Abstract
Background
World Health Organization (WHO) recognizes five groups of pulmonary hypertension (PH), categorized by etiology or comorbidity: 1-pulmonary arterial hypertension 2-left-heart disease, 3-hypoxia/lung disease, 4-chronic thromboembolic disease and 5-miscellaneous. The epidemiology of PH, apart from Group-1, is largely unknown.
Methods and Results
We describe incidence, prevalence, comorbidities, mortality and prescribing patterns for Groups 1–4 PH from 1993–2012. Case definitions are based on hospitalization and emergency department visits, using the Institute for Clinical Evaluative Sciences data, which comprises linked databases of universal coverage health service records for Ontario residents. This cohort included 50,529 PH patients. The annual incidence of adult PH increased from 2003 to 2012 from 24.1 to 28.7 cases/100,000 population and the annual prevalence from 1993 to 2012 from 99.8 to 127.3 cases/100,000 population, respectively. The most common form of adult PH was Group 2, alone (34.2%) or combined with Group 3 PH (29.3%). A diagnosis of PH increased the 1-year standardized mortality ratio 7.2-fold. Mortality in adults with PH was 13.0%, 36.4% and 62.4%, at 30-days, 1-year and 5-years, respectively. Mortality was highest in Groups 2 and 3 and lowest in Group 1. PH was present in only 3.6% of people with left heart disease, 0.7% with lung disease and 1.4% with thromboembolic disease, suggesting that PH is a relatively rare complication of these common diseases. Children (age <16 years) accounted for 3.6% of the cohort. In children Group 1 PH was most common (65.2%), and 5-year mortality was lower (21.4%) than in adults. Group 1-specific PH therapies were increasingly prescribed over time and paradoxically were often used in patients who appear to have Group 2, PH based on diagnostic codes indicating left heart disease.
Conclusions
The incidence and prevalence of adult PH is increasing. Group 2 and 3 are the most common and lethal forms of PH. This study identifies an emerging epidemic of pulmonary hypertension that likely has substantial adverse health and economic implications.
Introduction
The World Health Organization (WHO) defines pulmonary hypertension (PH) as a hemodynamic condition in which the resting mean pulmonary artery pressure (PAP) is ≥ 25mmHg.1 The diagnosis of PH cannot be reliably made by echocardiography alone, since it is defined by hemodynamic criteria that mandate right heart catheterization (RHC). While in theory the definition of PH requires RHC, in practice PH is usually identified by Doppler ultrasound, using the velocity of the tricuspid regurgitation jet to estimate the right ventricular systolic pressure and thereby infer PAP. The WHO recognizes five PH Groups:1 Group 1 due to pulmonary arterial hypertension (PAH); Group 2 PH related to left heart disease; Group 3 PH due to lung diseases and/or hypoxia; Group 4 due to chronic thromboembolic PH (CTEPH); and Group 5 which is a heterogeneous collection of PH syndromes (including sickle cell disease and sarcoidosis).
There are five classes of approved medications for Group 1 PH [calcium channel blockers (CCB), prostanoids, endothelin receptor antagonists (ETRA), phosphodiesterase-5 inhibitors (PDE5i) and soluble guanylate cyclase stimulator (sGCs)] and one for Group 4 PH (sGCs). The average annual health care costs associated with PH in the United States from 2004 to 2010 was US$100,000/Group 1 patient, $35,000 of which consisted of pharmacy costs.2 In 2012, drug therapy for Group 1 PH in Canada incurred substantial, albeit lower, annual costs/patient [US$ 4569± 1544].3 There are no PH-targeted medications approved for PH Groups 2 and 3, and therapy for these patients largely targets co-morbid conditions, such as systemic hypertension or valvular heart disease. Medications for Group 1 are expensive and may be ineffective (as in the case of ETRA) or harmful (as in the case of prostanoids) when used in Group 2 patients. 4, 5
Most previous epidemiological studies have focused on adult PH patients with Group 1 disease, including four American cohorts1, 6–9, five European cohorts10–14, two Chinese cohorts15, 16 and one Australian cohort17. These studies were largely conducted in specialized Group 1 referral centers and included patients in whom PH was diagnosed by echocardiography or right heart catheterization. Only two studies included information on groups other than Group 1.10, 17 A population-based Australian study of 10,000 patients identified by echocardiography17 found that Group 2 PH was the most common and lethal form. A Spanish study10 of Group 1 (866 PAH) and Group 4 (162 CTEPH) patients noted 1-, 3- and 5-yr survival rates of 87%, 75% and 65%, respectively, with no intergroup differences. The few epidemiological studies of PH performed in Canada have not been population-based and have focused on small cohorts of Group 1 patients. 18, 19
We conducted a population-based cohort study in Ontario to estimate the incidence, prevalence, mortality and prescribing patterns in WHO PH Groups 1–4 during the period from 1993 to 2012. To our knowledge, this is the first population-based study to comprehensively describe the epidemiology of PH in adults and children.
Methods
The Institute for Clinical Evaluative Sciences (ICES) data were used, which comprise databases of universal coverage health service records for Ontario residents who have Ontario Health Insurance Plan (OHIP) coverage. OHIP covers most physician and hospital services. Drug dispensing coverage for patients aged 65-years and over was obtained from the Ontario Drug Benefit Database (ODB). Hospitalization data was obtained from the Canadian Institute for Health Information Discharge Abstract Database, (CIHI-DAD) database and the National Ambulatory Care Reporting System (CIHI-NACRS) database for outpatient emergency department (ED) visits. Demographic information including mortality was obtained from the Registered Persons Database (RPDB). Databases were linked using unique individual identifiers and analyzed at the ICES. The authors declare that all supporting data are available within the article and its online supplementary files. The data set from this study is held securely in coded form at the ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full dataset creation plan is available from the authors upon request.
The study cohort consisted of Ontario residents with a primary or secondary PH diagnosis between January 1st 1993 and December 31st 2012. They were identified from hospitalizations (CIHI-DAD) and ED visits (CIHI-NACRS using ICD 9 (Code 416.0, 416.1, 416.8, 416.9) or ICD 10 (Codes I27.0, I27.1, I27.2, I27.8, I27.9). This strategy was also used to identify comorbidities. Patients missing information on gender or age were excluded from the cohort.
