Abstract
It is widely assumed that LPS lowers arterial pressure during sepsis by stimulating release of TNF-α and other vasoactive mediators from macrophages. However, recent data from this and other laboratories have shown that LPS hypotension can be prevented by inhibiting afferent impulse flow in the vagus nerve, by blocking neuronal activity in the nucleus of the solitary tract, or by blocking α-adrenergic receptors in the preoptic area/anterior hypothalamic area (POA). These findings suggest that the inflammatory signal is conveyed from the periphery to the brain via the vagus nerve, and that endotoxic shock is mediated through a central mechanism that requires activation of POA neurons. In the present study, we tested whether central cannabinoid 1 (CB1) receptors participate in the control of arterial pressure during endotoxemia based on evidence that hypothalamic neurons express CB1 receptors and synthesize the endogenous CB anandamide. We found that intracerebroventricular administration of rimonabant, a CB1 receptor antagonist, inhibited the fall in arterial pressure evoked by LPS significantly in both conscious and anesthetized rats. Rimonabant attenuated both the immediate fall in arterial pressure evoked by LPS and the second, delayed hypotensive phase that leads to tissue ischemia and death. Rimonabant also prevented the associated LPS-induced rise in extracellular fluid norepinephrine concentrations in the POA. Furthermore, rimonabant attenuated the associated increase in plasma TNF-α concentrations characteristic of the late phase of endotoxic hypotension. These data indicate that central CB1 receptors may play an important role in the initiation of endotoxic hypotension.
Keywords: Endotoxic shock, blood pressure, rimonabant, TNF, septic shock
INTRODUCTION
Septic shock is a complex medical condition that has long eluded the understanding of physicians and researchers. Despite great strides in our understanding of the molecular mechanisms that mediate septic shock, mortality continues to range between 40% and 50%, and sepsis remains a leading cause of death, accounting for 40,000 cases annually in the United States. New clinical trials directed to identified mediators of sepsis such as TNF-α and IL-1β have failed to improve survival (1). A better understanding of septic shock is clearly necessary to develop new methods for preventing its lethal effects.
The fall in arterial blood pressure that initiates septic shock is frequently attributed to an indirect effect of LPS, a constituent of the outer wall of Gram-negative bacteria, on the vasculature. LPS is thought to stimulate release of TNF-α, which induces the synthesis of NO, which causes vasodilation, thus lowering blood pressure (1, 2). In experimental animals, LPS administration characteristically produces a biphasic hypotensive response at doses of 1 mg/kg (i.v.) or higher. The first phase of the response starts almost immediately after LPS administration. Arterial pressure reaches its lowest value within approximately 10 min and subsequently returns toward baseline values within 30 to 40 min. The second phase of the response begins approximately 60 min after LPS injection. The second phase is greater in magnitude and longer in duration than the initial phase and leads to tissue ischemia and death.
One important caveat to the hypothesis that endotoxic hypotension is mediated by plasma TNF-α release is that LPS lowers arterial pressure within minutes of injection, whereas TNF-α is not detectable in plasma until at least 30 min later (3, 4). In a previous report, we tested an alternative hypothesis, that initiation of LPS hypotension is mediated through a central mechanism. We found that microinjection of the local anesthetic lidocaine or the α-adrenergic receptor antagonist phentolamine directly into the preoptic anterior hypothalamic area (POA) inhibited the initiation of LPS hypotension significantly (4, 5). Moreover, these pretreatments also inhibited the second, more prolonged phase of endotoxic hypotension and significantly attenuated the rise in plasma TNF-α plasma concentrations (4, 5). These data indicate that endotoxic hypotension is mediated through a central mechanism that involves noradrenergic neurotransmission in the POA.
The present study tested the hypothesis that central cannabinoid 1 (CB1) receptors also participate in the initiation of LPS hypotension. This hypothesis is based on evidence that POA neurons express CB1 receptors (6) and synthesize the endogenous CB receptor agonist anandamide (6, 7). Indeed, CB1 receptors are densely expressed in the POA, where they are thought to modulate both excitatory and inhibitory synaptic transmission (6). Cannabinoid 1 receptors are thus strategically located to modulate ongoing neuronal activity of POA neurons by controlling neurotransmitter release. There is also evidence that anandamide is released into blood by peripheral tissues during endotoxic shock (8) and contributes to the fall in blood pressure by activating CB1 receptors that cause vasodilation (9).
This study further tested the hypothesis that CB1 receptor activation prevents endotoxic hypotension by inhibiting nor-epinephrine (NE) release in the POA. This hypothesis is predicated on extensive evidence that systemic LPS administration stimulates NE release in the POA (reviewed in 10–13). Previous studies from this laboratory showed, by using microdialysis, that extracellular NE concentrations increase rapidly in the POA after intravenous LPS administration. The changes in NE levels were temporally correlated with the early phase of LPS fever (14), consistent with earlier evidence that LPS evokes parallel increases in POA NE levels and body temperature (11–13). Recently, we reported that microinjection of the nonselective α-adrenergic receptor antagonist phentolamine into the POA blocks initiation of the hypotensive response induced by LPS (5). Therefore, there is substantive evidence for involvement of central NE neurons in the activation of the POA during the host response against pathogens, including fever production.
Here, we report that intracerebroventricular injection of rimonabant, a CB1 receptor antagonist, prevents the initial fall in arterial pressure evoked by LPS in conscious and anesthetized rats. Rimonabant also attenuated the LPS-stimulated rise in extracellular fluid (ECF) NE concentrations in the POA, consistent with the idea that NE release within the POA mediates the initiation of LPS hypotension. Blockade of CB1 receptors also significantly inhibited the second, delayed fall in arterial pressure induced by LPS and simultaneously decreased the characteristic increase in plasma TNF-α concentrations. The present data thus indicate that the initial phase of endotoxic hypotension involves the stimulation of CB1 receptors and the activation of the noradrenergic neurotransmission within the POA.
