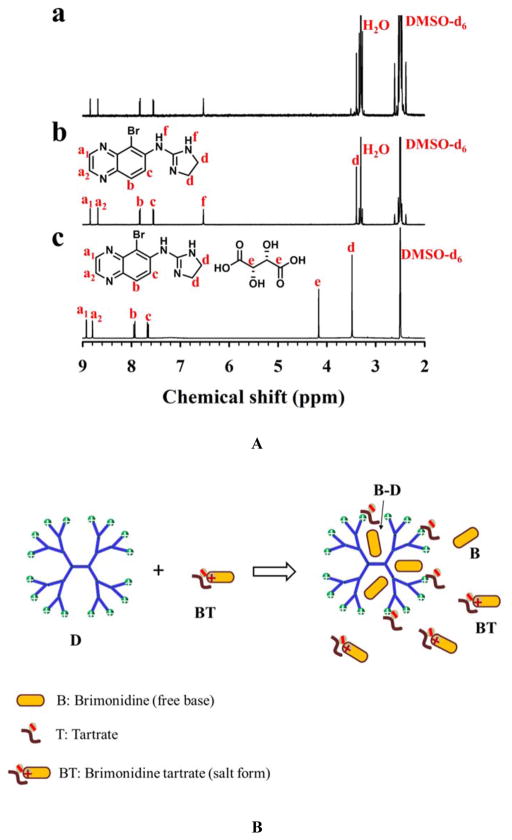
Abstract
In this work, we developed a mildly cross-linked dendrimer hydrogel (mcDH) via aza-Michael addition of polyamidoamine (PAMAM) dendrimer G5 and polyethylene glycol diacrylate (PEG-DA, Mn=575 g/mol). We chose the antiglaucoma drug brimonidine tartrate as a model drug and developed a new antiglaucoma drug formulation on the basis of mcDH. Cytotoxicity of the mcDH formulation to NIH3T3 fibroblasts, in vitro drug release kinetics and ex vivo drug permeability across the rabbit cornea were examined. We also studied interactions between PAMAM dendrimer and the drug using 1H NMR spectroscopy for a mechanistic understanding of brimonidine release from the mcDH. mcDH was found to be efficient unionizing brimonidine tartrate to form and encapsulate brimonidine free base for sustained release and enhanced corneal permeation.
Keywords: Dendrimer Hydrogel, Brimonidine Tartrate, Glaucoma, Topical Formulation
Graphical Abstract
A mildly cross-linked dendrimer hydrogel (mcDH) via the aza-Michael addition of polyamidoamine (PAMAM) dendrimer G5 and polyethylene glycol diacrylate (PEG-DA, Mn=575 g/mol) was developed and tested for delivery of the antiglaucoma drug brimonidine tartrate. mcDH was found to be efficient unionizing brimonidine tartrate to form and encapsulate brimonidine free base for sustained release and enhanced corneal permeation.
1. INTRODUCTION
Glaucoma is a group of diseases that can damage the eye’s optic nerve and result in blindness.1 WHO estimated that by 2020, global glaucoma patients will reach 80 million.2 Early detection and treatment can effectively curb the deterioration of glaucoma. To date, topical administration is the safest and effective treatment for most ocular diseases because of ease of use and low-cost manufacturing.3–5 Efforts have been devoted to develop drug delivery systems to extend drug activity, thus reducing dosing frequency and improving patient compliance.6–8 Topical drug vehicles are expected to be biocompatible, mucoadhesive, and have suitable viscosity.9, 10 Lipids, Hyaluronic acid, PEG, PLGA and chitosan are commonly used polymers as topical drug delivery carriers and have been fabricated into particles and hydrogels.11–19 In particular, particle-based colloidal drug delivery systems help overcome the cornea barrier and realize prolonged drug release.20–22 In addition, they do not cause blurriness.23 Dendrimers constituted of a focal core and successive branching units, are well-defined branched macromolecules with highly compact three-dimensional nanostructures. The monomolecular dendrimer itself is a nanoparticle. Dendrimers offer a high degree of flexibility in carrying drugs.24–27 Drugs can be either covalently conjugated to the dendrimer surface or physically encapsulated into the inner core.28–32 Also, the cationic dendrimers, for example, polyamidoamine dendrimer (PAMAM), can be preferentially captured at the negatively charged corneal surface and therefore enhance the permeability of the formulations.33–35 In-situ forming hydrogels are also often used as topical drug delivery systems because of ease of administration.36, 37 The application of hydrogels can result in prolonged pre-corneal residence time and increased ocular bioavailability.8, 38, 39 Our group has developed a hybrid dendrimer hydrogel/PLGA nanoparticles platform and used it to fabricate a brimonidine/timolol maleate combination formulation for topical application.38 This platform is capable of enhancing drug bioavailability and substantially sustaining drug activity following topical administration.
Recently we reported an in-situ forming PAMAM dendrimer hydrogel platform with tunable properties prepared via the highly efficient aza-Michael addition reaction.40 In this work, we chose the antiglaucoma drug brimonidine tartrate as a model drug and developed a new antiglaucoma drug formulation using a mildly cross-linked dendrimer hydrogel (mcDH) prepared by the aza-Michael addition reaction. We examined the cytotoxicity of the mcDH formulation, drug release kinetics and ex vivo drug influx across rabbit cornea. The results showed that a sustained in vitro brimonidine release and enhanced permeability had been realized by using this mcDH. For a mechanistic understanding of brimonidine release from the mcDH, we also studied interactions between PAMAM dendrimer and the drug.
