Abstract
Heart failure and related morbidity and mortality are increasing at an alarming rate, in large part, because of increases in aging, obesity and diabetes. The clinical outcomes associated with heart failure are considerably worse for patients with diabetes than for those without diabetes. In persons with diabetes, the presence of myocardial dysfunction in the absence of overt clinical coronary artery disease, valvular disease and other conventional cardiovascular risk factors such as hypertension and dyslipidemia has led to the descriptive terminology, “diabetic cardiomyopathy”. The prevalence of diabetic cardiomyopathy is increasing in parallel with the increase in diabetes. Diabetic cardiomyopathy is initially characterized by myocardial fibrosis, dysfunctional remodeling and associated diastolic dysfunction, later by systolic dysfunction, and eventually by clinical heart failure. Impaired cardiac insulin metabolic signaling, mitochondrial dysfunction, increases in oxidative stress, reduced nitric oxide bioavailability, elevations in advanced glycation end products and collagen – based cardiomyocyte and extracellular matrix stiffness, impaired mitochondrial and cardiomyocyte calcium handling, inflammation, renin angiotensin-aldosterone system activation, cardiac autonomic neuropathy, endoplasmic reticulum stress, microvascular dysfunction, and a myriad of cardiac metabolic abnormalities have all been implicated in the development and progression of diabetic cardiomyopathy. Molecular mechanisms linked to the underlying pathophysiological changes include abnormalities in AMP-activated protein kinase, peroxisome proliferator-activated receptors, O-linked N-acetylglucosamine, protein kinase C, microRNA, and exosome pathways. The aim of this review is to provide a contemporary view of these instigators of diabetic cardiomyopathy as well as mechanistically based strategies for the prevention and treatment of diabetic cardiomyopathy.
Keywords: Diabetes, Cardiac fibrosis, Heart failure
Diabetic cardiomyopathy is defined by the existence of abnormal myocardial structure and performance in the absence of other cardiac risk factors such as coronary artery disease, hypertension, and significant valvular disease in individuals with diabetes. It was first described in in 19721 in post-mortem pathological findings from four diabetic patients who manifested heart failure symptoms without evidence of coronary artery or valve disease and further confirmed in a 1974 Framingham Heart Study that demonstrated a higher incidence of heart failure in diabetic women (5 fold) and men (2.4-fold) after adjustment for other risk factors such as age, coronary heart disease, and hypertension.2 In 2013, the American College of Cardiology Foundation (ACCF), the American Heart Association (AHA)3 and the European Society of Cardiology (ESC) in collaboration with the European Association for the Study of Diabetes (EASD)4 defined diabetic cardiomyopathy as a clinical condition of ventricular dysfunction that occurs in the absence of coronary atherosclerosis and hypertension in patients with diabetes. In its early stages diabetic cardiomyopathy includes a hidden subclinical period characterized by structural and functional abnormalities, including left ventricular (LV) hypertrophy, fibrosis and cell signaling abnormalities. These pathophysiological changes of cardiac fibrosis and stiffness and associated subclinical diastolic dysfunction often evolve to heart failure with normal ejection fraction (HFpEF) and eventual systolic dysfunction accompanied by heart failure with reduced ejection fraction (HFrEF). This review summarizes recent research exploring molecular mechanisms, structural and functional changes and possible therapeutic approaches for the prevention and treatment of diabetic cardiomyopathy. It also highlights unmet needs and future research directions to better understand fundamental molecular abnormalities which promote this cardiomyopathy.