MATERIALS AND METHODS
Blood pressure and heart rate recordings
Male Sprague-Dawley rats (250–300 g; Charles River Laboratories, Wilmington, Mass) were anesthetized with 4% isoflurane and maintained with 1.5% isoflurane in 100% O2. The left femoral artery and left jugular vein were cannulated with PE-50 tubing filled with heparinized saline (100 U/mL) to record arterial pressure and administer drugs. The animals were allowed to recover from anesthesia, and 4 h later, the arterial cannula was connected to a volumetric pressure transducer, and blood pressure and heart rate were monitored and recorded at 1-min intervals using a MicroMed BPA-200 blood pressure analyzer (MicroMed, Louisville, Ky). Rats were treated with rimonabant (NIDA Drug Inventory and Supply, Research Triangle Park, NC) dissolved in 5 μL 1:1:18, EtOH (95%): emulphor: 0.9% intracerebroventricular saline or vehicle. A dose-response curve of rimonabant was performed, and we then selected the 250-ng dose because it was the minimal effective dose to block LPS hypotension (Fig. 1B). This dose of rimonabant has also been selected because it is extremely low and insufficient to affect LPS hypotension when injected systemically (8, 15). The dose used here is appropriate for blocking the effects of cannbinoid agonists on catecholamine release systemically (16, 17) and behavioral effects centrally (18). The half-life of rimonabant in clinical trials is approximately 6 to 7 days (19); for systemic administration, this is reduced to 72 h for intracerebroventricular administration in rodents (20). Rats were also treated with a CB1 receptor agonist WIN55,212-2 (WIN) or its vehicle (1:1:18 ratio of EtOH [70%; Sigma, St. Louis, Mo], Alkamuls [Rhodia, Cranbury, NJ], and saline) intracerebroventricularly. Vehicle for intracerebroventricular injections consisted of dimethyl sulfoxide 30% and saline (70%; pH 7.4).
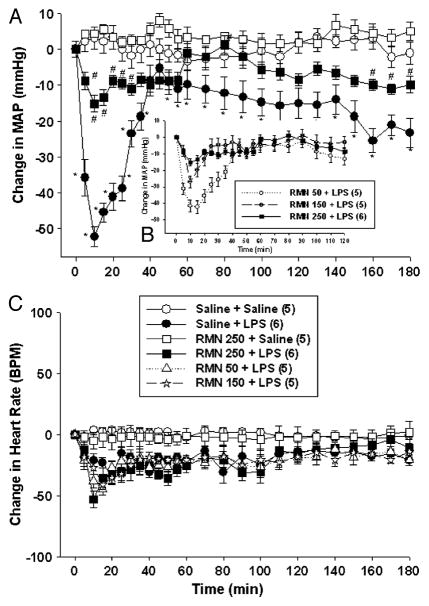
Fig. 1. Rimonabant (RMN) administration attenuates the fall in arterial pressure evoked by LPS in conscious rats.
A dose-response curve of rimonabant was conducted. To block endotoxic hypotension, 250 ng was the minimal effective dose (B). Thus, rats were treated with intracerebroventricular rimonabant (250 ng) or saline, followed 2 min later by intravenous LPS (1 mg/kg) or saline, and MAP (upper panel) and heart rate (lower panel) were monitored for 180 min. Data are presented as the mean ± SEM change in MAP or heart rate. Baseline MAP and heart rate values were vehicle + saline (n = 5), 127.9 ± 5.5 mmHg and 373 ± 31 bpm; vehicle + LPS (n = 5), 129.2 ± 4.1 mmHg and 366 ± 32 bpm; RMN + saline (n = 6), 132.3 ± 5.5 mmHg and 371 ± 21 BPM; RMN + LPS (n = 6), 128.8 ± 5.8 mmHg and 385 ± 21 BPM. Statistical analysis was performed using two-way repeated-measure ANOVA, followed by Tukey test. *P < 0.05, significantly different from the intracerebroventricular vehicle plus intravenous saline group.
The animals received LPS (1 mg/kg, from Escherichia coli 055:B5; Sigma) or saline (0.5 ml/kg) 2 min later. Intravenous and blood pressure and heart rate were recorded at 1-min intervals for 180 min. The animal protocols were conducted in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Intracerebroventricular injections
For intracerebroventricular injections, a 22-gauge stainless steel guide cannula was lowered through a burr hole drilled through the skull 1.5 mm lateral and 1.0 mm posterior to bregma, with its tip 4.5 mm below the surface of the skull (21). The cannula was fixed to the skull with acrylic cement and sealed with a stylette until the experiment was initiated approximately 4 h after. Intracerebroventricular injections were performed with a 28-gauge stainless steel injection cannula connected to a 5-μL Hamilton syringe with polyethylene tubing. The injection cannula was lowered through the guide cannula to a depth 1 mm below the tip of the guide cannula, and rimonabant or vehicle was injected at a constant rate during a 1-min period. The injection volume (0.5 μL) was monitored by observing the movement of an air bubble placed in the tubing, and the injection cannula was maintained in the guide cannula for 30 s after rimonabant, WIN, or their vehicle administration was complete.
The accuracy of intracerebroventricular injections was verified by adding 2% Chicago Sky Blue dye to the injection vehicle to mark the injection sites. At the end of each experiment, rats were euthanized with an overdose of isoflurane, the brain was removed, and the ventricles inspected for presence of the dye. Only data from confirmed cannula placements cannulas were included in the data analysis.
