Abstract
Chronic dairy intake is associated with improved cardiovascular outcomes while high dietary-sodium impairs endothelial function through increased oxidative stress and reduced nitric oxide (NO) bioavailability. The purpose of this study was to compare the effect of acute cheese consumption with consumption of sodium from non-dairy sources on microvascular function. We hypothesized that dairy-cheese ingestion would augment NO-dependent vasodilation compared to sodium from non-dairy sources. On 5 separate visits, 14 healthy subjects (61±2yrs, 8M/6F) consumed either 85g dairy cheese (560mg Na), 85g soy cheese (560mg Na), 65g pretzels (560mg Na), 170g dairy cheese (1120mg Na), or 130g pretzels (1120mg Na). Two intradermal microdialysis fibers were inserted in the ventral forearm for delivery of lactated Ringer’s or 10mM ascorbate (antioxidant) during local skin heating (~50 min). Red cell flux was measured continuously by laser-Doppler flowmetry (LDF) and cutaneous vascular conductance (CVC=LDF/MAP) was normalized as %CVCmax (28mM sodium nitroprusside). Following a plateau in CVC, 15mM NG-nitro-L-arginine methyl ester was perfused to quantify NO-dependent vasodilation (~45 min). NO-dependent vasodilation was greater following dairy (560mg Na 57±3%) (1120mg Na 55±5%) compared to soy (560mg Na 42±3%; p=0.002) or pretzel (560mg Na 43±4%; p=0.004) (1120mg Na 46±3%; p=0.04). Ascorbate augmented NO-dependent vasodilation following soy (control: 42±3 vs. ascorbate: 54±3%; p=0.01) or pretzel (560mg Na; control: 43±4 vs. ascorbate: 56±3%; p=0.006) (1120mg Na; control: 46±5 vs. ascorbate: 56±3%; p=0.02), but not dairy. Sodium ingestion in dairy was associated with greater NO-dependent vasodilation compared to non-dairy sodium, a difference that was ameliorated with ascorbate perfusion. Dairy nutrients may protect against sodium-induced reductions in NO-dependent dilation through ascorbate-sensitive mechanisms.
Keywords: dairy, cheese, sodium, nitric oxide, vascular health
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality in developed nations, with 40% of all deaths in the United States attributable to CVD. The annual health care burden of the treatment and management of CVD is greater than $656 billion and projected to increase as the population ages [1]. As such, identification of modifiable risk factors and non-pharmacological interventions are important for CVD prevention. Increasing dairy intake is an emerging lifestyle factor that is associated with a decreased CVD risk [2, 3]. Long term dairy consumption is associated with lower blood pressure in healthy, aged individuals [4], but its cardioprotective activities are also mediated independent of its blood pressure-lowering effect [5–14]. One putative mechanism through which dairy consumption may benefit vascular function is through the antioxidant properties of dairy peptides. Administration of dairy peptides in animal models reduces markers of inflammation and attenuates measures of oxidative stress, including total antioxidant capacity [9, 12, 13], suggesting that these mechanisms may decrease lifetime risk of cardiovascular morbidity and mortality.
A high dietary sodium intake is independently associated with elevations in arterial blood pressure [15] as well as increased cardiovascular morbidity and mortality [16]. Animal studies of the vascular effects of high dietary sodium implicate endothelium-derived oxidative stress, particularly the production of superoxide, in reduced NO bioavailability and endothelial dysfunction [17–21]. Similarly, human studies demonstrate that sodium restriction (≤1.5 g/day) reverses age-associated endothelial dysfunction by increasing NO-dependent vasodilation [22]. Similarly, non-invasive measures of conduit artery endothelial function show that low dietary sodium intake is associated with enhanced flow-mediated vasodilation in middle aged and older adults [23]. In contrast to sodium restriction, even short-term increases in dietary sodium (7 days) impair flow-mediated vasodilation in conduit arteries of otherwise healthy young adults [24–26]; and even a single high-salt meal can significantly suppress brachial artery flow-mediated dilation within 30 minutes in healthy young adults [27].
The human cutaneous circulation is an accessible vascular bed for examining mechanisms of microvascular dysfunction in vivo [28]. There is a significant relation between microvascular dysfunction measured in the skin and that measured invasively in the coronary and renal circulations, and intervention-induced improvements in vascular function are detectible in the cutaneous circulation prior to improvements in clinical outcomes [28–30]. Importantly, dietary sodium-induced impairments in endothelial function are detectable in the cutaneous microvasculature of otherwise healthy adults, independent of changes in blood pressure or blood chemistry [25, 31, 32]. These mechanistic in vivo human studies further demonstrate that even short term (7 day) increases in dietary sodium impair endothelial function and reduce NO bioavailability via an increase in oxidative stress [23, 25, 31].