2. MATERIALS AND METHODS
2.1. Materials
Diaminobutane (DAB) core PAMAM dendrimer generation 5 (PAMAM G5) was purchased from NanoSynthons (Mt. Pleasant, MI). Polyethylene glycol diacrylate (PEG-DA, Mn=575 g/mol), brimonidine (free base), sodium taurodeoxycholate hydrate (STDC hydrate), deuterated dimethyl sulfoxide (DMSO-d6) and tartrate were purchased from Sigma-Aldrich (St. Louis, MO). Brimonidine tartrate (BT) (salt form) was purchased from AvaChem Scientific (San Antonio, TX). Acetonitrile (ACN), water (HPLC grade), and phosphate-buffered saline (PBS) were purchased from Fisher Scientific (Pittsburgh, PA). SnakeSkin dialysis tubing with 3500 molecular weight cut-off (MWCO) was purchased from Thermo Fisher Scientific (Waltham, MA). Fresh rabbit whole eyes were purchased from Pel-Freeze Biologicals (Rogers, AR).
2.2 Formulation Preparation
Small batches of mcDH were prepared. For each batch, BT loaded dendrimer hydrogel formulations (BT/mcDH) were prepared in two steps: 1) 300 μg of PAMAM G5 and 200 μg of brimonidine tartrate in the presence or absence of 200 μg of permeation enhancer STDC hydrate41 were mixed in 100 μL of pH 7.4 PBS and vortexed vigorously; 2) 380 μg of PEG-DA was then added to the mixture and shaken overnight at 100 rpm. Brimonidine tartrate PBS solutions (BT/PBS) were also prepared on the basis of 200 μg per 100 μL and included as a control. The pH of each BT-containing formulation was measured by using an accumet™ AE150 pH benchtop meter.
2.3 Rheological Testing
Rheological testing was performed using a 20 mm diameter disk on a temperature controlled plate of an HR-3 Discovery Hybrid Rheometer (TA Instruments, New Castle, DE).35 Each set of samples was subjected to compression and shear stress at 25 °C. An amplitude sweep test was performed to ensure that all the measurements were conducted within the linear viscoelastic region. Oscillatory frequency sweep test was then carried out with a constant strain of 0.1% in the frequency region of 0.1–10 rad/s.
2.4 Scanning Electron Microscopy (SEM)
DH was lyophilized and mounted on an aluminum stub and sputter-coated with platinum for 90 s. The SEM images of DH were taken on a JEOL LV-5610 scanning electron microscope at 20 kV.
2.5 Cytotoxicity Assay
NIH3T3 fibroblasts were seeded at a density of 1×104 cells per well in a 96-well plate and cultured for one day to allow cell attachment. The cells were then treated with mcDH and BT/mcDH with STDC hydrate at various concentrations for 24 h and 48 h, respectively. Cell viability relative to untreated cells was then determined by using WST-1 proliferation assay.42
2.6 Ex Vivo Permeation Studies
Corneas were extracted from fresh rabbit eyes and mounted in a Franz diffusion cell system.23 The permeability of brimonidine tartrate in BT/mcDH and BT/PBS was evaluated. The receiver chamber was filled with PBS. BT-containing formulation (200 μL) was loaded to the donor chamber. At pre-determined time points up to 4 h, an aliquot of 500 μL from the receiver chamber was withdrawn and analyzed with HPLC. Fresh PBS (500 μL) was added to the receiver chamber following each sampling. Brimonidine tartrate in PBS (containing 200 μg of STDC hydrate) was also studied as a control. All the experiments were conducted in triplicate. The permeability coefficient, P, was then determined.43
2.7 In Vitro Drug Release Kinetics
BT/mcDH, BT/mcDH with STDC hydrate or BT/PBS (500 μL) was transferred to a dialysis tube (MWCO = 3500 Da) and suspended in 50-mL centrifuge tube filled with 30 mL of PBS. The tube was maintained at 37 °C. At the pre-determined time points (15 min, 30 min, 1 h, 2 h, 4 h, 6 h, 24 h, 48 h), an aliquot (1 mL) was withdrawn from outside the dialysis tube and filled with an equal volume amount of fresh PBS. The withdrawn samples were analyzed by using a reverse phase HPLC system equipped with UV detector.24 Cumulative release of brimonidine was then determined. All the experiments were performed in triplicate.
2.8 Study of Interactions between PAMAM Dendrimer and Brimonidine Tartrate
Brimonidine tartrate (5 mg) was added to 1 mL of pH 7.4 PBS solution containing 3 mg of PAMAM dendrimer G5 at room temperature. The resulting precipitate was separated from the mixture via centrifugation at 10 000 rpm, collected, washed with water, and freeze-dried. The 1H NMR spectrum of the precipitate in deuterated dimethyl sulfoxide (DMSO-d6) was obtained on a Bruker 600 MHz spectrometer. The 1H NMR spectra of brimonidine (free base) and brimonidine tartrate standards were also obtained for comparison.
