] [Google Scholar]
- 3.Ganz ML. The lifetime distribution of the incremental societal costs of autism. Arch Pediatr Adolesc Med. 2007;161:343–349. doi: 10.1001/archpedi.161.4.343. [DOI] [PubMed] [Google Scholar]
- 4.Buescher AVS, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168:721–728. doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- 5.Webb SL, Jones EJH, Kelly J, Dawson G. The motivation for very early intervention for infants at high risk for autism spectrum disorders. Int J Speech Lang Pathol. 2014;16:36–42. doi: 10.3109/17549507.2013.861018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogers SJ, Vismara L, Wagner AL, McCormick C, Young G, Ozonoff S. Autism treatment in the first year of life: A pilot study of infant start, a parent-implemented intervention for symptomatic infants. J Autism Dev Disord. 2014;44:2981–2995. doi: 10.1007/s10803-014-2202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green J, Charman T, Pickles A, Wan MW, Elsabbagh M, Slonims V, Taylor C, McNally J, Booth R, Gliga T, Jones EJH, Harrop C, Bedford R, Johnson MH. BASIS team, Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. Lancet Psychiatry. 2015;2:133–140. doi: 10.1016/S2215-0366(14)00091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, Rogers SJ, Rozga A, Sangha S, Sigman M, Steinfeld MB. A prospective study of the emergence of early behavioral signs of autism. J Am Acad Child Adolesc Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- 9.Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: Stability and change in clinical diagnosis and symptom presentation. J Child Psychol Psychiatry. 2013;54:582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Estes A, Zwaigenbaum L, Gu H, St John T, Paterson S, Elison JT, Hazlett H, Botteron K, Dager SR, Schultz RT, Kostopoulos P, Evans A, Dawson G, Eliason J, Alvarez S, Piven J IBIS Network. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J Neurodev Disord. 2015;7:24. doi: 10.1186/s11689-015-9117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504:427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landa RL, Gross AL, Stuart EA, Faherty A. Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child Dev. 2013;84:429–442. doi: 10.1111/j.1467-8624.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elison JT, Paterson SJ, Wolff JJ, Reznick JS, Sasson NJ, Gu H, Botteron KN, Dager SR, Estes AM, Evans AC, Gerig G, Hazlett HC, Schultz RT, Styner M, Zwaigenbaum L, Piven J IBIS Network. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am J Psychiatry. 2013;170:899–908. doi: 10.1176/appi.ajp.2012.12091150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 16.Wolff JJ, Gu H, Gerig G, Elison JT, Styner M, Gouttard S, Botteron KN, Dager SR, Dawson G, Estes AM, Evans A, Hazlett HC, Kostopoulos P, McKinstry RC, Paterson SJ, Schultz RT, Zwaigenbaum L, Piven J IBIS Network. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am J Psychiatry. 2012;169:589–600. doi: 10.1176/appi.ajp.2011.11091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff JJ, Gerig G, Lewis JD, Soda T, Styner MA, Vachet C, Botteron KN, Elison JT, Dager SR, Estes AM, Hazlett HC, Schultz RT, Zwaigenbaum L, Piven J IBIS Network. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain. 2015;138:2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen MD, Nordahl CW, Young GS, Wootton-Gorges SL, Lee A, Liston SE, Harrington KR, Ozonoff S, Amaral DG. Early brain enlargement and elevated extra-axial fluid in infants who develop autism spectrum disorder. Brain. 2013;136:2825–2835. doi: 10.1093/brain/awt166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsabbagh M, Mercure E, Hudry K, Chandler S, Pasco G, Charman T, Pickles A, Baron-Cohen S, Bolton P, Johnson MH. BASIS Team, Infant neural sensitivity to dynamic eye gaze is associated with later emerging autism. Curr Biol. 2012;22:338–342. doi: 10.1016/j.cub.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, Papademetris X, Constable RT. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18:1664–1671. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM. A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci. 2016;19:165–171. doi: 10.1038/nn.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emerson RW, Cantlon JF. Early math achievement and functional connectivity in the fronto-parietal network. Dev Cogn Neurosci. 2012;2:S139–S151. doi: 10.1016/j.dcn.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: Unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao W, Alcauter S, Smith JK, Gilmore JH, Lin W. Development of human brain corticalnetwork architecture during infancy. Brain Struct Funct. 2014;220:1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruett JR, Jr, Kandala S, Hoertel S, Snyder AZ, Elison JT, Nishino T, Feczko E, Dosenbach NUF, Nardos B, Power JD, Adeyemo B. Accurate age classification of 6 and 12 month-old infants based on resting-state functional connectivity magnetic resonance imaging data. Dev Cogn Neurosci. 2015;12:123–133. doi: 10.1016/j.dcn.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR, Jr, Barch DM, Petersen SE, Schlaggar BL. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fair DA, Nigg JT, Iyer S, Bathula D, Mills KL, Dosenbach NU, Schlaggar BL, Mennes M, Gutman D, Bangaru S, Buitelaar JK, Dickstein DP, Di Martino A, Kennedy DN, Kelly C, Luna B, Schweitzer JB, Velanova K, Wang YF, Mostofsky S, Castellanos FX, Milham MP. Distinct neural signatures detected for ADHD subtypes after controlling for micro-movements in resting state functional connectivity MRI data. Front. Syst Neurosci. 