Increased consumption of dairy products in the form natural cheese may inadvertently increase dietary sodium intake. Consequently, increasing dairy consumption, particularly in the form of cheese, may paradoxically hinder adherence to dietary sodium recommendations, while still mitigating CVD risk. It is currently unknown whether vasoprotective activities of dairy, provided as natural cheeses, protect against sodium-induced impairments in the vasculature. Therefore, we sought to examine the protective role of macronutrients in natural dairy-cheese against acute dietary sodium induced microvascular dysfunction. We hypothesized that acute natural dairy-cheese ingestion would improve NO-dependent vasodilation compared to an equal dietary sodium intake from non-dairy sources. Further, we hypothesized that this effect would be mediated by a reduction in ascorbate-sensitive oxidants.
METHODS
Subjects
All protocols were approved by the Institutional Review Board at The Pennsylvania State University and complied with the guidelines in the Declaration of Helsinki. All participants voluntarily provided written and verbal consent prior to the experiment. Fourteen subjects (61±2 years; 8 men, 6 women) participated in the study. Prior to participation, subjects underwent a medical screening that included a 12-lead electrocardiogram, fasting blood chemistry, and physical examination. Subjects also completed a 24 hour ambulatory blood pressure monitoring while enrolled in the study. Inclusion criteria required a daily dairy intake of less than 2 servings. Daily dairy intake was assessed with a modified food frequency questionnaire specific to dairy consumption. Subjects had a 2-day wash-in period where they did not consume any dairy. Experimental visits were separated by at least 3 days, to ensure the 2-day low-dairy wash-in before each visit. There were no recommendations regarding sodium intake during the wash-in. Subjects abstained from alcoholic and caffeinated beverages for 12h, vigorous physical activity for 24h, and food for 8h prior to each experiment. All subjects were non-smokers, non-diabetic, non-obese (body mass index < 30 kg m−2), and were not taking prescription medications that may alter vascular function (e.g. statins, antidepressants, antihypertensives, dietary supplements, aspirin, etc.). Women taking any form of hormone replacement therapy were excluded from the study. Subject characteristics are presented in Table 1.
Table 1.
Human subject characteristics. Mean ± SEM
| Sex (M,F) | (8,6) |
| Age (years) | 61±2 |
| BMI (kg m−2) | 25.9 ± 0.4 |
| SBP (mmHg) | 127 ± 2 |
| DBP (mmHg) | 77 ± 1 |
| Total Cholesterol (mg dL−1) | 200 ± 8 |
| HDL (mg dL−1) | 59 ± 4 |
| LDL (mg dL−1) | 128 ± 6 |
| HbA1c (%) | 5.7 ± 0.1 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, serum high density lipoprotein; LDL, serum low density lipoprotein; HbA1c, hemoglobin A1c.
Experimental Protocol
Figure 1 presents a schematic representation of the experimental protocol. On five separate visits, subjects arrived at the laboratory following an overnight (≥8 hours) fast. Each experimental visit spanned approximately 4 hours. Two intradermal microdialysis fibers (10mm, 20kDa cutoff membrane, MD 2000; Bioanalytical Systems, West Lafayette, IN) were placed into the dermal layer of the ventral left forearm for the local delivery of pharmacological agents[33]. Pharmacological agents were mixed just prior to use, dissolved in lactated Ringer’s solution, sterilized using syringe microfilters (Acrodisc; Pall, Ann Arbor, MI), and wrapped in foil to prevent degradation due to light exposure. Microdialysis sites were randomly assigned to receive either 10mM ascorbic acid (Sigma, St. Louis, MO) for local delivery of the non-specific antioxidant [31, 34]; or lactated Ringer’s solution to serve as control. Site-specific pharmacological solutions were perfused through the microdialysis fibers at a rate of 2μL/min [33, 35] (Bee Hive controller and Baby Bee microinfusion pumps; Bioanalytical Systems).
Figure 1.
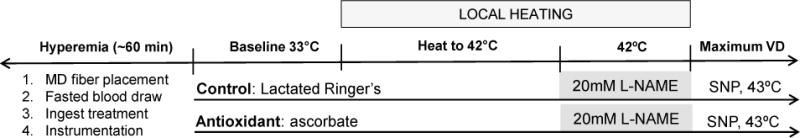
Schematic representation of the protocol. Subjects entered the lab fasted, had 2 intradermal microdialyis (MD) fibers placed, a fasted blood draw, and then ingested the treatment. Following the resolution of hyperemia, subjects were instrumented, and skin blood flow data were collected at baseline, throughout local heating, and during maximal vasodilation. The entire protocol lasted ~4 hours. Each arrow represents a microdialysis site. L-NAME, NG-nitro-L-arginine; SNP, sodium nitroprusside.
Following microdialysis fiber placement, subjects were instrumented with an intravenous catheter for blood collection. A fasted sample was collected before dietary treatment administration. Blood samples were then collected every 30 minutes post-treatment until the completion of the study. Whole blood samples were collected in EDTA treated tubes containing o-phenanthroline, p-hydroxy-mercuribenzoic acid, and pepstatin (Wake Forest University, Winston-Salem, NC). Whole blood samples were centrifuged, and plasma samples were frozen and stored at −80°C until future use. Plasma sodium was measured at baseline and at 90 minutes post-ingestion using an electrolyte analyzer (ProLyte, Diamond Diagnostics, Holliston, MA). Plasma angiotensin II concentrations were measured at baseline and at 90 minutes post-ingestion using a commercially available ELISA (Abcam, Cambridge, UK) according to the manufacturer’s instructions. Samples were analyzed in duplicate with an average CV <10%.