Patients with PH were classified into the four WHO PH groups based on diagnoses in the 5-years before their index date (using CIHI-DAD and/or CIHI-NACRS). Diagnostic codes for left heart disease, lung disease and venous thromboembolism were used to identify patients in Groups 2, 3 and 4, respectively (see Table S1 for ICD 9, ICD10). Group 5 PH (miscellaneous) was not studied because its heterogeneity precludes accurate identification in these databases. Patients were eligible to be included in multiple PH groups, except for Group 1, since Group 1 PH, by definition, should lack the diagnostic codes for the comorbidities that promote WHO Group 2–4 PH. It is acknowledged that a clinician might code a Group 1 patient as having some element of left heart or lung disease while ascribing the observed PH to PAH.
Four independent data abstractors validated our diagnostic algorithm. They were given a list of 20 diagnoses, including a mix of PH and systemic hypertension cases. The PH diagnoses were identified and coded with 100% specificity. Furthermore 100 random patients with a diagnosis of PH as captured through the database were case validated using hospital chart abstraction. We were able to verify the diagnosis of PH in all but 3 patients. The PH group inferred by our diagnostic algorithm was further verified by chart abstraction in the 97 patients who had a verified diagnosis of PH. The algorithm was consistent with the chart abstraction assignment to PH subgroups in 63/97 patients with 65% specificity. As a surrogate to the diagnosis of PH we explored the use of RHC in our incidence cohort using OHIP codes G297 and Z439. The Johns Hopkins Aggregated Diagnosis Groups (ADG) was used to measure disease morbidity burden.20 Socioeconomic status was determined using Canada Census data on neighborhood income quintiles. The prevalence of PH in Ontario was estimated for December 31, 2002 and 2012. In sensitivity analyses, the study period (January 1st 1993-December 31st 2012) was divided into two equal time periods to examine temporal trends.
Patients were considered prevalent cases if they had a diagnosis of PH prior to the specified date and were Ontario residents on the specified date. The denominator used to calculate prevalence was comprised of all Ontario residents who had OHIP coverage on December 31st of the specified year and a date of last healthcare contact within the previous 7-years. Analysis was conducted for pediatric (defined as <16 years of age) and adult cohorts. To see how common PH was in patients with known risk factors we determined the prevalence of PH amongst patients with the following co-morbidities (left heart disease, lung disease, and thromboembolic disease) for the period December 31, 2002–December 31, 2012.
A patient was considered to have incident PH if they had no diagnosis of PH in the 10-years prior to the first PH diagnosis. Annual incidence rates were calculated for 2003–2012. The denominator consisted of living Ontario residents with active OHIP coverage with a date of last healthcare contact within the previous 7-years. Incidence rates were stratified by age, PH group, and gender. A trend analysis for incidence rates was performed using a general additive model to determine any trends while accounting for non-linearity. Crude 30-day, 1-year and 5-year mortality following incident PH diagnosis were calculated by year and stratified by age, gender and PH group. Time to death (or date of last contact) was estimated for incident PH. Standardized Mortality Ratios (SMRs) were calculated for 2003 and 2011 using the 2007 Canadian population as the standard (see on-line supplement).21
Prescriptions for CCB, ETRA, PDE5i and prostanoids were identified for patients who were 65 years of age and over between 2003 and 2012. A PH-specific limited-use code for PDE5i allowed definition of prescription indication. The number of days for which a medication was prescribed was determined, both for monotherapy or combination therapy (on-line supplement). The average number of days prescribed/100 patients/calendar year was determined as the total number of days prescribed divided by the number of prevalent patients for that year × 100. The percentage of PH patients on different classes of medication was also calculated. Patients were considered to have been “dispensed” a medication if they were prescribed a medication from that class provided it was dispensed for a minimum cumulative duration of 30 days.
All analyses were performed at ICES using SAS software, version 9.2. Paired T tests or ANOVA was performed to compare between groups. This study was approved by the institutional review board at Sunnybrook Health Sciences Centre in Toronto, Canada, and Queen’s University Health Sciences Research Ethics Board.
Results
From January 1, 1993 to December 31, 2012, 50,529 PH patients were identified. Their mean age was 68.5 (±18.5) years and 54.5% were female (Table 1). Age, gender and ADG data for adult and pediatric PH are shown in Table 1. The frequency of WHO Groups within the adult PH population was: Group 2 (68.5%), Group 3 (47.0%) and Group 4 (9.0%). Many adult patients (35.4%) belonged to more than one group, with the most frequent overlap diagnosis being Groups 2 and 3 (29.3%) (Figure S1). PH patients with no diagnosis of left heart disease, lung disease, or CTEPH were identified as Group 1 (13.8%). Group 1 patients were younger and had lower ADG scores than other groups (Table 1). Among those WHO Groups that have recognized sub-groups, the leading etiologies in adults were: Group 1-idiopathic PH (50.4%), Group 2-systolic or diastolic LV dysfunction (86.8%) and Group 3-COPD (83%) (Table S2). In children, Group 1 PH was most common [65.2% (N=1,1198)] (Table 1) and 78.6% of cases were idiopathic (Table S2). However, 21.4% of children (N=394) had Group 2 PH (Table 1).
Table 1.