Microdialysis
A sterile, 17-mm-long, 17-gauge, thin-walled stainless steel guide cannula with a tightly fitting indwelling stylette was implanted stereotaxically into the medial POA (AP, 0.3 mm; L, 0.8 mm; V, −8.5 mm) according to the atlas of Paxinos and Watson (21) and fixed to the skull with four self-tapping, miniature, stainless steel screws and dental acrylic cement. Concentric microdialysis probes were constructed as previously described (22). Approximately 2 h before each experiment, the stylette was removed from the guide cannula and replaced with a sterile microdialysis probe, with the tip of the dialysis membrane protruding 1 mm below the end of the guide cannula. The probe was fixed to the skull with tissue adhesive and perfused with sterile artificial cerebrospinal fluid (composition in mM: NaCl, 148; KCl, 2.7; CaCl2, 1.2; MgCl2, 0.8; pH 7.4) prepared fresh daily and prewarmed to 38°C using sterile 1-mL tuberculin syringes clamped to a syringe pump (model A-99; Razel Scientific Instruments, Stamford, Conn).
The microdialysis probes were perfused for a 90-min thermal stabilization period at an initial flow rate of rate of 4 μL/min for 10 min, then at 3 μL/min for 10 min, then at 2 μL/min for the remainder of the experiment. The microdialysis effluents were collected at 10-min intervals into ice-cold polypropylene tubes containing 1 μL 5% perchloric acid and stored at −20°C until analysis. At the end of the experiment, each rat was ethanized with an overdose of isoflurane, the brain was rapidly removed, frozen on dry ice, and sections (50 μm) were cut with a microtome cryostat (Microm Model HM505E, Waldorf, Germany). Sections were mounted on slides, stained with eosin, air-dried, coverslipped, and the location of the tip of the microdialysis probe was confirmed. Only data from confirmed probe placements were included.
Norepinephrine analyses
The NE content of the samples was analyzed by using high-performance liquid chromatography (HPLC) with electrochemical detection. Samples (5 μL) were injected onto a 150 × 3-mm ODS C18 column (ESA Inc., Bedford, Mass) using a CMA 200 refrigerated automatic sampler (CMA Microdialysis, North Chelmsford, Mass). The column was perfused with a BAS 200A HPLC pump (BAS Inc., West Lafayette, Ind) at 0.25 mL/min with a mobile phase containing 80 mM sodium dihydrogen phosphate monohydrate, 2.0 mM 1-octanesulfonic acid sodium salt, 100 μL/L triethylamine, 5 nM EDTA, and 10% acetonitrile, pH 3.0. The HPLC was equipped with an ESA Coulochem II 5200A electrochemical detector with an ESA 5041 high-sensitivity microbore analytical cell and an ESA 5020 guard cell. Electrochemical detection was performed at 220 mV and 1.0 nA; the guard cell was set at 350 mV. Samples were analyzed in triplicate. The limit of detection for NE was 0.2 pg/5 μL. The chromatographic data were collected and analyzed with a PowerChrom system (AD Instruments, Castle Hill, NSW, Australia) and expressed as picogram per milliliter of sample. Basal values were defined as the average NE levels of the three samples before vehicle or LPS administration (pretreatment control period).
TNF assay
Blood (200 μL) was collected 0, 10, 60, 90, and 120 min after LPS administration, centrifuged to separate serum, and frozen at −50°C until analyzed. Serum TNF-α was measured with an enzyme immunometric assay kit (TNF-α [rat] enzyme immunoassay kit no. 900-086; Assay Designs, Inc., Ann Arbor, Mich) according to the manufacturer’s instructions. In brief, the samples and standards were added to microtiter plate wells, incubated for 1 h at 37°C, and washed seven times with wash solution, at which point, antibody was added to each well (except the blank) and incubated for 30 min at 4°C. After two additional wash procedures, substrate solution was added to each well, the plates were further incubated for 30 min at room temperature in the dark, at which point, stop solution was added to all wells. An ultraviolet spectrophotometer (model Ceres UV900 HDI; Bio-Tek Instruments, Inc., Winooski, Vt) was used to read plates at 450 nm.
In vivo microdialysis collection for the measurement of endogenous CBs
Rats (n = 6) were anesthetized (isoflurane, 1%–2% vapor), implanted with a jugular catheter, and stereotaxically implanted with a microdialysis probe (1-mm polyethyl sulfone dialysis membrane, 15 kDa molecular weight cutoff; SciPro Inc., Sanborn, NY) centered in the POA as previously stated. The artificial cerebrospinal fluid was delivered at 0.6 μL/min. Approximately 3 h after probe implantation, baseline microdialysate samples were collected at 10-min intervals for 60 min before administration of 0.5 mL/kg saline through the jugular catheter. After an additional 60 min of dialysate collection, each animal received a 1-mg/kg infusion of LPS, followed by an additional 60 min of dialysate collection.
Analysis of dialysate endocannabinoid content by liquid chromatography and mass spectroscopy
The dialysate content of anandamide and 2-arachidonoylglycerol (2-AG) were determined using a previously described liquid chromatography and mass spectroscopy method (23, 24). Microdialysate aliquots (5 μL) were spiked with 5 μL of 100 nM of N-(2-hydroxy-2methylethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide as an internal standard and injected onto a 1 × 100-mm microbore LC column (BetaBasic C-18; 100Å, 3-μm particles; ThermoHypersil-Keystone). The CBs were separated using an isocratic mobile phase consisting of 70% MeOH (v/v) containing 0.1% (v/v) acetic acid delivered at 10 μL/min. The column eluent was delivered into the mass spectrometer (Agilent 1100MSD) via a nanoelectrospray interface. Similar to findings by others (25), we find that the sodium adducts of anandamide (AEA) and 2-AG provide greater sensitivity than do their protonated forms. Thus, the following mass-charge ratios were scanned using selective ion monitoring: AEA, 369.9 (M + 1Na); 2-AG, 401.1 (M + 1Na); N-(2-hydroxy-2methylethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide, 384.9 (M + 1Na). External calibration curves were generated daily and were constructed from a minimum of three standard concentrations (each run in duplicate). The limits of quantitation were approximately 0.02 nM for each analyte.