Thirty minutes after microdialysis fibers were placed, subjects consumed either 85g cheddar dairy cheese (560mg Na), 85g soy cheese (560mg Na), 65g pretzels (560mg Na), 170g dairy cheese (1120mg Na), or 130g pretzels (1120mg Na) in randomized order. The treatment order was randomly assigned for each subject using a random number generator and was administered by the investigators. One subject did not consume soy cheese due to palatability issues. Our initial study design included a 170g soy cheese treatment (1120mg Na) however, several subjects refused this treatment due to palatability and we excluded it from further testing. The caloric, macronutrient, and sodium content of each dietary treatment is displayed in Table 2. The 30 minute time point for consumption was chosen such that the local heating plateau for our eNOS-dependent vascular stimulus [33] would occur 60-90 minutes post-treatment, corresponding with the time period after milk peptide ingestion when peak intestinal concentrations of bioactive peptides are recovered [36].
Table 2.
Sodium, calories, and macronutrient content of dietary treatments.
| 85g cheddar cheese |
85g soy cheese |
65g pretzel |
170g cheddar cheese |
130g pretzel |
|
|---|---|---|---|---|---|
| Sodium (mg) | 560 | 560 | 560 | 1120 | 1120 |
| Calories (kcal) | 360 | 210 | 255 | 720 | 509 |
| Fat (g) | 28 | 21 | 0 | 56 | 0 |
| Carbohydrate (g) | 0 | 6 | 56 | 0 | 112 |
| Protein (g) | 20 | 3 | 5 | 40 | 10 |
Sixty to 90 minutes were allowed for hyperemia associated with fiber placement to resolve before baseline data were collected, followed by a standard local heating protocol to induce endothelial nitric oxide synthase (eNOS)-dependent vasodilation as previously described [33, 37]. After ∼30–40 min of local heating, when skin blood flow reached an established plateau, 20mM NG-nitro-L-arginine (L-NAME; Calbiochem, San Diego, CA) was perfused at a rate of 4μL/min to quantify NO-dependent vasodilation at all sites [38, 39]. After infusion of L-NAME and subsequent stabilization of a post-L-NAME plateau in skin blood flow, 28mM sodium nitroprusside (Nitropress; Abbott Laboratories, Chicago, IL) was perfused and local temperature increased to 43°C to elicit maximal dilation (CVCmax) [37, 40]. Work in our laboratory and others has demonstrated that this protocol is highly specific to eNOS production of NO and allows the direct quantification of functional NO-dependent vasodilation in the cutaneous microcirculation [38, 41, 42].
Cutaneous red blood cell flux was continually measured directly over each microdialysis site with an integrated laser-Doppler flowmetry probe placed in a local heating unit (Moor Instruments SHO2). Mean arterial pressure (MAP) was measured at the brachial artery throughout the protocol using an automated blood pressure monitor (CardioCap, GE).Cutaneous vascular conductance (CVC) was calculated as red blood cell flux divided by MAP and expressed as a percent of site-specific maximal vasodilation (%CVCmax) [37, 43].
Data Acquisition & Statistical Analysis
Sample size was determined a priori by power analysis (p=0.8, α =0.05). Data were collected with Windaq (Windaq; Dataq Instruments) at a frequency of 40 Hz. A three-way (dietary treatment*local treatment*subject) repeated-measures mixed-model ANOVA was used to detect dietary treatment and local treatment differences in local heating plateau, NO-dependent vasodilation, and maximal CVC (version 9.1.3; SAS, Cary, NC). A two-way (dietary treatment*subject) repeated measures ANOVA was used to detect dietary treatment differences in plasma sodium and angiotensin II. Bonferroni post-hoc corrections were performed to account for multiple comparisons when necessary. Significance was accepted at α=0.05. All values are presented as mean ± SEM.
RESULTS
There was no difference in baseline or maximal (28mM SNP, 43ºC) CVC between microdialysis sites or across treatments.
Figure 2 shows original data records of the skin blood flow response normalized to maximal cutaneous vascular conductance (%CVCmax) during local heating in the control and ascorbate-treated microdialysis sites of one subject following non-dairy dietary sodium (pretzel, 1120mg Na) and dairy-cheese sodium (cheddar cheese, 1120mg Na) consumption. The percent decrease with NOS inhibition (L-NAME) following each treatment is indicated.
Figure 2.