3. RESULTS AND DISCUSSION
3.1. mcDH Possessing both Fluidity and Gel Viscoelasticity
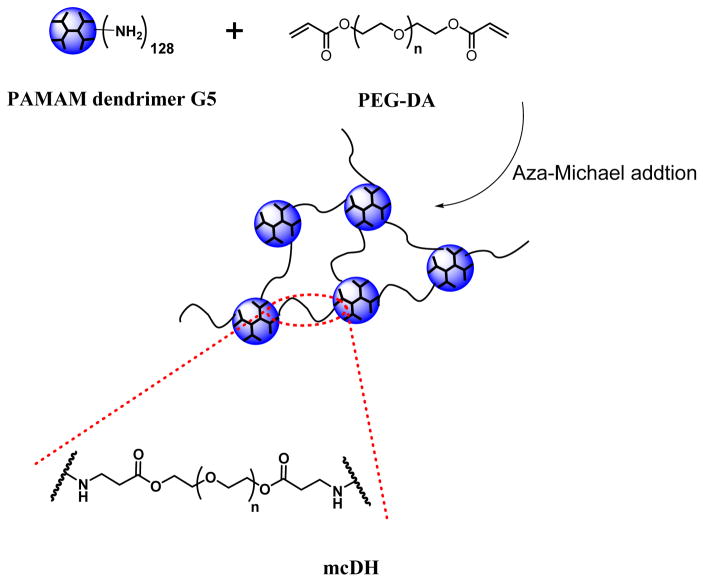
As shown in Scheme 1, a mildly cross-linked dendrimer hydrogel (mcDH) was prepared by reacting PAMAM G5 at a low concentration (0.3 wt% aqueous solution) with short chain PEG-DA (Mn=575 g/mol) at a molar ratio of 1:64. The resulting mcDH remained in the liquid form but displayed gel properties.
Scheme 1.
Preparation and structure illustration of the mcDH.
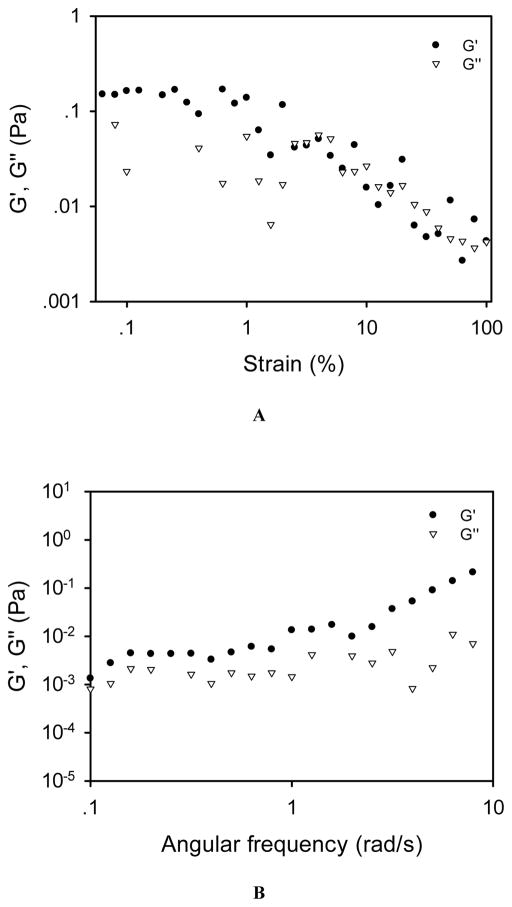
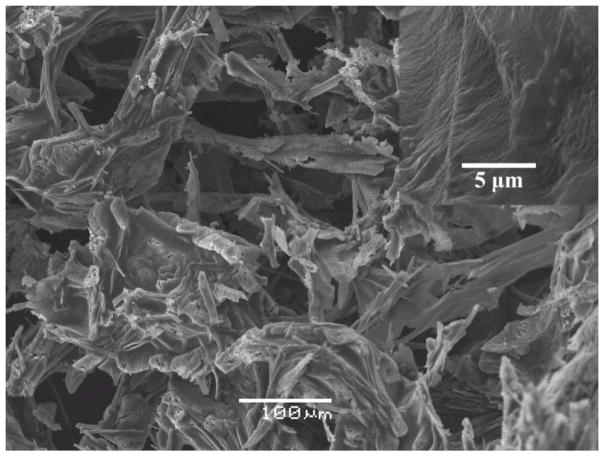
The linear viscoelastic region (LVR) of this liquid hydrogel was 0.1%–1% according to the amplitude sweep test result (Figure 1A). The frequency sweep test was then conducted at a fixed strain within the LVR, i.e., 0.1%. As shown in Figure 1B, mcDH displays a typical hydrogel viscoelastic behavior as its storage modulus (G′) is higher than its loss moduli (G″) with frequency-independence at a lower frequency range. The SEM image of mcDH (Figure 2) reveals the network morphology in the gel. The inset on the upper right corner of the figure shows the rough texture as a result of the cross-linking. The results presented above indicated that the formed mcDH possessed both fluidity and viscoelastic gel properties, making it a suitable platform for the development of topical ocular drug formulations.
Figure 1.
Rheological characterization of the mcDH. (A) Oscillatory amplitude sweep test. (B) Oscillatory frequency sweep test.
Figure 2.
SEM image of the mcDH.
3.2. Cytocompatibility of mcDH
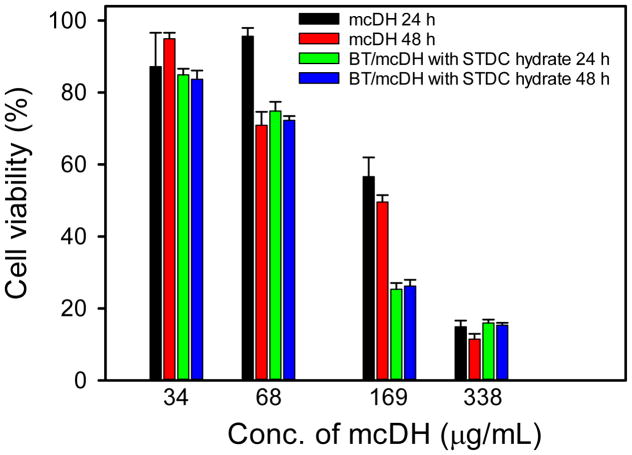
The cytotoxicity of mcDH and BT/mcDH containing STDC hydrate on NIH3T3 fibroblasts was tested. As shown in Figure 3, mcDH shows dose-dependent toxicity. However, it is highly cytocompatible and has no toxicity at 34 μg/mL following 24 h or 48 h exposure. It is nontoxic to the cells at 68 μg/mL following 24-h incubation. However, mcDH at 68 μg/mL shows minor cytotoxicity when incubation time is extended to 48 h. BT/mcDH with the addition of STDC hydrate has the similar dose-dependent toxicity. It has no toxicity at 34 μg/mL following 24 h or 48 h exposure. This result indicates that the BT loading and the addition of permeability enhancer do not affect to the cytocompatibility of the formulation.