Statistical analysis
Data are presented as mean ± SEM. Statistical analysis was performed using two-way repeated-measure ANOVA, followed by a post hoc Tukey test using SigmaStat 3.0 (SPSS, Chicago, Ill). A two-sided P value less than 0.05 was considered significant.
RESULTS
Rimonabant administration inhibits LPS hypotension
To test the hypothesis that CB1 receptors participate in LPS-evoked hypotension, conscious rats were pretreated with the CB1 receptor antagonist rimonabant (250 ng) or vehicle intracerebroventricularly 2 min before they received LPS (1 mg/kg) or intravenous saline. As shown previously (4), LPS evoked a rapid fall in arterial pressure, which reached a nadir within 10 min, returned toward baseline values by 40 min, then gradually declined during the remainder of the experiment (Fig. 1A). Rimonabant pretreatment inhibited the fall in arterial pressure evoked by LPS significantly [f (1,8) = 11.188; P < 0.001]. Both the immediate hypotensive response and the second, delayed fall in arterial pressure caused by LPS were attenuated in rimonabant-pretreated rats. Rimonabant did not affect arterial pressure significantly in control animals that received intravenous saline in lieu of LPS (Fig. 1A). Neither rimonabant nor LPS changed heart rate significantly (Fig. 1B). These data show that central rimonabant administration attenuates LPS hypotension without affecting heart rate and without influencing cardiovascular homeostasis in otherwise untreated animals.
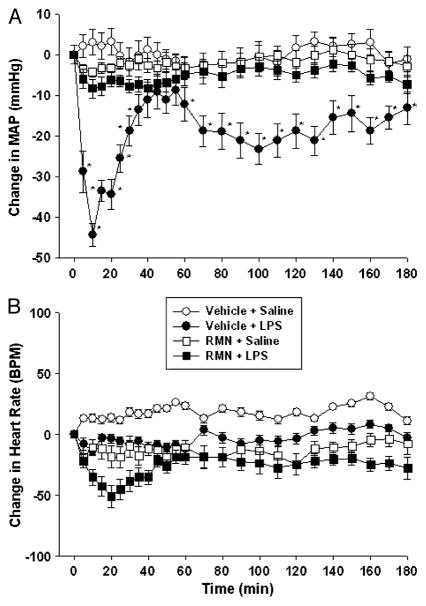
Previous studies on the role of the central nervous system in endotoxin-induced hypotension have been conducted largely in isoflurane-anesthetized rats (4). To determine whether rimonabant is also effective under these conditions, we performed parallel experiments in isoflurane anesthetized animals. Anesthetized rats were pretreated with rimonabant (250 ng) or vehicle intracerebroventricularly, followed 2 min later by LPS (1 mg/kg) or intravenous saline. As shown in conscious animals, LPS produced a biphasic fall in arterial pressure (Fig. 2A). Rimonabant pretreatment inhibited both phases of LPS hypotension [f (1,8) = 8.742; P < 0.01]. Indeed, MAP values in LPS-treated animals pretreated with rimonabant did not differ significantly from control values at any time point during the experiment in anesthetized rats. Rimonabant did not affect arterial pressure in control animals (Fig. 2A), and neither LPS, rimonabant, nor combined treatment altered heart rate significantly (Fig. 2B) in isoflurane-anesthetized animals.
Fig. 2. Rimonabant (RMN) prevents the fall in arterial blood pressure induced by LPS in anesthetized rats.
Isoflurane-anesthetized rats were sequentially treated with intracerebroventricular rimonabant (250 ng) or saline, followed 2 min later by intravenous LPS (1 mg/kg) or saline, and MAP (upper panel) and heart rate (lower panel) were monitored for 180 min. Data are presented as the mean ± SEM change in MAP or heart rate. Baseline MAP and heart rate values were vehicle + saline (n = 6), 121.1 ± 6.2 mmHg and 352 ± 17 bpm; vehicle + LPS (n = 6), 118.8 ± 4.2 mmHg and 338 ± 22 bpm; RMN + saline (n = 6), 108.9 ± 4.9 mmHg and 366 ± 27 bpm; RMN + LPS (n = 6), 116.5 ± 5.1 mmHg and 347 ± 28 BPM. Data were analyzed using two-way repeated-measure ANOVA, followed by Tukey test. *P < 0.05, significantly different from saline-treated control animals.
WIN administration does not affect LPS hypotension
To further study the role of CB1 receptors in LPS hypotension, conscious rats were pretreated with the CB1 receptor agonist WIN (250 ng) or vehicle intracerebroventricularly 2 min before they received LPS (1 mg/kg) or intravenous saline. WIN55,212-2 pretreatment did not affect the fall in arterial pressure evoked by LPS significantly (saline + LPS: maximum change in MAP, −37.6 ± 4.7 mmHg [n = 5]; WIN + LPS maximum change in MAP, −30.3 ± 5.8 mmHg [n = 5]). Heart rate was similarly unaffected (saline + LPS maximum change in BPM, −17.3.2 ± 8.3; WIN + LPS maximum change in BPM, −3.8 ± 3.1). WIN55,212-2 did not affect arterial pressure or heart rate significantly in control animals that received intravenous saline in lieu of LPS. These data show that central WIN administration does not alter the hypotensive response to LPS.