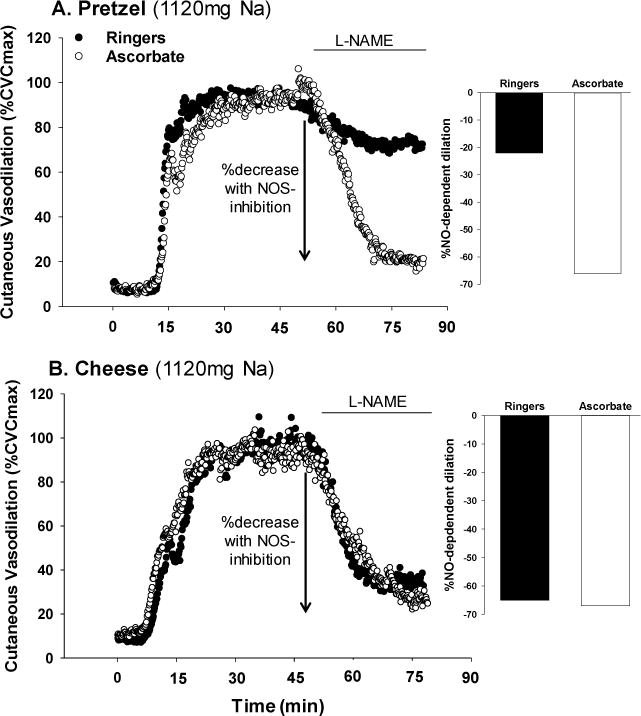
Representative tracing of skin blood flow (%CVCmax) during local heating in the control and ascorbate-treated microdialysis sites of one subject following non-dairy dietary sodium (panel A; pretzel, 1120mg Na) and dairy-cheese sodium (panel B, cheddar cheese, 1120mg Na) consumption. The difference between the local heating plateau and the post-NG-nitro-L-arginine methyl ester (L-NAME) plateau indicates the vasodilation attributed to the production of NO by eNOS (%NO-dependent dilation).
Figure 3 illustrates the total vasodilatory response to local heating (local heating plateau, %CVCmax) in Ringers (control) and ascorbate perfused microdialysis sites following each dietary treatment. There was no difference in the local heating plateau between sites or among dietary treatments (all p>0.05).
Figure 3.
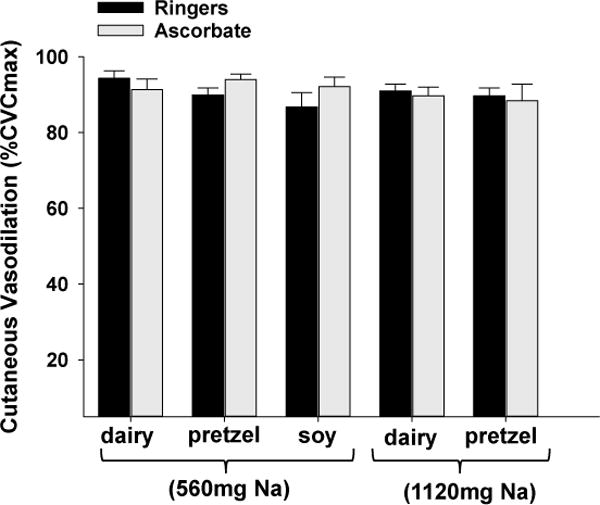
Group mean ± SEM (n=14) vasodilation response (%CVCmax) to local heating in Ringer’s (control) and ascorbate (antioxidant) perfused microdialysis sites following each dietary treatment.
Figure 4 shows the percent NO-dependent vasodilation during local heating in Ringers (control) and ascorbate perfused microdialysis sites following each dietary treatment. NO-dependent vasodilation was greater following 560mg Na contained in dairy cheese (57±3 %) compared to 560mg Na in soy cheese (42±3%; p=0.002) or 560mg Na in pretzels (43±4%; p=0.004). NO-dependent vasodilation was also greater following 1120mg Na contained in dairy cheese (55±5 %) compared to 1120mg Na in pretzels (46±5%; p=0.04). Local ascorbate perfusion augmented NO-dependent vasodilation compared to the control microdialysis site following Na ingestion in soy cheese (control: 42±3 vs. ascorbate: 54±3%) (p=0.01) and pretzel treatments with 560mg Na (control: 43±4 vs. ascorbate: 56±5%; p=0.006) and 1120mg Na (control: 46±5 vs. ascorbate: 56±3%; p=0.02). Local ascorbate perfusion did not augment NO-dependent vasodilation compared to the control microdialysis site following 560mg Na ingestion (control: 57±3 vs. ascorbate: 60±3%; p=0.6) and 1120mg Na ingestion (control: 55±5 vs. ascorbate: 54±3%; p=0.7) in dairy cheese.
Figure 4.
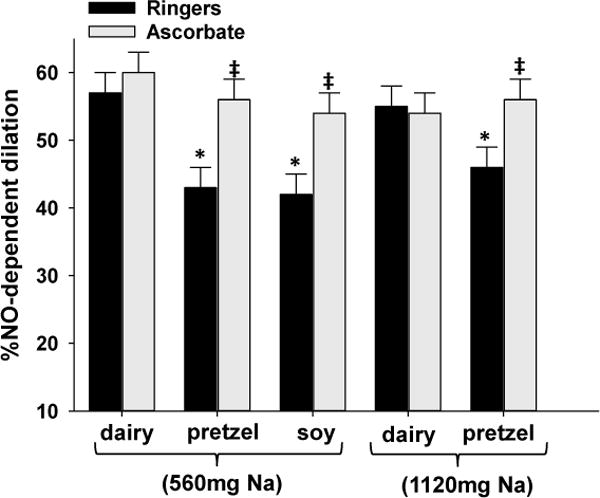
Group mean ± SEM (n=14) %NO-dependent vasodilation response to local heating in Ringer’s (control) and ascorbate (antioxidant) perfused microdialysis sites following each dietary treatment.*p<0.05 compared to dairy control within Na ingestion. ‡ p< 0.05 compared to Ringer’s site within dietary treatment.