2013;6:80. doi: 10.3389/fnsys.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greene DJ, Church JA, Dosenbach NUF, Nielsen AN, Adeyemo B, Nardos B, Petersen SE, Black KJ, Schlaggar BL. Multivariate pattern classification of pediatric Tourette syndrome using functional connectivity MRI. Dev Sci. 2016;1:1–18. doi: 10.1111/desc.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrieli JDE, Ghosh SS, Whitfield-Gabrieli S. Prediction as a humanitarian and pragmatic contribution from human cognitive neuroscience. Neuron. 2015;85:11–26. doi: 10.1016/j.neuron.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellanos FX, Di Martino A, Craddock RC, Mehta AD, Milham MP. Clinical applications of the functional connectome. Neuroimage. 2013;80:527–540. doi: 10.1016/j.neuroimage.2013.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Ryali S, Menon V. Salience network-based classification and prediction of symptom severity in children with autism. JAMA Psychiat. 2013;70:869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ecker C, Spooren W, Murphy DGM. Translational approaches to the biology of Autism: False dawn or a new era? Mol Psychiatry. 2013;18:435–442. doi: 10.1038/mp.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: A tutorial overview. Neuroimage. 2009;45:S199–S209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrus N, Underwood-Riordan H, Randall F, Zhang Y, Constantino JN. Lack of effect of risperidone on core autistic symptoms: Data from a longitudinal study. J Child Adolesc Psychopharmacol. 2014;24:513–518. doi: 10.1089/cap.2014.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: The early start Denver Model. Pediatrics. 2010;125:e17–e23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J IBIS Network. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542:348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence risk for autism spectrum disorders: A baby siblings research consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawarska K, Shic F, Macari S, Campbell DJ, Brian J, Landa R, Hutman T, Nelson CA, Ozonoff S, Tager-Flusberg H, Young GS, Zwaigenbaum L, Cohen IL, Charman T, Messinger DS, Klin A, Johnson S, Bryson S. 18-month predictors of later outcomes in younger siblings of children with autism spectrum disorder: A baby siblings research consortium study. J Am Acad Child Adolesc Psychiatry. 2010;53:1317–1327. doi: 10.1016/j.jaac.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, Di Lavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule–Generic:A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 40.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 41.American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; 2000. [Google Scholar]
- 42.Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: Comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 43.Mirenda P, Smith IM, Vaillancourt T, Georgiades S, Duku E, Szatmari P, Bryson S, Fombonne E, Roberts W, Volden J, Waddell C, Zwaigenbaum L Pathways in ASD Study Team. Validating the Repetitive Behavior Scale-Revised in young children with autism spectrum disorder. J Autism Dev Disord. 2010;40:1521–1530. doi: 10.1007/s10803-010-1012-0. [DOI] [PubMed] [Google Scholar]
- 44.Mullen EM. Mullen Scales of Early Learning: AGS Edition. American Guidance Service Publishing; 1995. [Google Scholar]
- 45.Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J Speech Lang Hear Res. 2002;45:1202–1218. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- 46.Philip RCM, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, Stanfield AC. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 2012;36:901–942. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Brain Development Cooperative Group, Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satterthwaite TD, Wolf DH, Ruparel K, Erus G, Elliott MA, Eickhoff SB, Gennatas ED, Jackson C, Prabhakaran K, Smith A, Hakonarson H, Verma R, Davatzikos C, Gur RE, Gur RC. Heterogeneous impact of motion on fundamental patterns of developmental changes in functional connectivity during youth. Neuroimage. 2013;83:45–57. doi: 10.1016/j.neuroimage.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- 54.Mathias A, Grond F, Guardans R, Seese D, Canela M, Diebner HH. Algorithms for spectral analysis of irregularly sampled time series. J Stat Softw. 2004;11:1–30. [Google Scholar]
- 55.Hastie T, Tibshirani R, Friedman J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. 2. Springer; 2009. [Google Scholar]
- 56.Newcombe RG. Two-sided confidence intervals for the single proportion: Comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 57.Greene DJ, Church JA, Dosenbach NU, Nielsen AN, Adeyemo B, Nardos B, Petersen SE, Black KJ, Schlaggar BL. Multivariate pattern classification of pediatric Tourette syndrome using functional connectivity MRI. Dev Sci. 2016;19:581–598. doi: 10.1111/desc.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Individual classification accuracies.
Fig. S2. Null distribution of classification accuracy.
Table S1. The Talairach coordinates for each of the ROIs.
Table S2. Comparison to independent high-risk sample.