Description of Ontario Adults and Children with Incident Pulmonary Hypertension Diagnosed in 1993–2012
| General Population* | All PH | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|---|
| All | 12,187,218 | 50,529 | 7,903 | 33,768 | 23,189 | 4,425 |
| Number | ||||||
| % of PH Population† | 15.6 | 66.8 | 45.9 | 8.8 | ||
| Female Gender (%) | 50.8 | 54.5 | 59.7 | 54.1 | 49.9 | 59.4 |
| Mean age at index date‡ (SD) | 37.3 (22.0) | 68.5 (18.9) | 55.4 (28.0) | 72.1 (14.9) | 71.4 (14.2) | 67.4 (17.0) |
| Mean total ADG Score (SD) | 8.4 (4.4) | 14.3 (4.2) | 12.7 (4.6) | 14.8 (4.1) | 15.1 (4.0) | 15.7 (4.1) |
| Mean major ADG Score (SD) | 1.0 (1.2) | 3.7 (1.5) | 3.3 (1.4) | 3.8 (1.5) | 3.9 (1.5) | 4.3 (1.4) |
| <16 Years | 2,484,107 | 1,837 | 1,198 | 394 | 295 | 65 |
| Number | ||||||
| % of PH Population† | 65.2 | 21.4 | 16.1 | 3.5 | ||
| Female Gender (%) | 48.7 | 46.9 | 45.9 | 52 | 48.8 | 43.1 |
| Mean age at index date‡ (SD) | 7.8 (4.5) | 1.4 (3.4) | 1.2 (3.3) | 1.7 (3.5) | 1.9 (3.7) | 1.8 (3.4) |
| Mean total ADG Score (SD) | 6.9 (3.3) | 8.9 (4.5) | 7.5 (3.8) | 11.9 (4.0) | 12.1 (4.7) | 13.2 (4.2) |
| Mean major ADG Score (SD) | 0.6 (0.8) | 3.0 (1.1) | 2.7 (0.9) | 3.5 (1.2) | 3.5 (1.1) | 4.1 (1.1) |
| ≥16 Years | 9,703,111 | 48,692 | 6,705 | 33,374 | 22,894 | 4,360 |
| Number | ||||||
| % of PH Population† | 13.8 | 68.5 | 47 | 9 | ||
| Female Gender (%) | 51.3 | 54.8 | 62.1 | 54.1 | 49.9 | 59.6 |
| Mean age at index date‡ (SD) | 44.8 (17.9) | 71.0 (14.0) | 65.1 (17.4) | 72.9 (12.8) | 72.3 (11.9) | 68.3 (15.1) |
| Mean total ADG Score (SD) | 8.8 (5.6) | 14.5 (4.1) | 13.7 (4.0) | 14.8 (4.0) | 15.1 (4.0) | 15.7 (4.0) |
| Mean major ADG Score (SD) | 1.2 (1.3) | 3.7 (1.5) | 3.3 (1.5) | 3.8 (1.5) | 3.9 (1.5) | 4.3 (1.4) |
The reference date for the general population is January 1, 2003
Percentage of patients in age group belonging to group
Index Date - date of first PH diagnosis between 1993–2012
Abbreviations: PH-pulmonary hypertension; SD-standard deviation; ADG-the Johns Hopkins Aggregated Diagnosis Groups Values in a column may add to more than 100% because patients may be classified as belonging to more than 1 WHO group
Annual PH prevalence increased from 87.6 to 114.9 cases/100,000 population between 2002 and 2012 (Table 2). In 2012, the mean age of all prevalent PH cases was 59.6 (±23.8) years, 57.3% were female and 43.6% were in the two lowest-income quintiles. In the adult PH cohort, PH prevalence increased over this period (99.8/100,000 to 127.3/100,000), primarily driven by increases in Group 2 PH (Table 2). The prevalence of pediatric PH in Ontario also increased over this period, from 39.6/100,000 population to 57.9/100,000 population.
Table 2.
Description of Ontario Patients with Prevalent Pulmonary Hypertension in 2002 and 2012
| < 16 years | ≥ 16 years | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2002 | 2012 | p-value | 2002 | 2012 | p-value | 2002 | 2012 | p-value | |
| Number | 986 | 1,404 | 9,710 | 14,108 | 10,696 | 15,512 | |||
| Prevalence* per 100,000 population | 39.6 | 57.9 | <.001 | 99.8 | 127.3 | <.001 | 87.6 | 114.9 | <.001 |
| Age in years | 0.7 (2.5) | 1.8 (3.0) | <.001 | 66.2 (14.7) | 65.4 (15.8) | <.001 | 60.2 (23.6) | 59.6 (23.8) | 0.07 |
| Female Gender (%) | 43.4% | 46.4% | 0.152 | 56.6% | 58.2% | 0.01 | 55.3% | 57.2% | 0.003 |
| Summation of All ADG Components | 7.1 (3.5) | 8.6 (4.7) | <.001 | 13.8 (4.1) | 14.1 (4.1) | <.001 | 13.2 (4.5) | 13.6 (4.5) | <.001 |
| Summation of Major ADG Components | 2.7 (0.8) | 2.9 (1.0) | <.001 | 3.6 (1.4) | 3.5 (1.5) | <.001 | 3.3 (1.4) | 3.4 (1.5) | <.001 |
| Income quintile (%) | 0.978 | 0.006 | 0.008 | ||||||
| 0th – 20th | 25.9% | 24.9% | 22.8% | 22.2% | 23.1% | 22.4% | |||
| 20th – 40th | 20.0% | 20.2% | 22.9% | 21.3% | 22.6% | 21.2% | |||
| 40th – 60th | 18.5% | 18.7% | 19.5% | 19.9% | 19.4% | 19.7% | |||
| 60th – 80th | 20.7% | 20.5% | 17.4% | 18.5% | 17.7% | 18.6% | |||
| 80th – 100th | 14.2% | 14.7% | 17.0% | 17.7% | 16.8% | 17.5% | |||
| PH group - Prevalence per 100,000 population | |||||||||
| Group 1 | 31.3 | 39.7 | <0.001 | 20.0 | 26.8 | <0.001 | 22.3 | 29.1 | <0.001 |
| Group 2 | 5.4 | 11.5 | <0.001 | 62.7 | 79.6 | <0.001 | 51.0 | 67.4 | <0.001 |
| Group 3 | 2.9 | 8.0 | <0.001 | 35.7 | 42.6 | <0.001 | 29.0 | 36.4 | <0.001 |
| Group 4 | 0.7 | 1.9 | <0.001 | 7.4 | 14.4 | <0.001 | 6.0 | 12.1 | <0.001 |
Values and mean±SD unless otherwise indicated
The denominator used for prevalence calculations is the number of people alive in Ontario on December 31st of that year with healthcare contact in the previous 7-years
P-Values report differences in prevalence, age etc. between 2002 and 2012 for each age group. Statistical comparisons were conducted using the student t-test for continuous variables, and the chi-square test for categorical variables.
Abbreviations: SD-standard deviation; ADG-the Johns Hopkins Aggregated Diagnosis Groups.
The percentage of the general population of Ontario with a predisposing factor for development of PH was: left heart disease (2.2%), lung disease (6%) and thromboembolic disease (1%) in 2012 (Table S3). However, a diagnosis of PH was present in only 3.6% of people with left heart disease, 0.7% of those with lung disease and 1.4% of those with thromboembolic disease patients. Fewer of these “at risk” patients had a PH diagnosis in 2012 versus 2002 (Table S3).
For the entire PH cohort, the annual incidence of PH increased significantly from 19.8 to 24.1 patients per 100,000 population between 2003 and 2012. This increase was predominantly noted over the 2008–2011 time period (Figure 1A, Figure S2) (Table S4). This increase was also evident in the adult cohort (Figure 1B). The female predominance of the PH cohort was consistent over time. Group 2 accounted for over 75% of all new PH cases. The incidence of adult Group 2 increased from 17.1 to 20.5 / cases/100,000/year over the study period. In the incident cohort 11,272 of the 27,577 (40.9%) patients had a record of RHC. Almost 40% of these patients belonged to Group 1.