There were no differences in plasma sodium or angiotensin II concentrations at baseline, or 90 minutes post ingestion, among any of the dietary treatments (sodium: p = 0.1 main effect of treatment, p = 0.2 main effect of time) (angiotensin II: p=0.6 main effect of treatment, p=0.8 main effect of time).
DISCUSSION
To our knowledge, this is the first study to directly examine the potential protective effect of cheddar cheese on dietary sodium-induced microvascular endothelial dysfunction. Our data demonstrate that acute (single-meal) dairy cheese consumption is protective against sodium-induced impairments in NO-dependent vasodilation in the microcirculation. Further, acute localized administration of the non-specific antioxidant ascorbate normalized NO-dependent vasodilation following non-dairy sodium ingestion, but had no effect on NO-dependent vasodilation after ingesting natural cheese, suggesting that non-sodium components of natural cheese protect against acute sodium-induced endothelial dysfunction in the microvasculature of healthy older adults. The primary finding of this study is that the presence of macronutrients in dairy-based natural cheese ameliorates acute sodium-induced reductions in NO-dependent vasodilation by reducing ascorbate-sensitive oxidants. These data suggest that paradoxically increasing dietary sodium by increasing cheese consumption may not confer the same CVD risk as dietary sodium consumption in the absence of dairy.
A high dietary sodium consumption is independently associated with elevated arterial blood pressure [15] as well as increased cardiovascular morbidity and mortality [16]. Individual sodium excretion resulting from increased dietary intake of sodium >5.8 g/day is strongly associated with increased systolic and diastolic pressures of 10-11 mmHg and 6 mmHg, respectively. High quality meta-analyses indicate that reducing sodium intake reduces blood pressure and the risk of stroke and fatal coronary heart disease [44].
Animal studies of the vascular effects of high dietary sodium implicate endothelium-derived oxidative stress, particularly the production of superoxide, in reduced NO bioavailability and endothelial dysfunction [17–21]. Similarly, human studies demonstrate that sodium restriction (≤1.5 g/day) reverses age-associated endothelial dysfunction by increasing NO-dependent vasodilation and decreasing superoxide dismutase expression [22]. Even short term increases in dietary sodium (7 days) induce microvascular dysfunction measured in the cutaneous circulation of healthy young adults [25]; an impairment that is mediated by increases in oxidant stress and occurs independent of changes in blood pressure [31]. Similar to our current findings, Dickinson et al demonstrated that a single high-salt meal significantly suppresses brachial flow-mediated dilation 30 and 60 minutes after ingestion in healthy adults [27]. Collectively, the animal and human literature agree that elevated dietary sodium consumption impairs endothelial function and reduces NO bioavailability via increased oxidant stress mechanisms, even in instances where patients do not have overt CVD. Our data add to this body of literature suggesting a single high sodium meal/snack from non-dairy sources acutely reduces NO-dependent vasodilation in healthy middle-aged adults.
The positive impact of chronic dairy consumption on cardiovascular health has been demonstrated in many population-based studies [45, 46]. Increased total dairy intake is associated with improvements in global measures of vascular health and function including blood pressure [47], pulse wave velocity [48], arterial compliance [47], and arterial stiffness [46]. The precise mechanism(s) by which dairy may confer cardiovascular benefits are currently unclear but include angiotensin converting enzyme (ACE) inhibition [6–8], protection and enhancement of bioavailable NO [9–11], and anti-inflammatory and anti-oxidant properties [9, 12–14] of dairy proteins and micronutrients. Because no single and specific mechanism has been definitively shown to account for the beneficial effects of increased dairy intake on vascular function, it is likely that lifetime increases in dietary dairy consumption confer cardiovascular benefits through several of these putative mechanisms. However, given the specific role of oxidant stress in dietary sodium-induced vessel dysfunction we focused our investigation on the antioxidant properties of dairy proteins. In support of this global hypothesis, our data suggest that the antioxidant properties of dairy peptides may play a primary role in the protection against dietary sodium induced reductions in NO bioavailability. Furthermore, we did not observe changes in circulating angiotensin II in response to our acute dietary treatments.
In the current study, we did not observe a reduction in the total vasodilator response to local heating of the skin, but rather an attenuation in the direct functional quantification of NO-mediated vasodilation with non-dairy sodium ingestion. These findings agree with earlier work performed in our laboratory that suggests that middle-aged adults maintain total vasodilator responsiveness to local heating but have reduced eNOS-mediated vasodilation compared to young adults [33]. The current data suggest that a secondary NO-independent pathway is upregulated to compensate for the decrease in eNOS function following acute sodium consumption. However, endothelial derived NO is synthesized ubiquitously throughout the vasculature and plays a crucial anti-atherogenic vasoprotective role. Given the putative role of NO in vascular health and vessel function, as well as possible age-associated reductions in other NO-independent endothelial pathways (prostaglandins, endothelium-derived hyperpolarizing factors, etc.) [49, 50], interventions that target the production and protection of NO at the endothelium are clinically relevant strategies to preserve vascular health and reduce CVD risk across the lifespan.