Figure 3.
Cytotoxicity of the mcDH and BT/mcDH with STDC hydrate on NIH3T3 fibroblasts (n = 4).
3.3. In Vitro Transport of Brimonidine across Cornea
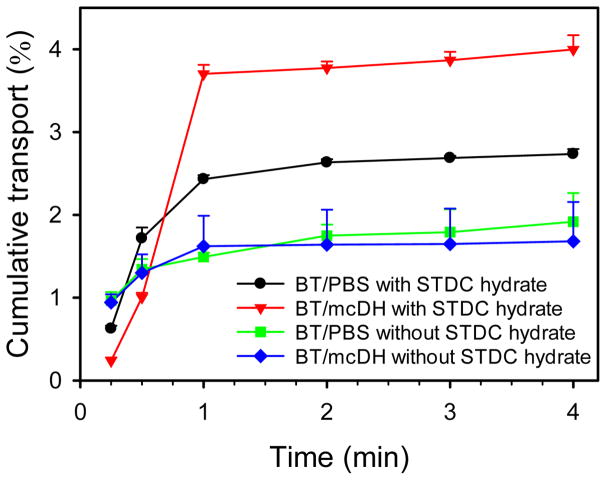
Transport of brimonidine across rabbit cornea was tested for BT/PBS and BT/mcDH. The flux of brimonidine in both PBS and DH kept increasing within the first 1 h and reached a plateau afterward (Figure 4). Permeability was then calculated according to the linear range (the first 1 h) of the cumulative flux curves. Without the use of permeation enhancer STDC hydrate, the cornea permeability of brimonidine in BT/mcDH was determined to be 6.4×10−7 cm/s, whereas the cornea permeability of brimonidine in BT/PBS was 4.5×10−7 cm/s. Permeation enhancer significantly improved brimonidine transport across the cornea. It increased the permeability of brimonidine in BT/mcDH by 5-fold to 3.4×10−6 cm/s. It also improved the permeability of brimonidine in BT/PBS by 4-fold to 1.6×10−6 cm/s. By using permeation enhancer STDC hydrate, about 15 μg of brimonidine from BT/mcDH permeated through the cornea, 50% more than that from BT/PBS.
Figure 4.
Ex vivo transport of brimonidine across the cornea with the aid of the mcDH.
3.4. In Vitro Drug Release Kinetics
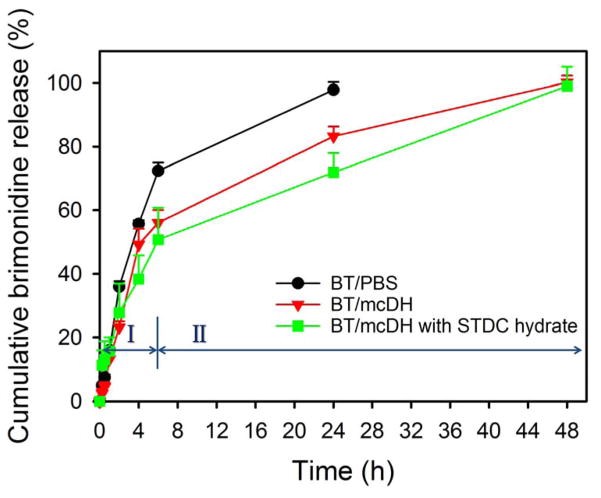
Brimonidine was released quickly from BT/PBS. More than 70% of the drug was released within 6 h and 100% drug release was achieved in 24 h (Figure 5). In contrast, a more sustained brimonidine release was achieved with BT/mcDH both with and without the permeability enhancer STDC hydrate. A two-phase release profile was evident: an initial burst release in the first 6 h and a more slowly release until 48 h.
Figure 5.
In vitro release kinetics of brimonidine from PBS, mcDH with and without STDC hydrate at 37 °C (n = 3).
To understand how mcDH achieves sustained brimonidine release, we investigated interactions between PAMAM dendrimer and brimonidine tartrate during the formulation process by using 1H NMR spectroscopy. Brimonidine free base has limited water solubility while BT has good water solubility (34 mg/mL at pH 6.5).