Clinical aspects of diabetic cardiomyopathy
Epidemiology of diabetes related heart failure
Clinical trials show the prevalence of heart failure in diabetic patients to range from 19% to 26%.5–7 The Framingham Heart Study found the incidence of heart failure was increased in both male and female diabetic patients when compared with age-matched individuals and this association was independent of obesity, hypertension, dyslipidemia, and coronary heart disease.2 One study found that the incidence of heart failure was higher in diabetic (39%) compared with non-diabetic (23%) patients, with a relative risk of 1.3 for developing heart failure following 43 months of observation.8 Further data derived from a population-based observational study in the Cardiovascular Health Study (CHS), the Strong Heart Study (SHS),9 and the Multi-Ethnic Study of Atherosclerosis (MESA)10 demonstrated differences in LV mass and wall thickness, and increased diastolic and systolic dysfunction between diabetic patients and normal individuals. Meanwhile, in type 1 diabetes each 1% increase in glycated hemoglobin A(1c) (HbA1c) was linked to a 30% increase for risk of heart failure11 whereas in type 2 diabetes each 1 % rise in HbA1c levels was associated with an 8% increase in risk, independent of other risk factors including obesity, smoking, hypertension, dyslipidemia, and coronary heart disease,12 suggesting that graded increases in glycemia are a powerful promoter of heart failure in diabetic patients.
Risk factors for diabetic cardiomyopathy
Hyperglycemia, systemic insulin resistance and impaired cardiac insulin metabolic signaling are major clinical abnormalities in diabetes, and all are involved in the pathogenesis of diabetic cardiomyopathy (Fig 1 and 2).13 In a prospective national survey of heart failure patients, 1811 individuals with and 2182 without pre-existing diabetes, glucose levels of 110–140, 140–200 and ≥200 mg/dL were associated with a 9%, 16% and 53% increased mortality risk when compared to an admission blood glucose< 110 mg/dL in patients with no pre-existing diabetes. There was a linear relationship between blood glucose level and long-term mortality in heart failure even in patients without a clinical diagnosis of diabetes. On the other hand, increased mortality risk was seen only in diabetics with glucose levels >200 mg/dL.14 In the UKPDS clinical study, a 1% reduction in HbA1c was associated with a 16% risk reduction for development of heart failure,12 suggesting that there is a time related log-linear relationship between long-term glycemic control and heart failure risk. Further, after 4 years of follow-up, a community based study of 6814 persons with no initial coronary artery disease demonstrated that increasing indices of metabolic syndrome tracked with increasing heart failure risk, with two-thirds of these patients developing HFpEF.15 The contemporary increase in dietary refined carbohydrate, and especially fructose, consumption may also impact development of diabetic cardiomyopathy as described in more in detail in the molecular mechanisms component of this review.16
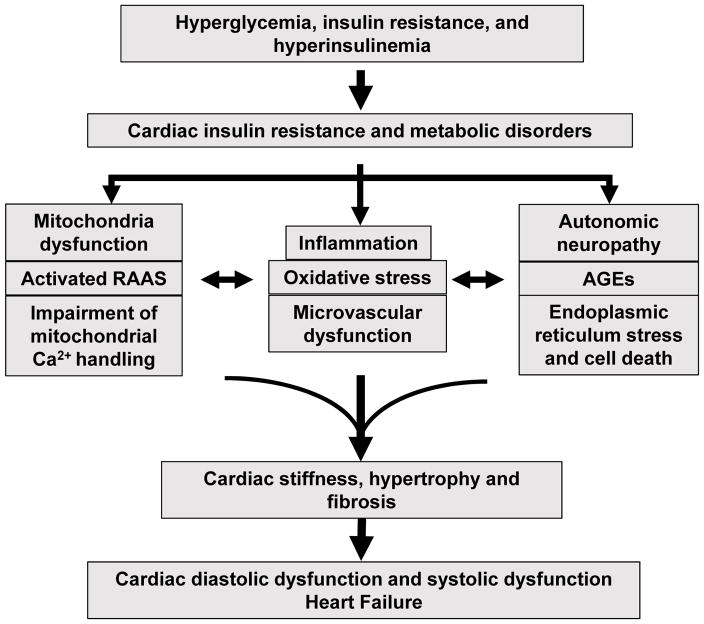
Fig. 1.