This study examined acute (single meal) vascular responses to dietary sodium with and without dairy. As expected, we did not observe a time or treatment-dependent change in plasma sodium concentrations. Plasma sodium concentration is tightly regulated, and our findings are consistent with other dietary studies in which high dietary sodium is associated with vessel dysfunction independent of changes in plasma sodium concentrations[31]. We selected our 2 sodium doses based on 2 and 4 servings of natural cheddar cheese. Interestingly, we did not observe a dose-response relation between dietary sodium from non-dairy sources and attenuated NO-dependent dilation. This may have occurred because our doses were relatively close together, or because our doses were high enough that we were at or near a ceiling effect. The dose-response relation between acute dietary sodium and endothelial dysfunction was not an experimental endpoint in this study, however further work in this area is warranted.
Our test foods were specifically matched for dietary sodium, with the soy cheese comparison included to account for potential differences with fat content and gastric emptying. There was a difference in protein content between cheddar cheese and soy cheese meals in this study. As such, we cannot rule-out the possibility that protein from non-dairy sources may similarly affect NO-dependent dilation. Furthermore, our study design did not account for differences in fat or carbohydrate composition between treatments, a factor that may influence the inflammatory milieu of the vascular endothelium [51, 52]. However, given the documented antioxidant properties of dairy peptide hydrolysates [53] and the beneficial effect of dairy peptide ingestion on vascular endothelial function in humans [5, 54], our findings fit within the broader hypothesis that specific antioxidant properties of hydrolyzed dairy peptides play a role in preserved or maintained vascular function. Collectively, our data strongly suggest that the macronutrients in dairy protect against acute sodium-induced endothelial dysfunction and that this protective effect is mediated by antioxidant properties of milk proteins. It is still unknown if this vasoprotection is maintained over longer periods of elevated dietary sodium intake. Furthermore, it is unclear if this effect is dependent on sodium being directly incorporated in the dairy (e.g. natural cheese) or if the same benefits would occur if the diet contained high dairy and high sodium separately (e.g. pretzels and milk). Further studies of the protective antioxidant mechanisms of dairy proteins, as well as their chronic role in vasoprotection against high dietary sodium are warranted.
Summary
Overall, the current study suggests that the macronutients in natural cheeses ameliorate sodium-induced vessel dysfunction following a high sodium meal, through anti-oxidant mechanisms. Increased dairy consumption is associated with decreased risk of cardiovascular morbidity and mortality, and our data suggest that the antioxidant properties of dairy likely contribute to this association by protecting against dietary sodium-induced impairments in vascular function. Consequently, increasing dietary dairy intake may represent a modifiable and non-pharmacological lifestyle factor that can increase vascular health and function through the production and protection of bioavailable NO.
Acknowledgments
The authors are grateful to the subjects for their time and effort and to Susan Slimak, RN, and Jane Pierzga, MS for their assistance.
FUNDING
This research was supported by Dairy Management Inc.
List of Abbreviations
- CVD
cardiovascular disease
- CVC
cutaneous vascular conductance
- L-NAME
NG-nitro-L-arginine methyl ester
- LDF
laser-Doppler flux
- MAP
mean arterial pressure
- NO
nitric oxide
- SNP
sodium nitroprusside
Footnotes
AUTHOR CONTRIBUTIONS
AES, designed research, conducted research, analyzed data and performed statistical analysis, wrote paper, had primary responsibility for final content; BKA, conducted research, analyzed data, wrote paper; WLK, designed research, wrote paper; LMA, designed research, analyzed data, wrote paper, had primary responsibility for final content. All authors read and approved the final version of the manuscript. All laboratory work was conducted at PSU.
CONFLICT OF INTEREST
None.