Figure 1.
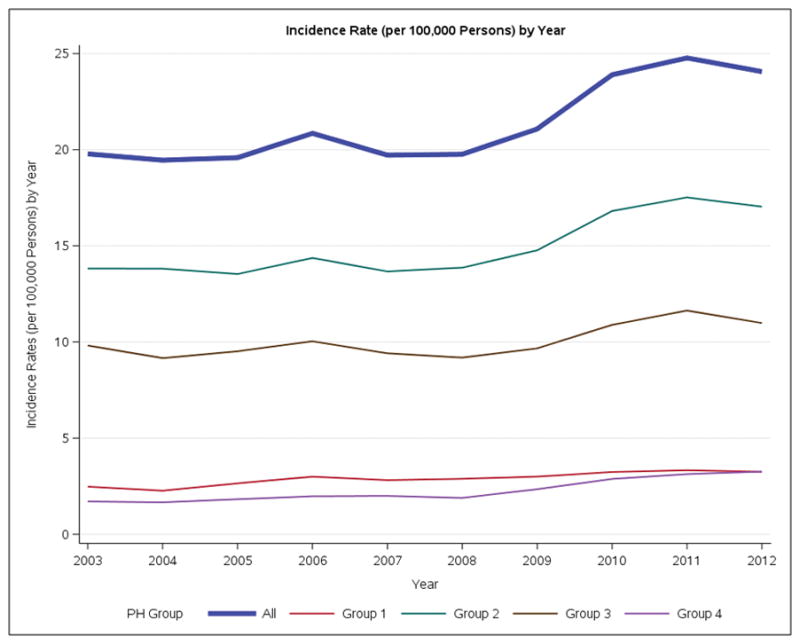
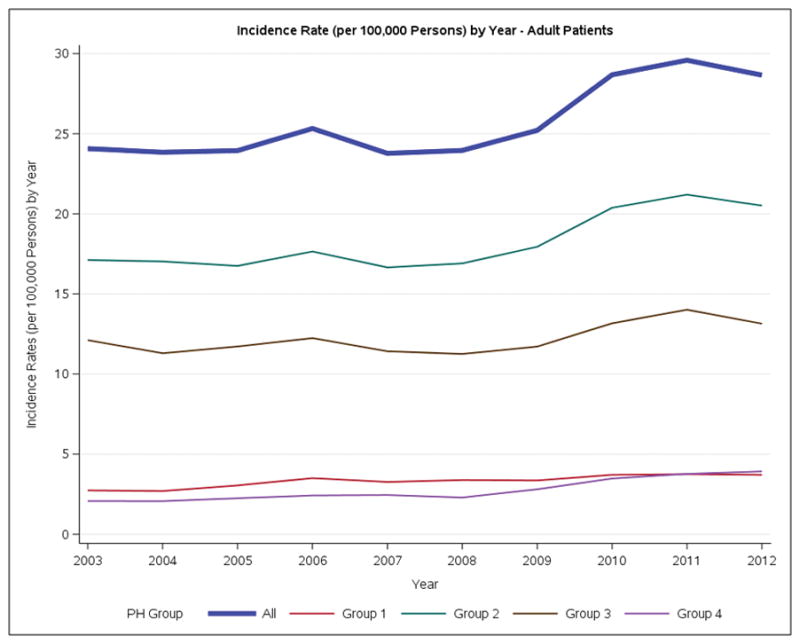
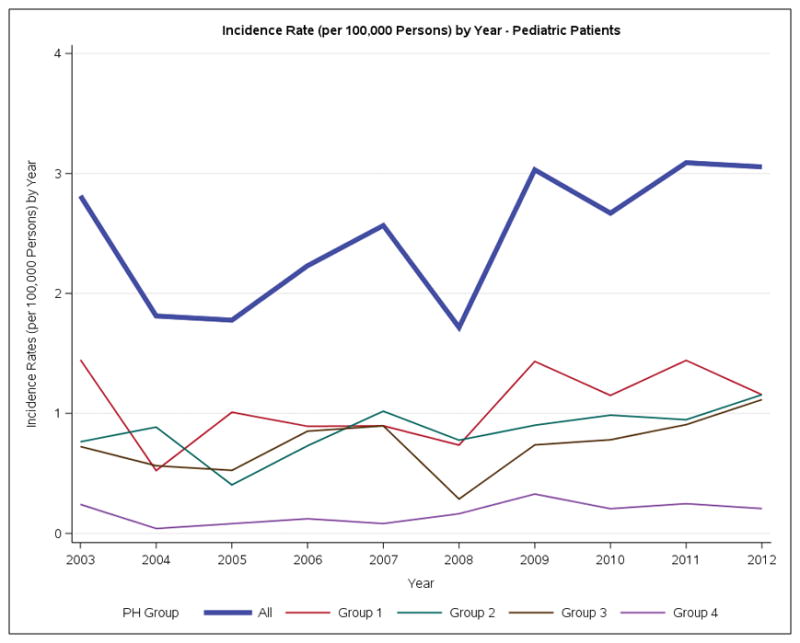
Incidence rates of Ontario Patients with Pulmonary Hypertension per 100,000 persons by year. A. Overall. B. Adults. C. Pediatric.
For the entire PH population, crude mortality rates at 30 days, 1-year and 5-years were 12.8%, 35.9% and 61.5%, respectively. These rates were stable over the study period (Figure S3). Group 1 patients had lower mortality rates than patients in other Groups (Table S5, Figure 2A). Similar mortality rates were observed for the adult-only PH cohort (Figure 2B). In children, the crude mortality rates at 30-days, 1-year and 5-years were 6.4%, 16.8% and 21.4% respectively (Table S5). There was a 10 fold increase in the risk of death (SMR=9.9, 95% CI 9.6–10.2) in the one year risk of death for patients diagnosed in 2003 compared to the general Canadian population. The one year SMR had declined by 2011 however a PH diagnosis still conferred a 7.2-fold increase in the 1-year risk of death (SMR=7.2, 95% CI: 7.0, 7.4). Group 1 patients had the lowest SMR and experienced the largest reduction in SMR over time [2003, SMR 9.1, 95%-CI (8.0, 10.2)] vs [2011, SMR [5.0, 95%-CI (4.4, 5.6)] (Table 3). Based on RHC, 1 year mortality in patients with group 1 who had a RHC was 11.7%.
Figure 2.