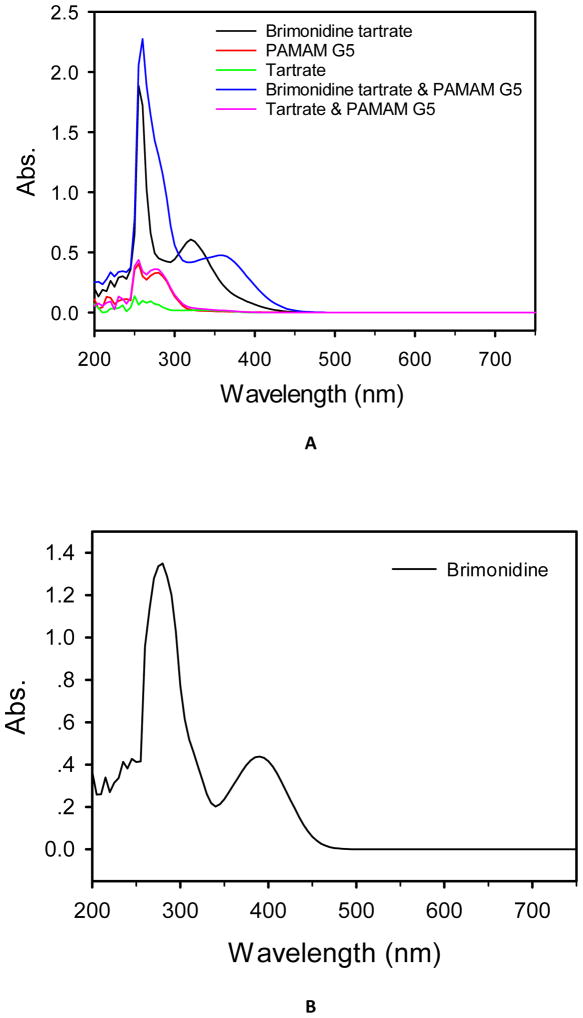
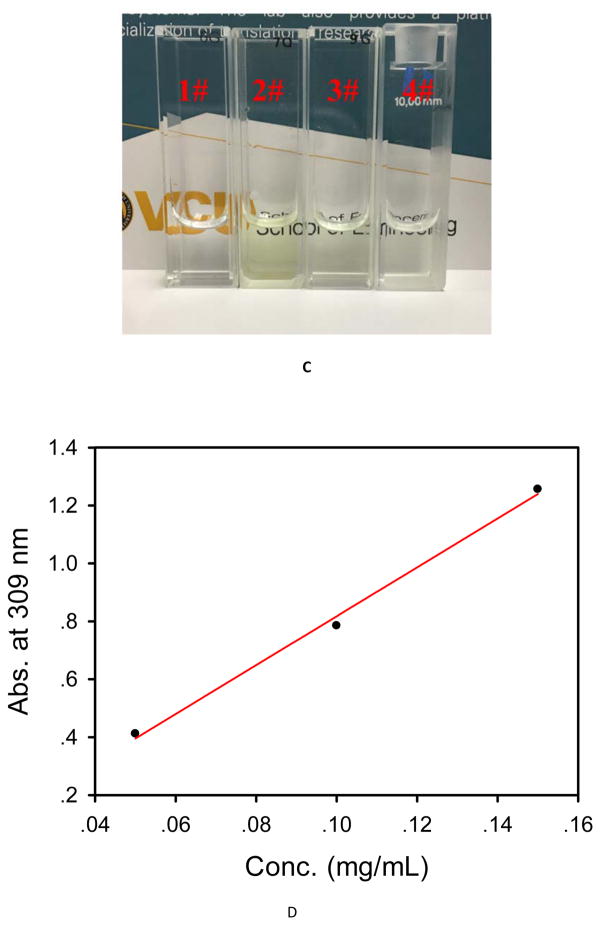
During the BT/mcDH preparation process, we noticed that mixing BT (2 mg/mL) and PAMAM dendrimer G5 (3 mg/mL) instantly turned the colorless solution into the yellowish green. A red-shift in UV-Vis spectrum was detected (Figure 6A). We attributed the color change and red-shift to the unionization of brimonidine tartrate (i.e., the formation of brimonidine free base) by PAMAM dendrimer. To validate this assumption, we increased the concentration of BT to 5 mg/mL in the presence of PAMAM dendrimer G5 (3 mg/mL), leading to the production of a precipitate. According to 1H NMR spectroscopy analysis (Figure 7A), the resulting precipitate was determined to be brimonidine free base because the spectrum matches that of brimonidine free base standard and does not show tartrate proton signals. Since excess loading BT to dendrimer could cause precipitation, we further investigated the full loading capacity of BT in the presence of PAMAM dendrimer G5 at 3 mg/mL. Especially, 3 mg of PAMAM G5 and different amounts of brimonidine tartrate (1 mg, 2 mg, 3 mg, 4 mg, 5 mg) were mixed in 1 mL of PBS and vortexed vigorously. The mixtures were centrifuged at 10 000 rpm. 50 μL of each supernatant was picked up and diluted to 1 mL. The UV absorbance at 309 nm of all the diluted solutions was measured at UV-Vis spectrometer. The absorbance of the first three diluted solutions keeps increasing, while the last two diluted solutions are the same as 1.47, which means adding 4 mg of BT has exceeded the loading capacity. We then plotted the first three absorbance values as a function of the concentration of BT (Figure 6D) and did a linear regression based on the Beer-Lambert law. The linear equation is y = 8.44x-0.0263. The BT loading capacity to G5 is then determined to be 3.6 mg BT/3 mg G5/1 mL PBS.
Figure 6.
(A) UV-Vis spectra of brimonidine tartrate (2 mg/mL), PAMAM dendrimer G5 (3 mg/mL), tartrate (0.7 mg/mL), the mixture of brimonidine tartrate (2 mg/mL) and PAMAM dendrimer G5 (3 mg/mL), and the mixture of tartrate (0.7 mg/mL) and PAMAM dendrimer G5 (3 mg/mL) in deionized H2O. (B) UV-Vis spectrum of brimonidine in DMSO. (C) Pictures of brimonidine tartrate aqueous solution (1#), brimonidine DMSO solution (2#), brimonidine tartrate and PAMAM G5 mixture aqueous solution (3#), brimonidine tartrate loaded into dendrimer hydrogel (4#). (D) UV-Vis absorbance at 309 nm as a function of the concentration of brimonidine tartrate in the mixture of brimonidine tartrate and PAMAM dendrimer G5.