Rimonabant inhibits the LPS-induced increase in ECF NE concentrations
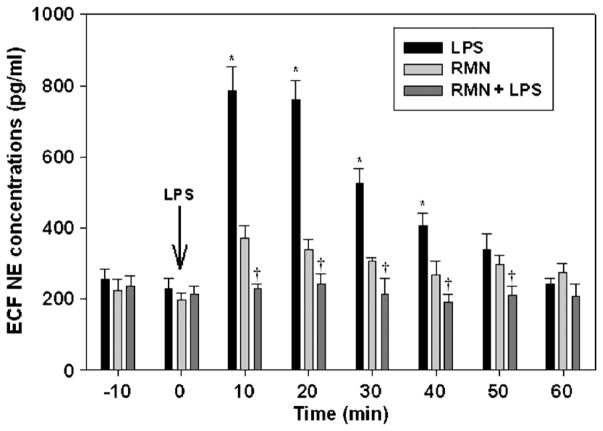
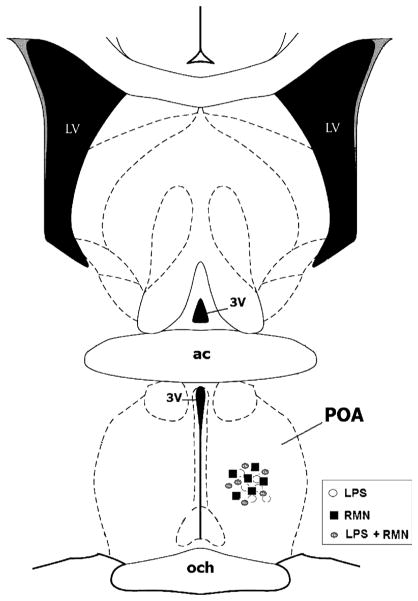
Previous studies have shown that intravenous LPS administration elevates ECF NE concentrations in the POA (14), and that microinjection of phentolamine, an α-adrenergic receptor antagonist into the POA, prevents the fall in arterial pressure evoked by LPS (5). To test the hypothesis that rimonabant prevents the onset of LPS hypotension by inhibiting NE release in the POA, we measured ECF NE levels by microdialysis in conscious animals. As in previous experiments, rats were treated with rimonabant (250 ng) or vehicle intracerebroventricularly 2 min before they received LPS (1 mg/kg) or intravenous saline. LPS administration increased ECF NE concentrations in the POA significantly within 10 min (Fig. 3). Extracellular NE achieved maximally elevated concentrations in samples collected 10 and 20 min after LPS injection and declined thereafter, reaching baseline values by the 50-min time point. Rimonabant inhibited the LPS-induced rise in ECF NE completely [f (14,105) = 16.093; P < 0.001]. Extracellular NE levels of rats treated with rimonabant and LPS did not differ significantly from baseline NE concentrations at any time point. Rimonabant administration to saline-treated control animals did not affect ECF NE concentrations significantly. Postmortem analysis confirmed that the microdialysis probes were located in the POA (Fig. 4). These data indicate that rimonabant pretreatment prevents the increase in ECF NE evoked by LPS in the POA.
Fig. 3. Rimonabant (RMN) administration prevents the rise in extracellular NE concentrations induced by LPS in conscious rats.
Groups of six rats were treated with intracerebroventricular rimonabant (250 ng) or saline, followed 2 min later by intravenous LPS (1 mg/kg) or saline. Extracellular fluid NE concentrations were sampled at 10-min intervals by microdialysis and analyzed by HPLC. Statistical analysis was performed using two-way repeated-measure ANOVA, followed by Tukey test. *P < 0.05, significantly different from basal concentrations of NE before LPS injection. †P < 0.05, significantly different from the LPS group.
Fig. 4. Schematic representation of a coronal section through the POA 0.36 mm from bregma, illustrating the location of the tip of the microdialysis probes for RMN-treated (filled squares) and saline-treated control (open circles) animals.
ac indicates anterior commissure; LV, lateral ventricle; 3V, third ventricle; och, optic chiasm.
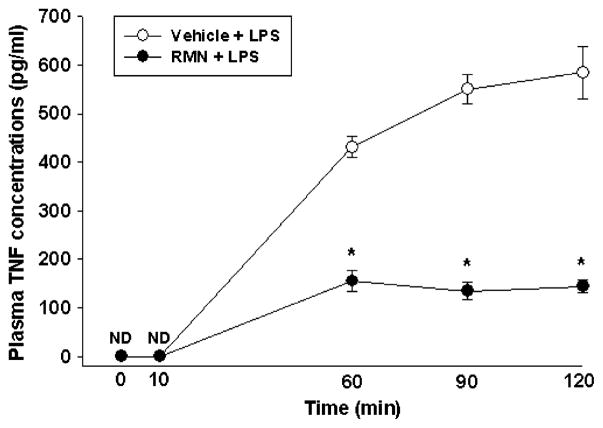
Rimonabant attenuates the delayed increase in plasma TNF-α concentrations caused by LPS
LPS is thought to lower arterial pressure, at least in part, by stimulating TNF-α release from macrophages and other immune cells (1, 2). Previous studies have shown that plasma TNF-α concentrations do not change detectably until 30 min or more after LPS administration, suggesting that elevated plasma TNF-α concentrations are correlated with the second, delayed phase of LPS hypotension (3, 4). Accordingly, we tested the hypothesis that rimonabant inhibits LPS hypotension, at least in part, by attenuating the delayed increase in plasma TNF-α concentrations caused by intravenous LPS administration. To test this, conscious rats were treated with rimonabant (250 ng) or vehicle intracerebroventricularly, followed 2 min later by LPS (1 mg/kg, i.v.). TNF-α was undetectable in plasma from control animals (detection limit, 5 pg/mL) and continued to be lower than the assay detection limit 10 min after intravenous LPS injection (Fig. 5). Plasma TNF-α concentrations increased approximately 500 to 600 pg/mL 60 min after LPS administration and increased steadily at 90- and 120-min time points. Intracerebroventricular rimonabant injection immediately before LPS administration inhibited the LPS-induced rise in plasma TNF-α levels significantly [f (12,97) = 11.225; P < 0.01; Fig. 5]. Central rimonabant administration thus attenuates the rise in plasma TNF-α concentrations evoked by LPS.