Pathophysiological mechanisms of diabetic cardiomyopathy. Hyperglycemia, insulin resistance, and hyperinsulinemia induce cardiac insulin resistance and metabolic disorders that increase mitochondria dysfunction, oxidative stress, AGEs, impairment of mitochondria Ca2+ handling, inflammation, activation of RAAS, autonomic neuropathy, endoplasmic reticulum stress, cardiomyocyte death, as well as microvascular dysfunction. These pathophysiological abnormalities promote cardiac stiffness, hypertrophy, and fibrosis, resulting in cardiac diastolic dysfunction, systolic dysfunction, and heart failure. Abbreviations: AGEs, advanced glycation end-products; RAAS, renin-angiotensin-aldosterone system.
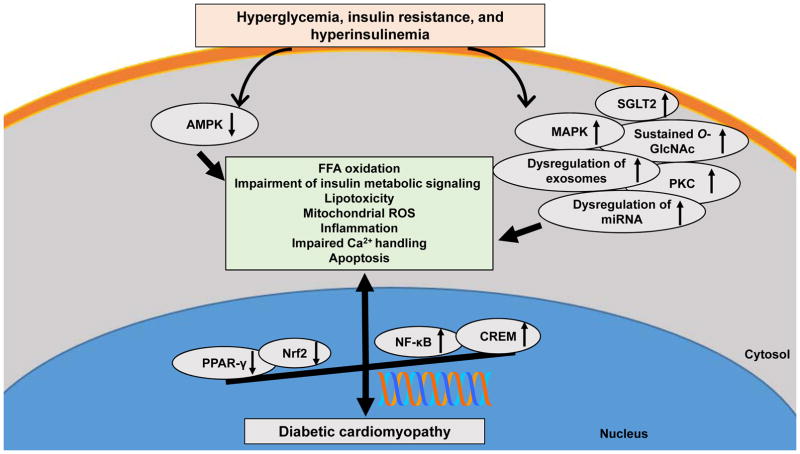
Fig. 2.
The molecular proteins and signaling pathways in hyperglycemia- and insulin resistance-diabetic cardiomyopathy. Increased PKC, MAPK, NF-κB, SGLT2, O-GlcNAc and CREM signaling, dysregulation of miRNA and exosomes, and reduction of AMPK, PPAR-γ and Nrf2 induce cardiac insulin resistance, subcellular component abnormalities, metabolic disorders, and structural changes, resulting in diabetic cardiomyopathy. Abbreviations: AMPK, AMP-activated protein kinase; PPAR, peroxisome proliferator-activated receptor; Nrf2, nuclear factor erythroid 2-related factor 2; PKC, protein kinase C; MAPK, mitogen-activated protein kinase; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; SGLT2, sodium-glucose cotransporter-2; O-GlcNAc, O-linked N-acetylglucosamine; CREM, cyclic adenosine 5′-monophosphate-responsive element modulator; miRNA; microRNA.