References
- 1.Mozaffarian D, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Crichton GE, Alkerwi A. Dairy food intake is positively associated with cardiovascular health: findings from Observation of Cardiovascular Risk Factors in Luxembourg study. Nutr Res. 2014;34(12):1036–44. doi: 10.1016/j.nutres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Markey O, et al. Dairy and cardiovascular health: Friend or foe? Nutr Bull. 2014;39(2):161–171. doi: 10.1111/nbu.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L, et al. Dietary intake of dairy products, calcium, and vitamin D and the risk of hypertension in middle-aged and older women. Hypertension. 2008;51(4):1073–9. doi: 10.1161/HYPERTENSIONAHA.107.107821. [DOI] [PubMed] [Google Scholar]
- 5.Ballard KD, Bruno RS. Protective role of dairy and its constituents on vascular function independent of blood pressure-lowering activities. Nutr Rev. 2015;73(1):36–50. doi: 10.1093/nutrit/nuu013. [DOI] [PubMed] [Google Scholar]
- 6.Xu JY, et al. Effect of milk tripeptides on blood pressure: a meta-analysis of randomized controlled trials. Nutrition. 2008;24(10):933–40. doi: 10.1016/j.nut.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Murakami M, et al. Structural analysis of a new anti-hypertensive peptide (beta-lactosin B) isolated from a commercial whey product. J Dairy Sci. 2004;87(7):1967–74. doi: 10.3168/jds.S0022-0302(04)70013-2. [DOI] [PubMed] [Google Scholar]
- 8.van der Zander K, et al. Fermented lactotripeptides-containing milk lowers daytime blood pressure in high normal-to-mild hypertensive subjects. J Hum Hypertens. 2008;22(11):804–6. doi: 10.1038/jhh.2008.59. [DOI] [PubMed] [Google Scholar]
- 9.Xia Z, et al. N-acetylcysteine attenuates TNF-alpha-induced human vascular endothelial cell apoptosis and restores eNOS expression. Eur J Pharmacol. 2006;550(1–3):134–42. doi: 10.1016/j.ejphar.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C, Hardebo JE, Salford LG. Role of endothelium in the responses of human intracranial arteries to a slight reduction of extracellular magnesium. Exp Physiol. 1992;77(1):209–11. doi: 10.1113/expphysiol.1992.sp003575. [DOI] [PubMed] [Google Scholar]
- 11.Turpeinen AM, et al. Antihypertensive effects of bioactive tripeptides-a random effects meta-analysis. Ann Med. 2013;45(1):51–6. doi: 10.3109/07853890.2012.663926. [DOI] [PubMed] [Google Scholar]
- 12.Jain SK, et al. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46(12):1633–8. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai GY, et al. Effect of N-acetylcysteine on the early expression of inflammatory markers in the retina and plasma of diabetic rats. Clin Experiment Ophthalmol. 2009;37(2):223–31. doi: 10.1111/j.1442-9071.2009.02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Mattia G, et al. Reduction of oxidative stress by oral N-acetyl-L-cysteine treatment decreases plasma soluble vascular cell adhesion molecule-1 concentrations in non-obese, non-dyslipidaemic, normotensive, patients with non-insulin-dependent diabetes. Diabetologia. 1998;41(11):1392–6. doi: 10.1007/s001250051082. [DOI] [PubMed] [Google Scholar]
- 15.Elliott P, et al. Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. Intersalt Cooperative Research Group. BMJ. 1996;312(7041):1249–53. doi: 10.1136/bmj.312.7041.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res. 2015;116(6):1046–57. doi: 10.1161/CIRCRESAHA.116.303771. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension. 1999;33(2):686–8. doi: 10.1161/01.hyp.33.2.686. [DOI] [PubMed] [Google Scholar]
- 18.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res. 2002;39(1):41–50. doi: 10.1159/000048992. [DOI] [PubMed] [Google Scholar]
- 19.Nurkiewicz TR, et al. Decreased arteriolar tetrahydrobiopterin is linked to superoxide generation from nitric oxide synthase in mice fed high salt. Microcirculation. 2010;17(2):147–57. doi: 10.1111/j.1549-8719.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J, et al. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol. 2004;286(2):H575–83. doi: 10.1152/ajpheart.00331.2003. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. J Vasc Res. 2007;44(5):382–90. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 22.Jablonski KL, et al. Dietary sodium restriction reverses vascular endothelial dysfunction in middle-aged/older adults with moderately elevated systolic blood pressure. J Am Coll Cardiol. 2013;61(3):335–43. doi: 10.1016/j.jacc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablonski KL, et al. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Ther Adv Cardiovasc Dis. 2009;3(5):347–56. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tzemos N, et al. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51(6):1525–30. doi: 10.1161/HYPERTENSIONAHA.108.109868. [DOI] [PubMed] [Google Scholar]
- 25.DuPont JJ, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31(3):530–6. doi: 10.1097/HJH.0b013e32835c6ca8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eisenach JH, et al. Sex differences in salt sensitivity to nitric oxide dependent vasodilation in healthy young adults. J Appl Physiol (1985) 2012;112(6):1049–53. doi: 10.1152/japplphysiol.01197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson KM, Clifton PM, Keogh JB. Endothelial function is impaired after a high-salt meal in healthy subjects. Am J Clin Nutr. 2011;93(3):500–5. doi: 10.3945/ajcn.110.006155. [DOI] [PubMed] [Google Scholar]