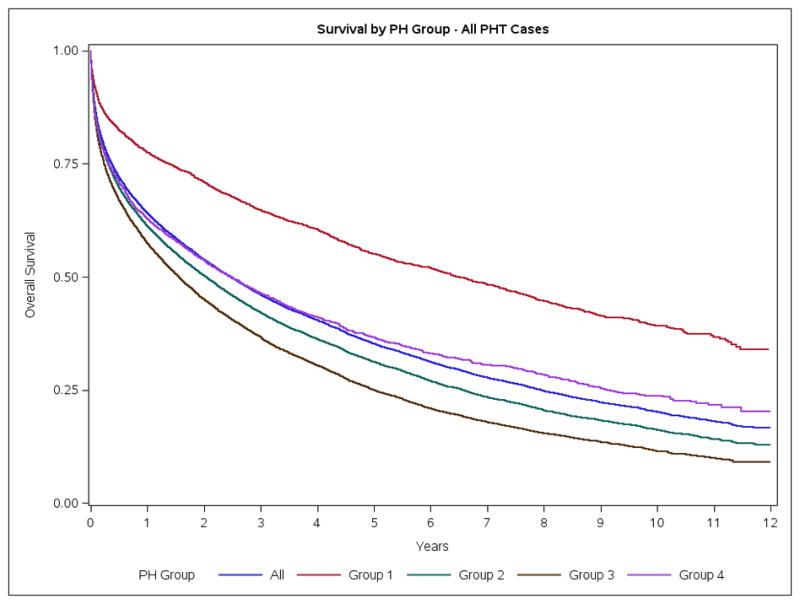
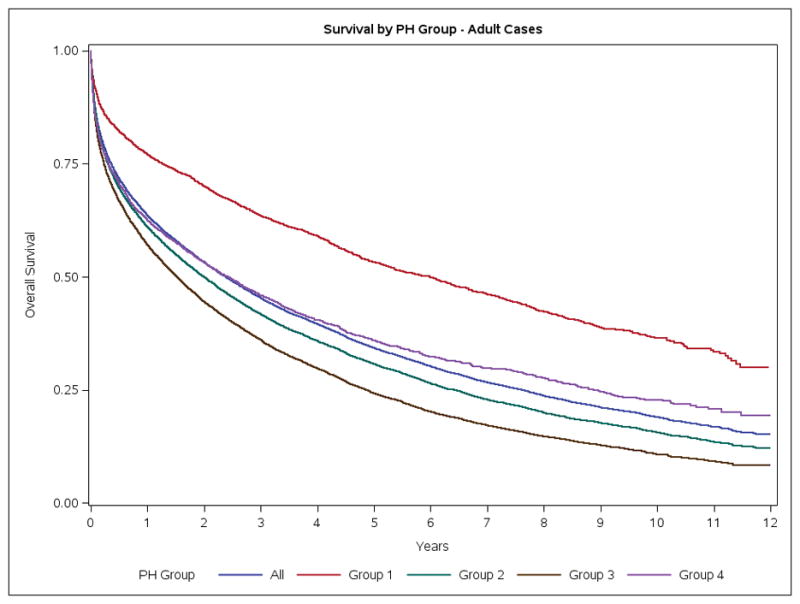
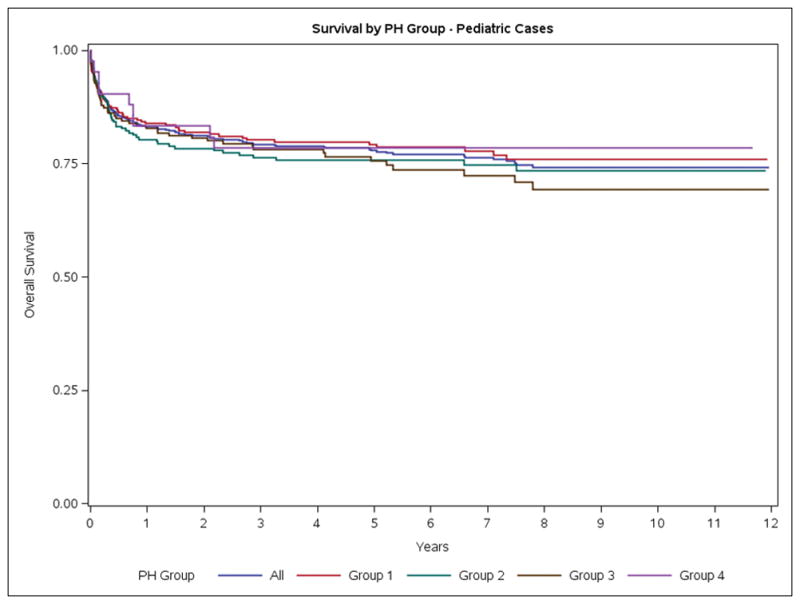
Survival of Ontario Patients with Pulmonary Hypertension. A. Overall. B. Adults. C. Pediatric.
Table 3.
Standardized Mortality Ratios for Ontario Patients with Incident Pulmonary Hypertension Diagnosed in 2003 and 2012 Compared to the Age and Sex Matched Canadian Population
| Group | Year | |||
|---|---|---|---|---|
| 2003 | 2011 | |||
| SMR | 95% Confidence Interval | SMR | 95% Confidence Interval | |
| All PH | 9.9 | (9.6, 10.2) | 7.2 | (7.0, 7.4) |
| Group 1 | 9.1 | (8.0, 10.2) | 5.0 | (4.4, 5.6) |
| Group 2 | 9.4 | (9.0, 9.8) | 7.1 | (6.8, 7.3) |
| Group 3 | 11.1 | (10.6, 11.6) | 8.9 | (8.6, 9.3) |
| Group 4 | 12.6 | (11.2, 14.1) | 8.4 | (7.7, 9.1) |
Abbreviations: PH-pulmonary hypertension
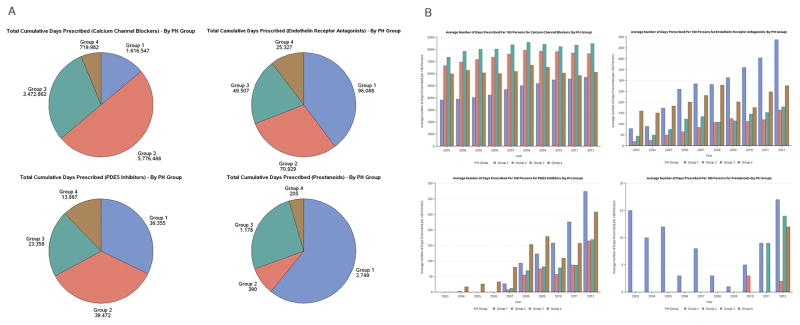
Prescription data were available for the 29,137 PH patients who were 65 years or older (57.5% of the PH cohort). Prescription of CCB, ETRA, PDE5i and prostanoids occurred in all Groups (Figures 3A, 3B) and increased over time (Figure 3B). The most common combination used was CCB plus ETRA, followed by CCBs plus PDE5i. From 2003–2012 the largest dispensing of ETRA (based upon number of total days prescribed) were to Group1 > Group 2 >Group 3 patients (total days prescribed 96,088 >70,929>49,507), respectively (Figure 3A). Similarly, PDE5i were consumed by Groups 2 > Group 1 > Group 3 patients (total days prescribed 39,472 > 36,355 > 23,358 respectively). Prescriptions for CCB plateaued in 2008 (Figure 3B) whilst the prescriptions for ETRA and PDE5i continued to increase through the study period. Over 40% of Group 1 patients and 34.2% of group 2 patients were dispensed calcium channel blockers. Although the percentage was small, ETRA, PDE5i usage was distributed across all PH groups (Table 4). Of those Group 1 patients, 65 years or older who had a RHC, the use of PH specific therapy (ETRA, PDE5i and prostanoids) was 17.2 percent.