Evolution of diabetic cardiomyopathy to clinical heart failure
Diabetic cardiomyopathy is usually asymptomatic in the early stages of its evolution.13 One of the earliest manifestations is LV hypertrophy and/or decreased LV compliance characterized by impaired early diastolic filling, increased atrial filling, and prolonged isovolumetric relaxation.13 LV dilation and symptomatic heart failure occur after the development of systolic dysfunction.13 Indeed, our recent data support the notion that diastolic dysfunction, as observed by cine magnetic resonance imaging in rodents, is associated with impaired cardiac insulin metabolic signaling.17 Cardiomyocyte stiffness and hypertrophy, as well as myocardial fibrosis all contribute to this cardiac abnormality (Fig. 1). The Cardiovascular Health Study found that, in a cohort of 5201 men and women, the ventricular septal and left posterior myocardial wall thicknesses were greater in diabetic patients than in nondiabetic individuals and that this was associated with compromised systolic or diastolic function.18
There is a considerable body of epidemiological evidence that implicates obesity, linked to increased intake of refined carbohydrates and decreased exercise, in the increasing prevalence of diabetes and related heart disease throughout the world. Lifestyle changes such as aerobic exercise, weight control and smoking cessation are efficacious therapeutic approaches in the prevention of diabetic cardiomyopathy. Sustained glycemic control reduces the prevalence of diabetic cardiomyopathy and reduces cardiovascular disease (CVD). For example, normalization of glycaemia with insulin therapy reduced cardiomyocyte hypertrophy, collagen content and diastolic dysfunction and limited progression of diabetic cardiomyopathy in type 1 diabetic rodent models.19 There is emerging evidence that some glycemic therapies may have specific benefits. In a retrospective cohort study of 10,920 patients, metformin use was associated with a low risk of mortality in diabetic individuals with heart failure.20 Sodium–glucose cotransporter (SGLT) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists have beneficial effects on CVD outcomes in type 2 diabetic patients.13 A meta-analysis of randomized controlled trials found that dipeptidyl peptidase 4 inhibitors and peroxisome proliferator-activated receptor (PPAR) agonists increased the risk of heart failure in patients with or at risk for type 2 diabetes mellitus. The EMPA-REG OUTCOME trial showed that SGLT2 antagonist treatment with empagliflozin reduced primary outcomes such as nonfatal myocardial infarction, nonfatal stroke, and CVD related mortality in 7020 diabetic patients.21 Finally, treatment with two long acting GLP-1 receptor agonists significantly reduced CVD events and heart failure in high risk diabetic patients.22, 23 The results suggest that these anti-diabetic drugs may have a role in preventing and treating diabetic cardiomyopathy and associated CVD in type 2 diabetic patients.
Functional phenotype of diabetic cardiomyopathy
The first stage of diabetic cardiomyopathy is clinically asymptomatic and is characterized by increased fibrosis and stiffness; there is a reduction of early diastolic filling and an increase in atrial filling and enlargement, as well as an elevated LV end-diastolic pressure.24 Underlying pathological factors include hyperglycemia, systemic and cardiac insulin resistance, increased free fatty acid (FFA) levels, systemic and tissue inflammation, oxidative stress and activation of the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system (SNS) (Fig 1).13 Reduced calcium (Ca2+) pump activity-induced inefficient sequestration of sarcoplasmic reticulum Ca2+ is regarded as an important contributor to the development of the cardiac diastolic dysfunction.25
The second stage of diabetic cardiomyopathy is characterized by LV hypertrophy, cardiac remodeling, advancing cardiac diastolic dysfunction, and the consequent emergence of clinical indications of HFpEF.13 With progression of diabetic cardiomyopathy, diastolic dysfunction and reduced cardiac compliance may co-exist with systolic dysfunction leading to reduced ejection fraction, prolonged pre-ejection performance, an enlarged LV chamber, shortened ejection period, and the latter by an increased resistance to filling with increased filling pressures.13 Abnormalities in contractile and regulatory protein expression are responsible for the mechanical defects in cardiac contraction. For example, decreased Ca2+ sensitivity along with shifts in cardiac myosin heavy chain from V1 to V3 isoforms contributes to the impaired cardiac systolic dysfunction, a long-term complication of diabetes.26 Phosphorylation of troponin also contributes to depressed myocardial contractility since myosin light chain-2 and troponin I are involved in regulating cardiomyocyte contraction.27
The phenotypes and underlying mechanisms of diabetic cardiomyopathy in type 2 diabetes have been mostly investigated in db/db mice, ob/ob mice, Zucker diabetic fatty rats, and diabetic patients.13 The impact of type 1 diabetes on systolic and diastolic function is less clear. As in type 2 diabetes, diastolic dysfunction is also often observed in type 1 diabetes.28 The underlying mechanisms of diabetic cardiomyopathy in type 1 diabetes probably mostly overlap but different alterations exist in hearts of type 2 diabetes.29 For instance, systolic function was preserved and cardiac hypertrophy was not observed in type 1 Akita diabetic mice, although hearts were smaller compared to non-diabetic controls. 30 Cardiomyocyte autophagy was enhanced in type 1 but suppressed in type 2 diabetes.28 Clearly, further studies are necessary to understand the potential differences in phenotype and underlying mechanisms for diabetic cardiomyopathy in type 1 and type 2 diabetes.