Fig. 5. Rimonabant (RMN) attenuates the rise in plasma TNF-α concentrations caused by LPS administration.
Groups of five rats were treated with intracerebroventricular rimonabant (250 ng) or vehicle, followed 2 min later by LPS (1 mg/kg, i.v.). Blood was collected at the indicated time interval after LPS (1 mg/kg, i.v.) administration, and plasma TNF-α concentrations were analyzed by enzyme immunoassay. Data were analyzed by using two-way repeated-measure ANOVA, followed by Tukey test. *P < 0.05, significantly different from the vehicle + LPS group. ND indicates nondetectable.
Effect of LPS injection on tonic endogenous CB levels in the POA
Baseline levels of AEA and 2-AG in POA dialysates were 1.0 ± 0.2 and 4.7 ± 0.9 nM, respectively, and these levels were unaltered after the intravenous administration of saline [AEA, F(5,6) = 1.703; 2-AG, F(5,6) = 0.892]. Subsequent challenge with 1 mg/kg LPS did not significantly alter dialysate AEA levels [F(5,6) = 0.757] and led to a transient nonsignificant decrease in dialysate 2-AG levels with an onset 20-min post-LPS administration.
DISCUSSION
The results of the present study show that intracerebroventricular administration of the CB1 receptor antagonist rimonabant prevents the initial fall in arterial blood pressure induced by LPS in conscious and anesthetized animals. Rimonabant also blocked the rapid increase in ECF NE concentrations caused by LPS in the POA, providing further evidence that LPS hypotension involves NE release in the POA. Rimonabant injection attenuated development of the delayed fall in arterial pressure evoked by LPS and simultaneously inhibited the rise in plasma TNF-α concentrations that characterize the second, decompensatory phase of LPS hypotension. Together, these data indicate that the initial phase of endotoxic hypotension is mediated by central CB1 receptors that influence NE release within the POA.
This study was predicated on evidence that POA neurons express CB1 receptors and synthesize the endocannabinoid anandamide (6). Endocannabinoids are thought to function primarily as retrograde inhibitors of neurotransmitter release (26). Indeed, endogenous cannabinoid signaling is becoming increasingly recognized as an important negative-feedback regulator of brain activity (26). In the POA, CB1 receptors are located on excitatory and inhibitory afferents and are thus strategically positioned to modulate network activity (6). The finding that rimonabant inhibits the rise in ECF NE concentrations in the POA after LPS injection is thus consistent with the notion that activation of CB1 receptors by endogenously released endocannabinoids normally modulates NE release in the POA.
To further explore the role of CB1 receptors in LPS hypotension, we administered the CB1 receptor agonist WIN 55,212-2 intracerebroventricular before LPS to determine if a CB1 agonist can produce a more dramatic decrease in blood pressure. Interestingly, WIN did not affect LPS hypotension. Although these results may seem counterintuitive, they were somewhat predictable considering the high dose of LPS injected, which probably induces the maximal hypotensive response. Thus, this drop in blood pressure cannot be enhanced any further. Indeed, we have seen (unpublished data) that administration of 15 mg/kg (i.v.) LPS produces a hypotensive response that is similar in magnitude to that seen with 1 mg/kg LPS (i.v.). Other investigators have reproduced these results (8). Experiments using a lower LPS dose and WIN are currently being performed in our laboratory to clarify this point.
Nevertheless, endogenous CBs are thought to be involved in a number of physiological responses that are mediated by noradrenergic neurons (27–29). There is also evidence that Δ9-tetrahydrocannabinol alters hormone release by influencing NE uptake and release in the hypothalamus (30) These data are consistent with the finding reported here that CB1 receptors may influence arterial blood pressure during septic shock by affecting noradrenergic activity within the POA. The fast onset of action of endogenous CBs seen here is consistent with studies demonstrating that under physiological conditions, the window of activation of these lipid messengers is approximately 50 ms (31, 32).
The data also support the hypothesis that the initial phase of LPS hypotension is an essential preface for development of the delayed, second fall in arterial pressure LPS produces. Nonetheless, the second phase of LPS-induced hypotension does not seem to be mediated directly by NE within the POA. This conclusion is based on the finding (5) that microinjection of phentolamine into the POA 45 min after LPS administration failed to prevent development of the second hypotensive response. Alternatively, activation of neurotransmission within the POA may initiate a signaling cascade that results in the second phase of hypotension.
The second phase of LPS hypotension seems to be dependent on circulating TNF-α. It is well understood that TNF-α initiates a series of biochemical and immunological responses that culminate in lethal hypotensive shock (1, 2). However, TNF-α is not detectable in general circulation until at least 30 min after LPS injection, suggesting that TNF-α is primarily associated with the second phase of hypotension (3, 4). The present study showed that circulating TNF-α levels are significantly lower in animals given rimonabant intracerebroventricular, which suggests that rimonabant inhibits TNF-α release by acting centrally. This hypothesis is consistent with earlier reports that central administration of α-melanocyte stimulating hormone (33) or nonsteroidal anti-inflammatory drugs (34) inhibit cytokine-induced cutaneous inflammation, presumably by activating sympathetic neurons (35, 36). Activation of vagus nerve efferents also inhibits systemic inflammation. Electrical stimulation of the vagus nerve or central administration of the anti-inflammatory drug CNI-1493 attenuates LPS-induced TNF-α release and prevents the development of endotoxin-induced septic shock (37, 38). It is therefore plausible to hypothesize that the POA can play a role in controlling the efferent mechanisms that mediate the release of TNF-α during endotoxic shock, although this concept requires further elucidation.