Figure 3.
A. Cumulative number of days Pulmonary Hypertension medications were dispensed to Ontario Patients with Pulmonary Hypertension (2003–2012). B. Average number of days Pulmonary Hypertension Medications were dispensed per 100 Ontario Patients with Pulmonary Hypertension each year (2003–2012).
Table 4.
Percentage of patients 65 and Older with Pulmonary Hypertension in Ontario By Medication Use
| Class | All PH | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| Calcium Channel Blockers | 35.9 | 43.8 | 34.2 | 35.1 | 33.3 |
| Endothelin Receptor Antagonists | 1.1 | 3.6 | 0.7 | 0.8 | 1.2 |
| PDE5 Inhibitors | 0.7 | 2.3 | 0.4 | 0.4 | 1 |
| Prostanoids | <0.1 | 0.1 | <0.1 | <0.1 | <0.1 |
Abbreviations: PH-pulmonary hypertension
Note: The denominators used for the percentage calculations are PH patients in each Group
Almost as many patients qualified for a combination diagnosis of Group 2+3 PH (9,155) as had isolated Group 2 (9,956) or isolated Group 3 (3,641) PH (Table S6). Approximately 51% of Group 2+3 were females. The Group 2+3 cohort were older 74.4(±11.6) years. Their comorbidity scores were also greater [Major ADG score 4.2(1.5)] compared to isolated Group 2 [3.8(±1.5)] or Group 3 PH [3.8(±1.5)] (Table S6). In the incidence cohort, survival of Group 2+3 patients was worse than isolated Group 2 or 3 patients (Figure S4). The SMR for Group 2+3 was higher than for isolated Group 2 patients (Table S7).
Discussion
This is the first population-based study to describe the epidemiology of PH in adults and children. There are five important findings. First, the incidence and prevalence of PH in adults increased significantly over the past two decades, driven primarily by increases in WHO Group 2. Second, any diagnosis of PH, regardless of WHO group, portended an adverse impact, with the worst outcomes observed in adults with Group 2 and 3 PH. Although the increase in SMR declined in the second decade of this study it remains high with any diagnosis of PH conferring over a 7-fold increase in adjusted mortality (Table 3). Thirds, a substantial cohort of PH patients qualified for inclusion in more than one PH Group (usually group 2 and 3). This overlap is not surprising in light of common risk factors for left heart disease and COPD, notably cigarette smoking. Individuals with criteria for inclusion in both Groups 2 and 3 were older with more comorbidities than those in the isolated Group 2 or 3 and they had a worse prognosis (Table S6). Fourth, substantial departure from prescribing guidelines was noted, including high rates of CCB use in Group 1 PH and the use of Group 1-specific therapeutic agents (ETRA and PDE5i) in Groups 2 and 3 PH (Figure 3A). Fifth, pediatric PH was much less common and had better survival than adult disease. The predominant type of pediatric PH is PAH (Group 1) (Table S2).
Between 2003 to 2012 the annual incidence of PH increased, with the greatest increase being in Group 2 (Figure 1A). In Ontario in 2012, over 15,000 patients were living with a diagnosis of PH (Table 2). The temporal increase in incidence was most pronounced from 2008–2011 and may be related to increased rates of diagnosis related to improved disease awareness, increased treatment options and the greater availability of less invasive diagnostic techniques. The annual incidence of Group 1 PH increased from 2.5 to 3.2 patients per 100,000 population between 2003 and 2012. The fact that we captured primary or secondary diagnoses of PH patients at a population level and included both admitted patients and those seen in the Emergency Department may explain the somewhat higher rates of Group 1 disease in our study versus reports from cohorts that only capture patients from referral centers. The range of reported incidences for Group 1 PH (0.07/100,000 to 0.37/100,0006, 10, 12–14) in these referral centre cohorts is up to 5–10 fold less than in our study, although in the same range as our findings.
PH, when considered in its totality (i.e. including Group 1–4 patients) is not rare; occurring at 7.9% of the incidence of congestive heart failure (306.1/100,000 people)22 and 2.9% of the incidence of COPD (820/100,000 people)23 in Ontario. Compared to the general population, PH patients are older, more often female and have more comorbidities (Table 1). We suspect that the rise in PH prevalence relates in part to the ubiquitous availability of Doppler echocardiography, which permits noninvasive quantification of pulmonary artery pressure. However, in addition to improved noninvasive detection it is likely the true incidence and prevalence of PH are increasing. The most common isolated form of PH was Group 2, followed by Group 3 PH. The high burden of heart failure, especially diastolic left ventricular dysfunction24 in our aging population likely underlies much of the increased prevalence of PH and will likely lead to continued growth in the absolute and relative importance of Group 2 PH. However, PH is still a relatively rare complication in patients identified based solely on the presence of a predisposing comorbidities, such as COPD (<5%) (Table S3). It is interesting to note that between 2002 and 2012 fewer patients with risk factors for PH in fact developed PH, likely indicating early detection and better control of comorbid conditions including left heart disease, COPD and thromboembolic disease. We interpret this as indicating a complex interplay of factors determine whether a patient with left heart disease or chronic lung disease will develop PH. It is likely PH becomes significant in left heart disease only when left atrial pressures increase substantially and other factors, such as endothelial dysfunction, are present. Likewise, in COPD it is probable that PH only becomes manifest when disease is severe with significant hypoxia and loss of arteriolar and capillary bed volume.