Thus, cardiac dysfunction in diabetic hearts progresses from subclinical cardiac abnormalities such as LV fibrosis to diastolic dysfunction and eventually systolic dysfunction accompanied by reduced ejection fraction. A number of non-invasive techniques including echocardiography, computed tomography, and cinematic magnetic resonance imaging have been applied to detect changes of cardiac structure (i.e. fibrosis) and function.13 Further, elevated levels of atrial natriuretic peptide, brain natriuretic peptide, and O-linked N-acetylglucosamine (O-GlcNAc), among others, may also serve as markers for diabetic cardiomyopathy and heart failure.13
Molecular mechanisms underlying diabetic cardiomyopathy
Cardiac structural abnormalities
The mechanism promoting cardiomyocyte stiffness in the diabetic heart include impaired insulin metabolic signaling that decreases glucose transporter type 4 (GLUT4) recruitment to the plasma membrane and glucose uptake, thus lowering sarcoplasmic reticulum Ca2+ pump activity and increasing cardiomyocyte intracellular Ca2+.13 Meanwhile, abnormal insulin metabolic signaling also decreases insulin-stimulated coronary endothelial nitric oxide (NO) synthase (eNOS) activity and NO production increasing cardiomyocyte intracellular Ca2+/Ca2+ sensitization and reducing sarcoplasmic Ca2+uptake.13 Reduction of NO bioavailability may also lead to phosphorylation of titin increasing the ratio of stiff titin isoform N2B/N2BA (compliant) expression. These pathophysiological abnormalities increase cardiac stiffness and impair relaxation, cardinal manifestations of diabetic cardiomyopathy.13 Other pertinent abnormalities include hyperglycemia, insulin resistance and oxidative stress that promote expression of a number of cardiomyocyte hypertrophic genes such as β-myosin heavy chain, insulin-like growth factor 1 (IGF-1) receptor, and B-type natriuretic peptide.31 High insulin levels induce cardiomyocyte hypertrophy by binding to the IGF-1 receptor. IGF-1, produced by cardiomyocytes, can also stimulate cardiomyocyte hypertrophy via the insulin receptor, extracellular signal-regulated kinase 2 (Erk1/2) and phosphatidylinositol 3-kinase (PI3K) signaling pathways.32 Crosstalk between the IGF-1 and insulin signaling pathways plays an important role in hyperglycemia/insulin resistance-induced cardiac hypertrophy and fibrosis in diabetic cardiomyopathy, as described in more detail later in this review.