The finding that LPS increases ECF NE concentrations in the POA is supported by evidence that noradrenergic neurons in the A1 and A2 medullary cell groups densely innervate the POA and other hypothalamic subregions (39). Furthermore, LPS administration induces c-fos expression in A1 and A2 neurons (40) and stimulates NE release in the POA (10–13). In earlier studies, we showed, by using microdialysis, that ECF NE concentrations increase rapidly in the POA after intravenous LPS administration, and that changes in NE levels are temporally correlated with the early phase of LPS-induced fever (14), consistent with an earlier report that LPS causes parallel elevations in POA ECF NE levels and fever (10). There is, therefore, substantive evidence for involvement of central NE neurons in the activation of the POA during LPS fever, although the present data are, to our knowledge, the first evidence that LPS initially lowers arterial pressure through a comparable mechanism. The finding that NE release within the POA mediates the systemic inflammatory response to pathogens is consistent with evidence that α-adrenergic receptors are expressed by POA neurons (14), and that blockade of α-receptors in the POA inhibits LPS hypotension (5). Hence, the present results support the conclusion that noradrenergic neurotransmission within the POA participates in LPS hypotension.
Based on evidence that POA neurons synthesize AEA and express CB1 receptors (6, 7), we hypothesized that LPS-induced increases in preoptic NE release and resultant decreases in arterial blood pressure may result from increased anandamide formation in the POA. However, this hypothesis was not fully supported by our analyses of interstitial AEA and 2-AG levels in the POA after LPS administration. Dialysate AEA levels were unaltered during a 60-min post-LPS sampling period, and dialysate 2-AG levels were transiently decreased during this period. Although we have previously observed relatively long-lasting pharmacologically induced alterations in rat and mouse brain interstitial endocannabinoid levels by in vivo microdialysis sampling (23, 24, 41), it is conceivable that the nature of an LPS-induced alteration in endocannabinoid formation precludes observation by this methodology. For example, a rapid and transient increase in either AEA or 2-AG formation may be diluted by the relatively long sampling intervals required for endocannabinoid microdialysis (10 min in this study). It is also possible avid metabolic clearance processes for AEA and 2-AG sufficiently counter a localized increase in endocannabinoid so as to prevent an overall change in interstitial cannabinoid levels. However, an alternative explanation of the present results may be that the CB1 receptor–mediated effects of endotoxemia on preoptic NE levels are not mediated by preoptic CB1 receptors. Indeed, several lines of evidence demonstrate that endocannabinoids stimulate the firing of noradrenergic cells in the locus ceruleus through a CB1 receptor–dependent mechanism (27, 42, 43), although it remains possible that CB1 receptors modulate noradrenergic activity in the A1 and A2 medullary cell groups that innervate the POA. Future evaluations of altered LPS hypotension after site-specific Rimonabant infusions will help clarify the specific neural mechanisms through which CB1 receptors and their endogenous ligands modulate endotoxemia.
In conclusion, the present study shows that blockade of central CB1 receptors inhibits endotoxic hypotension. This inhibition was associated with a significant attenuation of LPS-induced rises in preoptic NE and plasma TNF-α levels. These novel findings may help to better understand the convoluted mechanisms of hypotension during endotoxic shock.
Acknowledgments
This research was supported, in part, by an intramural grant from the Albany College of Pharmacy and a scholarship provided by the American Foundation for Pharmaceutical Education to Alex Villanueva and Carlos Feleder.
References
- 1.Kirkeboen KA, Strand OA. The role of nitric oxide in sepsis—an overview. Acta Anaesthesiol Scand. 1999;43:275–288. doi: 10.1034/j.1399-6576.1999.430307.x. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Tracey KJ. The “cytokine profile”: a code for sepsis. Trans Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Perlik V, Feleder C, Tang Y, Blatteis CM. Kupffer cell–generated PGE2 triggers the febrile response of guinea pigs to intravenously injected LPS. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1262–R1270. doi: 10.1152/ajpregu.00724.2005. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz MS, Millington WR, Feleder C. The preoptic anterior hypothalamic area mediates initiation of the hypotensive response induced by LPS in male rats. Shock. 2008;29:232–237. doi: 10.1097/shk.0b013e3180caac7e. [DOI] [PubMed] [Google Scholar]
- 5.Yilmaz MS, Goktalay G, Millington WR, Myer BS, Cutrera RA, Feleder C. Lipopolysaccharide-induced hypotension is mediated by a neural pathway involving the vagus nerve, the nucleus tractus solitarius and alpha-adrenergic receptors in the preoptic anterior hypothalamic area. J Neuroimmunol. 2008;203:39–49. doi: 10.1016/j.jneuroim.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Wittmann G, Deli L, Kallo I, Hrabovszky E, Watanabe M, Liposits Z, Fekete C. Distribution of type 1 cannabinoid receptor (CB1)–immunoreactive axons in the mouse hypothalamus. J Comp Neurol. 2007;503:270–279. doi: 10.1002/cne.21383. [DOI] [PubMed] [Google Scholar]
- 7.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, et al. Endocannabinoid leptin–regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 8.Varga K, Wagner JA, Bridgen DT, Kunos G. Platelet- and macrophage-derived endogenous cannabinoids are involved in endotoxin-induced hypotension. FASEB J. 1998;12:1035–1044. doi: 10.1096/fasebj.12.11.1035. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JA, Varga K, Kunos G. Cardiovascular actions of cannabinoids and their generation during shock. J Mol Med. 1998;76:824–836. doi: 10.1007/s001090050287. [DOI] [PubMed] [Google Scholar]