Consistent with real-world clinical practice, it was often difficult to definitively categorize patients with concomitant heart and lung disease into a single WHO Group based on diagnostic codes. Patients who had comorbidities that qualified them to be counted in both Groups 2 and 3 accounted for over 9,000 patients (20.5% of the entire PH cohort). This Groups 2+3 cohort is older with more comorbidities than the relevant isolated PH Groups (Table S6). Accordingly, their survival is worse than their counterparts with isolated Group 2 or 3 disease (Figure S4). This overlap group merits further consideration. Although most therapeutic focus is on Group 1 and Group 4 PH they account for only 13.8% and 9.0% of the adult cohort, respectively. Thus, an approach to the public health burden of PH will require an increased focus on patients with Group 2 and 3 disease.
Little is known about the epidemiology of children with PH. We identified 1837 children with PH, representing 3.6% of all PH cases. In children, Group 1 PH is the most common form of disease. Children have substantially better 5-year survival than adults. These data begin to fill the void in understanding the epidemiology of pediatric PH that was identified in the first scientific statement on PH in children.25
In previous studies, the calculated 1-year mortality of Group 1 PH was 8–33%6–13, 15–17. This variability is depended on the period of study, the type of patient and expertise of the center. At a population level our study noted a 1 year mortality rate in adults of 22.9%, which is congruent with previous literature. Mortality was lower in Group 1 than in all other WHO Groups (Figure 2A). This better survival may reflect the natural history of Group 1 disease, the increasing use of Group 1-specific pharmacologic treatments, the relative paucity of comorbidities in Group 1 (Table 1) and/or the fact that Group 1 patients are approximately a decade younger than other groups. We noted a generalized temporal decline in SMR in all Groups over the study period after adjustment for age- and gender (Table 3), similar to trends in PAH registries.26
PH, like COPD, heart disease, diabetes and hypertension27, is more prevalent in low-income quintiles. The disparity in PH patients between the top 2 and bottom 2 income quintiles was 7.5% in our study. Patients with lower income have higher prevalence of smoking, inactivity and obesity.27 Although the basis for the increase in PH in those with low socioeconomic status was not explored it likely relates to disease factors such as smoking, obesity and hypertension. It is unlikely the effect of income is due to impaired access, since health care access is relatively uniform across income groups in Ontario, Canada.27
There are several strengths and limitations of our study. This is the largest PH study to date, including over 50,000 individuals. It is the first population-based study to describe the epidemiology of PH in adults and children. In a population study such as this we cannot determine whether there is a true change in disease incidence and prevalence versus better case ascertainment due to some combination of enhanced disease awareness by practitioners and increased utilization of cardiac ultrasound (which enhances disease detection in population). Although PH codes are intended to be specific, it is possible that patients with other diagnoses may be misclassified as having PH leading to over or underestimation of rates. The lack of specificity of our diagnostic algorithm due to the absence of data regarding right heart catheterization and reliance predominantly upon echocardiograms is a limitation.28
Although we could not case-validate for such a large study the veracity of the diagnostic code approach we used was validated by noting that a diagnosis of any form of PH conferred up to a 10-fold increase in SMR. This is important because if the PH we captured was physiologically insignificant and solely due to an echo surveillance bias one would not have expected a diagnosis of PH to have conferred the observed dramatic risk of mortality. Also over 40% of incident PH patients we captured had a RHC and of those 40% were identified as Group 1. Over 17% of Group 1 patients who had a RHC were dispensed PH specific therapies. These findings support the specificity of identifying PH patients using administrative codes. Furthermore, the incidence, prevalence and mortality rates noted for Group 1 PH in our study are in line with those in the literature obtained from standard registries. A second limitation is that we are unable to differentiate whether the indication for CCB use was PH versus systemic hypertension. However, the capture of PDE5i usage in PH was indication-specific and distinct from its use in erectile dysfunction. While we acknowledge that the current WHO PH classification does not recognize combined co-morbidities as drivers of PH (i.e. the Group 2–3 patients) we suspect that it is this multiple-hit pathophysiology (such as the combination of systemic hypertension and sleep apnea) that renders this patient subset vulnerable to developing PH. Supporting the validity of recognizing combined Group 2–3 disease, this cohort had a particularly poor prognosis. Even in registries, such as REVEAL, created in specialized Group 1 PH-focused studies, where all patients are assessed and classified by a PH expert physician, multiple comorbidities, including COPD and sleep apnea, are common.29 A limitation of our study is our inability to quantify severity of the comorbidities or attribute primary causality to one versus another of the major identified comorbidities. The fact that a comorbidity (COPD versus left heart disease) was sufficiently severe to merit diagnostic coding suggests that it was deemed physiologically relevant by the physician.
The rising incidence and prevalence of PH has several implications for health policy and practice. In terms of policy there should be a greater focus on prevention and treatment of Groups 2 and 3 patients, since they are the most rapidly growing cohort and have the highest standardized mortality ratios. The substantial use of PH-specific therapies, approved solely for use in WHO Group 1, notably ETRA and PDE5i, in the large and growing cohorts of WHO Group 2 and 3 patients, suggests a departure from treatment guidelines is occurring. An alternative explanation is that the prescribing physician considered the left heart disease and lung disease minor and thus treated the patient as a Group1 patient, despite acknowledging the co-morbidity with a diagnostic code.If this occurred, the patients would have been coded as a Group 2 or Group 3 PH patient, respectively in our study, potentially creating the appearance of off-label application of Group 1–specific drugs in other PH groups. Conversely, if an echocardiogram led to a diagnosis of PH in a patient deemed either too well or too ill to merit investigation with RHC or therapy and if no comorbidity was noted, the case would be classified as Group 1 PH. This would lead to underestimation of the use of PAH-targeted therapy. In our study, where severity of the comorbid condition and the proportion of the PH that should be attributed to a comorbidity cannot be ascertained, this may have led to overestimation of the off-label use of PH-targeted therapies in our study. However, in this case it would suggest practicing physicians are choosing to ignore left heart and lung disease in Group 1 PH, which is inconsistent with guidelines. Education for prescribers may be worthwhile considering the cost and potential toxicity of off-label prescription of Group 1 PH-targeted therapeutics.
Supplementary Material
What is Known
World Health Organization (WHO) recognizes five groups of pulmonary hypertension (PH), categorized by etiology or comorbidity.
The epidemiology of PH, apart from Group 1 disease (pulmonary arterial hypertension), is largely unknown and most data derive from specialized registries and referral programs, rather than from the population.