Development of myocardial fibrosis in diabetic cardiomyopathy involves the deposition of stiff collagen and its cross linking, cardiac interstitial fibrosis, progressive abolition of muscular fibrils, perivascular fibrosis, thickened and sclerotic small coronary vessels, and basement membrane thickening, as well as coronary microvascular sclerosis and microaneurysms.33, 34 Activation of the RAAS and SNS, stimulation of advanced glycation end products (AGE)-mediated signaling via RAGE (cell surface receptor for AGE), hyperinsulinemia and hyperglycemia collectively lead to activation of transforming growth factor beta 1 (TGF-β1) pathway and dysregulation of extracellular matrix (ECM) degradation.35 Some biomarkers of collagen synthesis, including inflammation cytokines, connective tissue growth factor, metalloproteinases, and galectin-3 can be used clinically in the determination of myocardial fibrosis.36 As discussed in more detail later, reduced bioavailable NO, increased oxidative stress, and an activated TGF-β1/SMAD signaling pathway, in concert with impaired insulin metabolic signaling pathways, increase the myocardial fibronectin and collagen content and cardiac interstitial fibrosis characteristic of diabetic cardiomyopathy.17
Cardiac insulin resistance and diabetic cardiomyopathy
Cardiac insulin signaling mediates cellular homeostasis via control of protein synthesis, substrate utilization, and cell survival. Glucose transport in cardiac tissue is mediated via GLUT4 as in skeletal muscle, liver and fat tissue. Insulin, binding to the insulin receptor, activates insulin signaling/docking molecule insulin receptor substrate (IRS)-1/2 and downstream PI3K/protein kinase B (Akt), stimulating GLUT4 translocation to the cell membrane and subsequent glucose uptake.13 Further, normal coronary artery and myocardial insulin metabolic signaling promotes eNOS activation and bioavailable NO necessary for optimal coronary microvascular flow and myocardial function.13, 17 Cardiac insulin receptor knockout decreases cardiac glucose uptake, increases cardiac reactive oxygen species (ROS) production, and induces mitochondrial dysfunction.37, 38 Double IRS-1/2 knockout reduces cardiomyocyte adenosine triphosphate (ATP) content, impairs cardiac metabolism and function, and increases fibrosis and cardiac failure.37, 38 Reduced PI3K/Akt signaling and reduced GLUT4 expression and translocation have also been found in ventricular muscle biopsies obtained from patients with type 2 diabetes.39 The E3 ubiquitin ligase, mitsugumin 53 (MG53), may play an important negative role in the maintenance of insulin signaling.40 Elevated cardiac MG53 protein levels in a type 2 diabetic mouse model were correlated with increased proteosomal degradation of the insulin receptor and IRS-1. Further, cardiomyocyte-specific overexpression of MG53 inhibited insulin signaling and increased cardiac fibrosis,41 suggesting down-regulation of cardiac MG53 may be a potential therapeutic strategy in the prevention of diabetic cardiomyopathy and/or progression to clinically manifested heart failure.
Risk factors such as obesity and inappropriate activation of RAAS can impair cardiac insulin metabolic signaling through enhanced activation of the mammalian target of rapamycin (mTOR)/S6 kinase 1/(S6K1) signaling pathway,13, 17 which increases serine phosphorylation and reduces tyrosine phosphorylation of IRS-1/2 and impairs PI3K engagement and Akt/eNOS activation and NO production.13, 17 Lower NO production impairs coronary vessel relaxation and insulin-mediated capillary recruitment, both of which are important for the delivery of insulin and glucose necessary for normal myocardial energetics.42–45 Impairment of NO production also leads to increased activation of collagen cross linking enzymes such as transglutaminase thereby promoting cardiac fibrosis and stiffness.1,10 Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), have been reported to induce cardiac insulin resistance through activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and c-Jun N terminal kinase (JNK) that induce phosphorylation of IRS-1.13 Activation of FoxO1 (forkhead box-containing protein, O subfamily) directly regulates IRS1 signaling and decreases PI3K/Akt signaling, leading to insulin resistance in mice fed a high fat diet.46 Further, deletion of cardiac FoxO1 largely prevented heart failure in these animals.47 Thus, FoxO1 may also provide a novel therapeutic or preventive strategy for treating individuals with diabetic cardiomyopathy.47
Role of fructose in the pathogenesis of diabetic cardiomyopathy