- 10.Linthorst ACE, Flachskamm C, Holsboer F, Reul MHM. Intraperitoneal administration of bacterial endotoxin enhances noradrenergic neurotransmission in the rat preoptic area: relationship with body temperature and hypothalamic-pituitary-adrenocortical axis activity. Eur J Neurosci. 1995;7:2418–2430. doi: 10.1111/j.1460-9568.1995.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 11.Dunn AJ, Wang J. Cytokine effects on CNS biogenic amines. Neuroimmunomodulation. 1995;2:319–328. doi: 10.1159/000097211. [DOI] [PubMed] [Google Scholar]
- 12.Dunn AJ, Hall NRS. CNS biogenic amines and the immune system. In: Marsh JA, Kendall MD, editors. The Physiology of Immunity. Boca Raton, FL: CRC; 1999. pp. 45–156. [Google Scholar]
- 13.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. New York: Academic Press; 2001. pp. 645–666. [Google Scholar]
- 14.Feleder C, Perlik V, Blatteis CM. Preoptic norepinephrine mediates the febrile response of guinea pigs to lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1135–R1143. doi: 10.1152/ajpregu.00067.2007. [DOI] [PubMed] [Google Scholar]
- 15.Kadoi Y, Goto F. Effects of AM281, a cannabinoid antagonist, on circulatory deterioration and cytokine production in an endotoxin shock model: comparison with norepinephrine. J Anesth. 2006;20:284–289. doi: 10.1007/s00540-006-0428-3. [DOI] [PubMed] [Google Scholar]
- 16.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 17.Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ducobu J, Sternon J. Rimonabant (acomplia), specific inhibitor of the endocannabinoid system. J Pharm Belg. 2005;60:89–91. [PubMed] [Google Scholar]
- 20.Braida D, Pozzi M, Parolaro D, Sala M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur J Pharmacol. 2001;413:227–234. doi: 10.1016/s0014-2999(01)00766-x. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Elsevier Academic Press; 2005. [Google Scholar]
- 22.Feleder C, Perlik V, Blatteis CM. Preoptic alpha 1– and alpha 2–noradrenergic agonists induce, respectively, PGE2-independent and PGE2-dependent hyperthermic responses in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1156–R1166. doi: 10.1152/ajpregu.00486.2003. [DOI] [PubMed] [Google Scholar]
- 23.Caillé S, Alvarez L, Polis I, Stouffer D, Parsons LH. Specific alterations of extracellular endocannabinoid levels in nucleus accumbens by ethanol, heroin and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrer B, Bermudez-Silva J, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Navarro M, Parsons LH, et al. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- 26.Mendizabal VE, Adler-Graschinsky E. Cannabinoids as therapeutic agents in cardiovascular disease: a tale of passions and illusions. Br J Pharmacol. 2007;151:427–440. doi: 10.1038/sj.bjp.0707261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;20(102):18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 29.Tran PV, Bymaster FP, McNamara RK, Potter WZ. Dual monoamine modulation for improved treatment of major depressive disorder. J Clin Psychopharmacol. 2003;23:78–86. doi: 10.1097/00004714-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Rettori V, Aguila MC, Gimeno MF, Franchi AM, McCann SM. In vitro effect of delta 9-tetrahydrocannabinol to stimulate somatostatin release and block that of luteinizing hormone-releasing hormone by suppression of the release of prostaglandin E2. Proc Natl Acad Sci U S A. 1990;87:10063–10066. doi: 10.1073/pnas.87.24.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinbockel T, Brager DH, Reich CG, Zhao J, Muralidharan S, Alger BE, Kao JP. Endocannabinoid signaling dynamics probed with optical tools. J Neuroscience. 2005;25:9449–9459. doi: 10.1523/JNEUROSCI.2078-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poddar MK, Dewey WL. Effects of cannabinoids on catecholamine uptake and release in hypothalamic and striatal synaptosomes. J Pharmacol Exp Ther. 1980;214:63–67. [PubMed] [Google Scholar]
- 33.Delgado Hernandez R, Dimitri MT, Carlin A, Meazza C, Villa P, Ghezzi P, Lipton JM, Catania A. Inhibition of systemic inflammation by central action of the neuropeptide α-melanocyte stimulating hormone. Neuroimmunomodulation. 1999;6:187–192. doi: 10.1159/000026381. [DOI] [PubMed] [Google Scholar]
- 34.Catania A, Arnold J, Macaluso A, Hiltz ME, Lipton JM. Inhibition of acute inflammation in the periphery by central action of salicylates. Proc Natl Acad Sci U S A. 1991;88:8544–8547. doi: 10.1073/pnas.88.19.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceriani G, Macaluso A, Catania A, Lipton JM. Central neurogenic anti-inflammatory action of α-MSH: modulation of peripheral inflammation induced by cytokines and other mediators of inflammation. Neuroendocrinology. 1994;59:138–143. doi: 10.1159/000126650. [DOI] [PubMed] [Google Scholar]
- 36.Macaluso A, McCoy D, Ceriani G, Watanabe T, Biltz J, Catania A, Lipton JM. Antiinflammatory influences of α-MSH molecules: central neurogenic and peripheral actions. J Neurosci. 1994;14:2377–2382. doi: 10.1523/JNEUROSCI.14-04-02377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 38.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:25–28. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez-Galaz C, Dyer RG, Herbison AE. Analysis of brainstem A1 and A2 noradrenergic inputs to the preoptic area using microdialysis in the rat. Brain Res. 1994;636:227–232. doi: 10.1016/0006-8993(94)91021-9. [DOI] [PubMed] [Google Scholar]
- 40.Hollis JH, Lightman SL, Lowry CA. Lipopolysaccharide has selective actions on sub-populations of catecholaminergic neurons involved in activation of the hypothalamic-pituitary-adrenal axis and inhibition of prolactin secretion. J Endocrinol. 2005;184:393–406. doi: 10.1677/joe.1.05839. [DOI] [PubMed] [Google Scholar]
- 41.Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2008;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 43.Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–2394. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]