Figure 7.
Interactions of PAMAM dendrimers and brimonidine tartrate. (A) It is postulated that upon mixing, PAMAM dendrimer (D) unionizes brimonidine tartrate (BT), leading to formation of brimonidine free base (B) in the solution as well as entrapment of brimonidine free base into the dendrimer core (B–D) via hydrophobic interactions. Brimonidine exists in three forms: BT, B, and B–D in the presence of PAMAM dendrimer. (B) 1H NMR spectroscopy analysis confirms that PAMAM dendrimer converts brimonidine tartrate to brimonidine free base. a) 1H NMR spectrum of the precipitates resulting from mixing of brimonidine tartrate and PAMAM dendrimer G5, δ (ppm) 8.85 (d, J = 1.9 Hz, 1H), 8.69 (d, J = 1.9 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.55 (d, J = 9.0 Hz, 1H), 6.53 (s, 2H), 3.40 (s, 4H) b) 1H NMR spectrum of brimonidine free base standard, δ (ppm) 8.85 (d, J = 1.9 Hz, 1H), 8.69 (d, J = 1.9 Hz, 1H), 7.83 (d, J = 9.0 Hz, 1H), 7.55 (d, J = 9.0 Hz, 1H), 6.53 (s, 2H), 3.40 (s, 4H); and c) 1H NMR spectrum of brimonidine tartrate standard. δ (ppm) 8.93 (d, J = 1.9 Hz, 1H), 8.80 (d, J = 1.9 Hz, 1H), 7.94 (d, J = 9.0 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 4.17 (s, 2H), 3.48 (s, 4H).
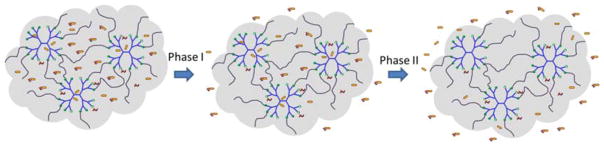
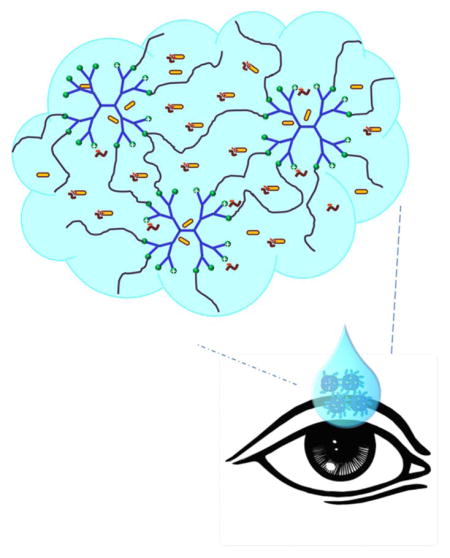
BT/PBS had a pH of 7.1, which was relatively neutral. The pH of BT/mcDH was 7.6, closer to the pH of the lacrimal fluid (7.45). The pH values of the two formulations are within the ocular comfort range (6.6–7.8).44 Our study suggests that PAMAM dendrimer is efficient unionizing brimonidine tartrate at a pH within the ocular comfort range. Driven by the hydrophobic interaction, brimonidine free base is entrapped into the dendrimer’s hydrophobic core upon the unionization by the dendrimer (Figure 7B). Brimonidine exists in three forms: free base form, salt form and encapsulation form. Brimonidine free base is a lipid soluble form. Increasing pH causes a higher proportion of brimonidine free base form. The proportions of brimonidine free base form and salt form (pKa=7.8) are affected by pH and can be theoretically estimated by using the Henderson-Hasselbalch equation. Because of an elevated pH and drug encapsulation by PAMAM dendrimer, a higher fraction of the brimonidine free base form (39% vs. 17%) can be generated in BT/mcDH, accounting for enhanced corneal permeation. Brimonidine free base form and salt form in the loosely 3-D network of the mcDH are released primarily in the burst release phase I. Brimonidine entrapped in the dendrimer core is released slowly and its release lasts longer and extends to phase II (Figure 8). This mcDH formulation enables the two-phase drug release kinetics. The burst release in phase I within the first 6 hours allows the drug to rapidly reach an effective concentration, whereas the sustained release in phase II continuously supplies the drug to maintain the therapeutic drug level over an extended period of time.
Figure 8.
Two-phase brimonidine release from the mcDH. Phase I: burst release of brimonidine that exists as free base and salt form in the cross-linked network; Phase II: more sustained release of brimonidine free base that is entrapped in dendrimer core.
4. CONCLUSIONS
In this work, a novel mildly cross-linked dendrimer hydrogel through aza-Michael addition was developed for topical delivery of brimonidine tartrate. The dendrimer hydrogel can unionize brimonidine tartrate to form and encapsulate brimonidine free base for sustained release and enhanced corneal permeation.
Acknowledgments
This work was supported by the National Institutes of Health (R01EY024072).