High fructose diets induce cardiomyocyte autophagy, oxidative stress, and impaired insulin metabolic PI3K/Akt/eNOS signaling and interstitial fibrosis.16, 48 Generally, fructose is readily absorbed and rapidly metabolized by the human liver through GLUT2 and 5.13 Fructose 1-phosphate is cleaved to dihydroxyacetone phosphate by aldolase B, which can then be isomerized into glyceraldehyde 3-phosphate and acetyl-coenzyme A (CoA). Acetyl-CoA is either oxidized in the tricarboxylic acid cycle or directed towards FFA synthesis.49, 50 Phosphorylation of fructose also decreases ATP production.49, 50 Enhanced cellular fructose metabolism promotes a number of subcellular hexose sugar-related protein modifications, such as O-GlcNAc, and formation of advanced glycation end products (AGE) that contribute to impaired insulin metabolic signaling, reductions in NO production, and increased cardiac fibrosis.51
Decreased flexibility in substrate utilization in diabetic cardiomyopathy
Under normal physiological circumstances, the heart displays considerable metabolic substrate flexibility, utilizing energy from various substrates such as FFAs, glucose, ketone bodies, lactate, and some amino acids to produce ATP, the predominant source of energy for cardiac energetics.13 Mitochondria normally occupy approximately 20 to 30 % of the total cell volume of cardiomyocytes.13 Typically, mitochondrial oxidative phosphorylation produces more than 95% of ATP.52 The citric acid cycle usually accounts for the remaining 5% of ATP produced from glucose and lactate in the heart.53 However, in the setting of hyperglycemia, insulin resistance and hypertriglyceridemia, there is a reduction in the myocardium’s ability to use glucose as an energy source, and it subsequently switches to FFAs.53 This energy substrate switch is accompanied by impaired oxidative phosphorylation and a mitochondrial proton leak that results in increased production of ROS. As the heart has very limited anti-oxidant capacity, increased mitochondrial ROS production leads to NO destruction and reduced bioavailable NO, hallmarks of diabetic cardiomyopathy.1, 22
The role of abnormal FFA metabolism in diabetic cardiomyopathy
Increased FFA release from adipose tissue and increased capacity of myocyte sarcolemmal FFA transporters also contribute to the development of diabetic cardiomyopathy.13, 53 Cluster of Differentiation 36 (CD36), a predominantly membrane-located protein and transporter that promotes FFA uptake in both sarcolemma and endosomal membranes, is increased in diabetic hearts.54 Increased subcellular vesicular recycling of CD36 from endosomes to the plasma membrane increases the rate of cellular FFA uptake in diabetic hearts.13, 54 CD36 is also paramount in AMP-activated protein kinase (AMPK)-mediated stimulation of FFA uptake in cardiomyocytes. Indeed, CD36-knockout mice show a 70% reduction in FFA uptake in cardiomyocytes55 and CD36 deficiency rescues lipotoxic cardiomyopathy.56, 57 Activation of AMPK is responsible for early activation of glucose uptake and glycolysis and improves cardiac function in diabetic patients.58 However, AMPK activation is attenuated in diabetes increasing FFA uptake and triacylglycerol accumulation and reducing glucose utilization, also characteristic of diabetic cardiomyopathy.59
Several lipid metabolites such as diacylglycerols (DAGs) and ceramides impair insulin metabolic signaling contributing to and exacerbating diabetic cardiomyopathy. Insulin sensitivity in obese humans can be correlated to elevated DAG content and protein kinase C (PKC)ε activation.60 DAG increases lipid-associated endoplasmic reticulum stress.60 A high fat diet increases DAG in the membrane fraction, activating PKCε and inducing insulin resistance,61 and lowering NO production.62 Comparative gene identification 58 (CGI-58) is a lipid droplet-associated protein that promotes triglyceride hydrolysis and adipose triglyceride lipase activation.63 Inhibition of CGI-58 induced hepatic steatosis and increased total DAG content in the absence of hepatic insulin resistance.63 Studies have found that CGI-58 gene knock out prevents DAG accumulation at the plasma membrane, PKCε activation, and the impairment of hepatic insulin metabolic signaling.61, 63 Meanwhile, ceramide can directly activate atypical PKCs and inhibit insulin metabolic Akt/PKB signaling, attenuating GLUT4 translocation and insulin-stimulated glucose uptake in diabetic hearts62. These data support a role for intracellular compartmentation of DAG and ceramide in causing lipotoxicity and metabolic insulin resistance in a number of tissues including the heart.