- 28.Holowatz LA, Thompson-Torgerson CS, Kenney WL. The human cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2008;105(1):370–2. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- 29.RG IJ, et al. Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest. 2003;33(7):536–42. doi: 10.1046/j.1365-2362.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 30.Abularrage CJ, et al. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005;42(3):574–81. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Greaney JL, et al. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590(Pt 21):5519–28. doi: 10.1113/jphysiol.2012.236992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DuPont JJ, Farquhar WB, Edwards DG. Intradermal microdialysis of hypertonic saline attenuates cutaneous vasodilatation in response to local heating. Exp Physiol. 2011;96(7):674–80. doi: 10.1113/expphysiol.2011.058404. [DOI] [PubMed] [Google Scholar]
- 33.Bruning RS, et al. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol. 2012;112(12):2019–26. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol. 2007;293(2):H1090–6. doi: 10.1152/ajpheart.00295.2007. [DOI] [PubMed] [Google Scholar]
- 35.Holowatz LA, Thompson CS, Kenney WL. Acute ascorbate supplementation alone or combined with arginase inhibition augments reflex cutaneous vasodilation in aged human skin. Am J Physiol Heart Circ Physiol. 2006;291(6):H2965–70. doi: 10.1152/ajpheart.00648.2006. [DOI] [PubMed] [Google Scholar]
- 36.Boutrou R, et al. Sequential release of milk protein-derived bioactive peptides in the jejunum in healthy humans. Am J Clin Nutr. 2013;97(6):1314–23. doi: 10.3945/ajcn.112.055202. [DOI] [PubMed] [Google Scholar]
- 37.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol (1985) 2001;91(4):1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 38.Alexander LM, Kutz JL, Kenney WL. Tetrahydrobiopterin increases NO-dependent vasodilation in hypercholesterolemic human skin through eNOS-coupling mechanisms. Am J Physiol Regul Integr Comp Physiol. 2013;304(2):R164–9. doi: 10.1152/ajpregu.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanhewicz AE, et al. Sex- and limb-specific differences in the nitric oxide-dependent cutaneous vasodilation in response to local heating. Am J Physiol Regul Integr Comp Physiol. 2014;307(7):R914–9. doi: 10.1152/ajpregu.00269.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson JM, et al. Effect of local warming on forearm reactive hyperaemia. Clin Physiol. 1986;6(4):337–46. doi: 10.1111/j.1475-097x.1986.tb00239.x. [DOI] [PubMed] [Google Scholar]
- 41.Bruning RS, et al. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol (1985) 2012;112(12):2019–26. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kellogg DL, Jr, Zhao JL, Wu Y. Roles of nitric oxide synthase isoforms in cutaneous vasodilation induced by local warming of the skin and whole body heat stress in humans. J Appl Physiol (1985) 2009;107(5):1438–44. doi: 10.1152/japplphysiol.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Minson CT. Thermal provocation to evaluate microvascular reactivity in human skin. J Appl Physiol. 2010;109(4):1239–46. doi: 10.1152/japplphysiol.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aburto NJ, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ. 2013;346:f1326. doi: 10.1136/bmj.f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore LL, et al. Dietary Approaches to Stop Hypertension (DASH) eating pattern and risk of elevated blood pressure in adolescent girls. Br J Nutr. 2012;108(9):1678–85. doi: 10.1017/S000711451100715X. [DOI] [PubMed] [Google Scholar]
- 46.Livingstone KM, et al. Does dairy food intake predict arterial stiffness and blood pressure in men?: Evidence from the Caerphilly Prospective Study. Hypertension. 2013;61(1):42–7. doi: 10.1161/HYPERTENSIONAHA.111.00026. [DOI] [PubMed] [Google Scholar]
- 47.Yoshizawa M, et al. Additive beneficial effects of lactotripeptides and aerobic exercise on arterial compliance in postmenopausal women. Am J Physiol Heart Circ Physiol. 2009;297(5):H1899–903. doi: 10.1152/ajpheart.00433.2009. [DOI] [PubMed] [Google Scholar]
- 48.Crichton GE, et al. Relations between dairy food intake and arterial stiffness: pulse wave velocity and pulse pressure. Hypertension. 2012;59(5):1044–51. doi: 10.1161/HYPERTENSIONAHA.111.190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holowatz LA, Thompson-Torgerson C, Kenney WL. Aging and the control of human skin blood flow. Front Biosci (Landmark Ed) 2010;15:718–39. doi: 10.2741/3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minson CT, et al. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol (1985) 2002;93(5):1644–9. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 51.Botham KM, Wheeler-Jones CP. Postprandial lipoproteins and the molecular regulation of vascular homeostasis. Prog Lipid Res. 2013;52(4):446–64. doi: 10.1016/j.plipres.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Mah E, et al. Postprandial hyperglycemia impairs vascular endothelial function in healthy men by inducing lipid peroxidation and increasing asymmetric dimethylarginine:arginine. J Nutr. 2011;141(11):1961–8. doi: 10.3945/jn.111.144592. [DOI] [PubMed] [Google Scholar]
- 53.Zhang QX, et al. Hydrophobicity of whey protein hydrolysates enhances the protective effect against oxidative damage on PC 12 cells. J Dairy Res. 2015;82(1):1–7. doi: 10.1017/S0022029914000405. [DOI] [PubMed] [Google Scholar]
- 54.Ballard KD, et al. Low-fat milk ingestion prevents postprandial hyperglycemia-mediated impairments in vascular endothelial function in obese individuals with metabolic syndrome. J Nutr. 2013;143(10):1602–10. doi: 10.3945/jn.113.179465. [DOI] [PubMed] [Google Scholar]