References
- 1.Gupta CP, Glaucoma D. Am Fam Physician. 2016;93:663. [PubMed] [Google Scholar]
- 2.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jian HJ, Wu RS, Lin TY, Li YJ, Lin HJ, Harroun SG, Lai JY, Huang CC. Super-cationic carbon quantum dots synthesized from spermidine as an eye drop formulation for topical treatment of bacterial keratitis. ACS Nano. 2017;11:6703. doi: 10.1021/acsnano.7b01023. [DOI] [PubMed] [Google Scholar]
- 4.Li M, Xin M, Guo C, Lin G, Wu X. New nanomicelle curcumin formulation for ocular delivery: improved stability, solubility, and ocular anti-inflammatory treatment. Drug Dev Ind Pharm. 2017;43:1846. doi: 10.1080/03639045.2017.1349787. [DOI] [PubMed] [Google Scholar]
- 5.Sheybani ND, Yang H. Pediatric ocular nanomedicines: Challenges and opportunities. Chinese Chem Lett. 2017 doi: 10.1016/j.cclet.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickmann L. Ocular therapeutics: Drug delivery and pharmacology. Mol Pharm. 2016;13:2875. doi: 10.1021/acs.molpharmaceut.6b00703. [DOI] [PubMed] [Google Scholar]
- 7.Mandal A, Cholkar K, Khurana V, Shah A, Agrahari V, Bisht R, Pal D, Mitra AK. Topical formulation of self-assembled antiviral prodrug nanomicelles for targeted retinal delivery. Mol Pharm. 2017;14:2056. doi: 10.1021/acs.molpharmaceut.7b00128. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Lei Y, Dai Z, Liu X, Huang T, Wu J, Xu ZP, Sun X. Sustained release of brimonidine from a new composite drug delivery system for treatment of glaucoma. ACS Appl Mater Interfaces. 2017;9:7990. doi: 10.1021/acsami.6b16509. [DOI] [PubMed] [Google Scholar]
- 9.Chi H, Gu Y, Xu T, Cao F. Multifunctional organic–inorganic hybrid nanoparticles and nanosheets based on chitosan derivative and layered double hydroxide: cellular uptake mechanism and application for topical ocular drug delivery. Int J Nanomedicine. 2017;12:1607. doi: 10.2147/IJN.S129311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousry C, Elkheshen SA, El-laithy HM, Essam T, Fahmy RH. Studying the influence of formulation and process variables on Vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur J Pharm Sci. 2017;100:142. doi: 10.1016/j.ejps.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Battaglia L, Serpe L, Foglietta F, Muntoni E, Gallarate M, Del Pozo Rodriguez A, Solinis MA. Application of lipid nanoparticles to ocular drug delivery. Expert Opi Drug Deliv. 2016;13:1743. doi: 10.1080/17425247.2016.1201059. [DOI] [PubMed] [Google Scholar]
- 12.Diebold Y, Jarrín M, Sáez V, Carvalho ELS, Orea M, Calonge M, Seijo B, Alonso MJ. Ocular drug delivery by liposome-chitosan nanoparticle complexes (LCS-NP) Biomaterials. 2007;28:1553. doi: 10.1016/j.biomaterials.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Li J, Cheng B, Wu Q, Pan H. Ex vivo and in vivo evaluation of the effect of coating a coumarin-6-labeled nanostructured lipid carrier with chitosan-N-acetylcysteine on rabbit ocular distribution. Mol Pharm. 2017;14:2639. doi: 10.1021/acs.molpharmaceut.7b00069. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Dozois MD, Chang CN, Ahmad A, Ng DLT, Hileeto D, Liang H, Reyad MM, Boyd S, Jones LW, Gu FX. Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol Pharm. 2016;13:2897. doi: 10.1021/acs.molpharmaceut.6b00445. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Yan J, Zhou Z, Amsden BG. Dithiol-PEG-PDLLA micelles: preparation and evaluation as potential topical ocular delivery vehicle. Biomacromolecules. 2014;15:1346. doi: 10.1021/bm4018879. [DOI] [PubMed] [Google Scholar]
- 16.Rusanu A, Tama, Isabela A, Vulpe R, Rusu A, Butnaru M, Vereştiuc L. Biocompatible and biodegradable hydrogels based on chitosan and gelatin with potential applications as wound dressings. J Nanosci Nanotechnol. 2017;17:4584. [Google Scholar]
- 17.Kalam MA. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int J Biol Macromol. 2016;89:127. doi: 10.1016/j.ijbiomac.2016.04.070. [DOI] [PubMed] [Google Scholar]
- 18.Sánchez-López E, Egea MA, Cano A, Espina M, Calpena AC, Ettcheto M, Camins A, Souto EB, Silva AM, García ML. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen-in vitro, ex vivo and in vivo characterization. Colloids Surf B Biointerfaces. 2016;145:241. doi: 10.1016/j.colsurfb.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Qin J, Fan Y, Qin X, Jiang Y, Wu Z. Carboxymethyl chitosan-modified polyamidoamine dendrimer enables progressive drug targeting of tumors via pH-sensitive charge inversion. J Biomed Nanotechnol. 2016;12:667. doi: 10.1166/jbn.2016.2206. [DOI] [PubMed] [Google Scholar]
- 20.Bravo-Osuna I, Andrés-Guerrero V, Pastoriza Abal P, Molina-Martínez IT, Herrero-Vanrell R. Pharmaceutical microscale and nanoscale approaches for efficient treatment of ocular diseases. Drug Deliv Transl Res. 2016;6:686. doi: 10.1007/s13346-016-0336-5. [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Marcano DC, Shin CS, Hua X, Isenhart LC, Pflugfelder SC, Acharya G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano. 2015;9:1749. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]
- 22.Mou X, Ali Z, Li S, He N. Applications of magnetic nanoparticles in targeted drug delivery system. J Nanosci Nanotechnol. 2015;15:54. doi: 10.1166/jnn.2015.9585. [DOI] [PubMed] [Google Scholar]