What the Study Adds
The incidence and prevalence of PH (all Groups) is increasing over the past two decades.
The most common form of adult PH is Group 2 PH, occurring in isolation or in combination with Group 3 disease.
A diagnosis of any form of PH increases 1-year standardized mortality ratio 7.2-fold.
Acknowledgments
This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). We wish to acknowledge the administrative support rendered by Susan Roland from ICES. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed herein are those of the author, and not necessarily those of CIHI. We wish to acknowledge the superb clinical care of our PH patients at Kingston Health Sciences Centre at Queen’s University, by Dr. Christine D’Arsigny. We also thank our PH clinic patients for their inspiration of this study and input to study design based on the questions they posed in clinic.
Funding Sources: SLA and this research are supported by a CIHR Foundation Grant, NIH-RO1-HL071115, 1RC1HL099462, a Tier 1 Canada Research Chair in Mitochondrial Dynamics, the William J Henderson Foundation and the CIHR Vascular Network and the Canadian Vascular Network - Bayer Research Fellowship Award.
Footnotes
Disclosures: No relevant disclosures
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Sikirica M, Iorga SR, Bancroft T, Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;14:676. doi: 10.1186/s12913-014-0676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaid HM, Camacho X, Granton JT, Mamdani MM, Yao Z, Singh S, Juurlink DN, Gomes T. The Characteristics of Treated Pulmonary Arterial Hypertension Patients in Ontario. Can Respir J. 2016;2016:6279250. doi: 10.1155/2016/6279250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Califf RM, Adams KF, McKenna WJ, Gheorghiade M, Uretsky BF, McNulty SE, Darius H, Schulman K, Zannad F, Handberg-Thurmond E, Harrell FE, Wheeler W, Soler-Soler J, Swedberg K. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: The Flolan International Randomized Survival Trial (FIRST) American Heart Journal. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 5.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E, Trial R. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–87. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 7.Kane GC, Maradit-Kremers H, Slusser JP, Scott CG, Frantz RP, McGoon MD. Integration of clinical and hemodynamic parameters in the prediction of long-term survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1285–93. doi: 10.1378/chest.10-1293. [DOI] [PubMed] [Google Scholar]
- 8.Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Koerner SK, Levy PC, Reid LM, Vreim CE, Williams GW. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216–23. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 9.Thenappan T, Shah SJ, Rich S, Gomberg-Maitland M. A USA-based registry for pulmonary arterial hypertension: 1982–2006. Eur Respir J. 2007;30:1103–10. doi: 10.1183/09031936.00042107. [DOI] [PubMed] [Google Scholar]
- 10.Escribano-Subias P, Blanco I, Lopez-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, Castillo-Palma MJ, Segovia J, Gomez-Sanchez MA, Barbera JA investigators R. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. 2012;40:596–603. doi: 10.1183/09031936.00101211. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, Held M, Grohe C, Lange TJ, Behr J, Klose H, Wilkens H, Filusch A, Germann M, Ewert R, Seyfarth HJ, Olsson KM, Opitz CF, Gaine SP, Vizza CD, Vonk-Noordegraaf A, Kaemmerer H, Gibbs JS, Pittrow D. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;168:871–80. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–30. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 13.Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke-Zaba J, Sheares KK, Corris PA, Fisher AJ, Lordan JL, Gaine S, Coghlan JG, Wort SJ, Gatzoulis MA, Peacock AJ. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–6. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 14.Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–9. doi: 10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 15.Jing ZC, Xu XQ, Han ZY, Wu Y, Deng KW, Wang H, Wang ZW, Cheng XS, Xu B, Hu SS, Hui RT, Yang YJ. Registry and survival study in chinese patients with idiopathic and familial pulmonary arterial hypertension. Chest. 2007;132:373–9. doi: 10.1378/chest.06-2913. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Dai LZ, Xie WP, Yu ZX, Wu BX, Pan L, Yuan P, Jiang X, He J, Humbert M, Jing ZC. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest. 2011;140:301–9. doi: 10.1378/chest.10-2327. [DOI] [PubMed] [Google Scholar]
- 17.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, Gabbay E. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98:1805–11. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pope JE, Lee P, Baron M, Dunne J, Smith D, Docherty PS, Bookman A, Abu-Hakima M. Prevalence of elevated pulmonary arterial pressures measured by echocardiography in a multicenter study of patients with systemic sclerosis. The Journal of Rheumatology. 2005;32:1273–1278. [PubMed] [Google Scholar]
- 19.Shimony A, Fox BD, Langleben D, Rudski LG. Incidence and significance of pericardial effusion in patients with pulmonary arterial hypertension. Can J Cardiol. 2013;29:678–82. doi: 10.1016/j.cjca.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC, van Walraven C, Wodchis WP, Newman A, Anderson GM. Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:932–9. doi: 10.1097/MLR.0b013e318215d5e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Statistics-Canada. [Accessed December 26th 2017];Deaths and mortality rates, by age group and sex, Canada, provinces and territories. 2015 http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=1020504.
- 22.Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE, Tu JV. Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ. 2012;184:E765–73. doi: 10.1503/cmaj.111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stats Canada. Population aged 35 and over who reported being diagnosed by a health professional with chronic bronchitis, emphysema or chronic obstructive pulmonary disease (COPD) [Accessed December 26th 2017];Table 105-0501 - Health indicator profile, annual estimates, by age group and sex, Canada, provinces, territories, health regions (2013 boundaries) and peer groups, occasional, CANSIM (database) 2015 http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1050501&&pattern=&stByVal=1&p1=1&p2=-1&tabMode=dataTable&csid=#F81.
- 24.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–70. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thebaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL American Heart Association Council on Cardiopulmonary CCP, Resuscitation, Council on Clinical C, Council on Cardiovascular Disease in the Y, Council on Cardiovascular R, Intervention, Council on Cardiovascular S, Anesthesia and the American Thoracic S. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132:2037–99. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 26.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, Pulido T, Rich S, Rosenkranz S, Suissa S, Humbert M. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62:D51–9. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Health-Quality-Ontario. Income and Health: Opportunities to achieve health equity in Ontario. Toronto: 2016. [Accessed December 26th 2017]. http://www.hqontario.ca/Portals/0/documents/system-performance/health-equity-report-en.pdf. [Google Scholar]
- 28.Fisher MR, Forfia PR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler Echocardiography in the Hemodynamic Assessment of Pulmonary Hypertension. American Journal of Respiratory and Critical Care Medicine. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. CHEST Journal. 2013;144:169–176. doi: 10.1378/chest.11-3241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.