- 23.Park CG, Kim YK, Kim MJ, Park M, Kim MH, Lee SH, Choi SY, Lee WS, Chung YJ, Jung YE, Park KH, Choy YB. Mucoadhesive microparticles with a nanostructured surface for enhanced bioavailability of glaucoma drug. J Control Release. 2015;220:180. doi: 10.1016/j.jconrel.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Lancina MG, Singh S, Kompella UB, Husain S, Yang H. Fast dissolving dendrimer nanofiber mats as alternative to eye drops for more efficient antiglaucoma drug delivery. ACS Biomater Sci Engineering. 2017;3:1861. doi: 10.1021/acsbiomaterials.7b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez Villanueva J, Navarro MG, Rodríguez Villanueva L. Dendrimers as a promising tool in ocular therapeutics: Latest advances and perspectives. Int J Pharm. 2016;511:359. doi: 10.1016/j.ijpharm.2016.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Lancina MG, Yang H. Dendrimers for ocular drug delivery. Can J Chem. 2017;95:897. doi: 10.1139/cjc-2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao S, Castro R, Rodrigues J, Shi X, Tomas H. PAMAM dendrimer/pDNA functionalized-magnetic iron oxide nanoparticles for gene delivery. J Biomed Nanotechnol. 2015;11:1370. doi: 10.1166/jbn.2015.2101. [DOI] [PubMed] [Google Scholar]
- 28.Caminade AM, Turrin CO. Dendrimers for drug delivery. J Mater Chem B. 2014;2:4055. doi: 10.1039/c4tb00171k. [DOI] [PubMed] [Google Scholar]
- 29.Choudhary S, Gupta L, Rani S, Dave K, Gupta U. Impact of dendrimers on solubility of hydrophobic drug molecules. Front Pharmaco. 2017;8:261. doi: 10.3389/fphar.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madaan K, Kumar S, Poonia N, Lather V, Pandita D. Dendrimers in drug delivery and targeting: Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied Sci. 2014;6:139. doi: 10.4103/0975-7406.130965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She W, Pan D, Luo K, He B, Cheng G, Zhang C, Gu Z. PEGylated dendrimer-doxorubicin cojugates as pH-sensitive drug delivery systems: synthesis and in vitro characterization. J Biomed Nanotechnol. 2015;11:964. doi: 10.1166/jbn.2015.1865. [DOI] [PubMed] [Google Scholar]
- 32.Maturavongsadit P, Bi X, Gado TA, Nie YZ, Wang Q. Adhesive peptides conjugated PAMAM dendrimer as a coating polymeric material enhancing cell responses. Chinese Chem Lett. 2016;27:1473. [Google Scholar]
- 33.Bravo-Osuna I, Vicario-de-la-Torre M, Andrés-Guerrero V, Sánchez-Nieves J, Guzmán-Navarro M, de la Mata FJ, Gómez R, de las Heras B, Argüeso P, Ponchel G, Herrero-Vanrell R, Molina-Martínez IT. Novel water-soluble mucoadhesive carbosilane dendrimers for ocular administration. Mol Pharm. 2016;13:2966. doi: 10.1021/acs.molpharmaceut.6b00182. [DOI] [PubMed] [Google Scholar]
- 34.Romero GB, Keck CM, Müller RH, Bou-Chacra NA. Development of cationic nanocrystals for ocular delivery. Eur J Pharm Biopharm. 2016;107:215. doi: 10.1016/j.ejpb.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Jeong H, Lee ES, Jung G, Park J, Jeong B, Ryu KH, Hwang NS, Lee H. Bioreducible-cationic poly(amido amine)s for enhanced gene delivery and osteogenic differentiation of tonsil-derived mesenchymal stem cells. J Biomed Nanotechnol. 2016;12:1023. doi: 10.1166/jbn.2016.2223. [DOI] [PubMed] [Google Scholar]
- 36.Iohara D, Okubo M, Anraku M, Uramatsu S, Shimamoto T, Uekama K, Hirayama F. Hydrophobically modified polymer/α-cyclodextrin thermoresponsive hydrogels for use in ocular drug delivery. Mol Pharm. 2017;14:2740. doi: 10.1021/acs.molpharmaceut.7b00291. [DOI] [PubMed] [Google Scholar]
- 37.Lai JY, Luo LJ. Antioxidant gallic acid-functionalized biodegradable in situ gelling copolymers for cytoprotective antiglaucoma drug delivery systems. Biomacromolecules. 2015;16:2950. doi: 10.1021/acs.biomac.5b00854. [DOI] [PubMed] [Google Scholar]
- 38.Yang H, Tyagi P, Kadam RS, Holden CA, Kompella UB. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano. 2012;6:7595. doi: 10.1021/nn301873v. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, He Z, Liang R, Ma Y, Huang W, Jiang R, Shi S, Chen H, Li X. Fabrication of a micellar supramolecular hydrogel for ocular drug delivery. Biomacromolecules. 2016;17:798. doi: 10.1021/acs.biomac.5b01526. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, He H, Cooper RC, Yang H. In situ-forming polyamidoamine dendrimer hydrogels with tunable properties prepared via aza-Michael addition reaction. ACS Appl Mater Interfaces. 2017;9:10494. doi: 10.1021/acsami.7b00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Zolotarskaya OY, Yeudall WA, Yang H. Click hybridization of immune cells and polyamidoamine dendrimers. Adv Healthc Mater. 2014;3:1430. doi: 10.1002/adhm.201300515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Q, Fu Y, Kao WJ, Janigro D, Yang H. Transbuccal delivery of CNS therapeutic nanoparticles: synthesis, characterization, and in vitro permeation studies. ACS Chem Neurosci. 2011;2:676. doi: 10.1021/cn200078m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carney LG, Hill RM. Human tear pH. Diurnal variations. Arch Ophthalmol. 1976;94:821. doi: 10.1001/archopht.1976.03910030405011. [DOI] [PubMed] [Google Scholar]