Abnormalities in ketogenesis in diabetic cardiomyopathy
Type 2 diabetes is often associated with decreased ketogenesis because of systemic insulin resistance and hyperinsulinemia.64 Ketones, such as B-hydroxybutyrate, may play a key role in maintaining bio-energetic homeostasis in diabetic cardiomyopathy where there is reduced cardiac glucose utilization.65 In this regard, treatment with empagliflozin, a SGLT2 antagonist, increased ketone levels providing a more efficient energy source in the failing myocardium of diabetic patients with heart failure,66 perhaps compensating for an impairment in mitochondrial energy transduction related to decreased myocardial glucose utilization.
Abnormalities in cardiac glucose FFA cycling
The glucose fatty-acid cycle (Randle cycle) is a metabolic mechanism which involves competition between glucose and FFAs for their oxidation and uptake and is thus related to insulin resistance and type 2 diabetes. In this regard, activation of lipolysis provides FFAs as the preferred fuel source leading to ketogenesis during fasting whereas inhibition of glucose oxidation contributes to a glucose-sparing effect that is an essential survival mechanism during times of starvation.67 In the fed state or during exercise, glucose is rerouted to glycogen and muscle glycogen content is increased.67 Typically, FFA oxidation increases the mitochondrial ratios of acetyl-CoA/CoA and nicotinamide adenine dinucleotide (NADH)/NAD+ that inhibit pyruvate dehydrogenase activity and impair glucose metabolism, resulting in accumulation of cytosolic citrate, increased glucose 6-phosphate and inhibition of hexokinase.67, 68 Meanwhile, glucose oxidation produces citrate that can be metabolized to malonyl-CoA; malonyl-CoA controls the entry and oxidation of long chain FFA favoring FFA esterification.67, 69, 70
Mitochondrial dysfunction in the genesis of diabetic cardiomyopathy
Mitochondrial dysfunction plays a pivotal role in the development of diabetic cardiomyopathy and associated heart failure.71 Mitochondrial oxidative phosphorylation provides 90% of intracellular ATP production in cardiomyocytes but in type 2 diabetes, mitochondria switch from glucose to FFA oxidation for ATP production (Fig. 1).13 This is accompanied by increased mitochondrial ROS generation and impaired oxidative phosphorylation. Altered mitochondrial Ca2+ handling further promotes mitochondrial respiratory dysfunction leading to cell death.72 Metabolic stress-induced mitochondrial dysfunction also increases Ca2+ overload-induced opening of the mitochondrial permeability transition pores resulting in cardiomyocyte autophagy and cardiac necrosis.73
Mitochondrial oxidative stress in the pathogenesis of diabetic cardiomyopathy
Oxidative stress promotes development and progression of cardiac insulin resistance, diabetic cardiomyopathy and heart failure (Fig. 1). Mitochondrial ROS are a natural byproduct of oxygen metabolism at complexes I and III within the electron transport chain.13 Under normal physiological conditions, the major electrochemical proton gradient is utilized to synthetize ATP.13 However, hyperglycemia and insulin resistance increase the NADH and flavin adenine dinucleotide flux to the mitochondrial respiratory chain, resulting in hyperpolarization of the mitochondrial inner membrane, inhibition of electron transport in complex III and excess ROS production.74 Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase is another important source of cardiomyocyte ROS. Increased cardiomyocyte NADPH oxidase activity, has been reported in diet-induced obesity and systemic and cardiac insulin resistance.75 Increased RAAS-mediated NADPH oxidase activity may also directly promote cardiac fibrosis by activation of a pro-fibrotic TGF-β1/Smad 2/3 signaling pathway.76, 77 Other sources of ROS in diabetic cardiomyopathy include increases in xanthine oxidase, and microsomal P-450 enzyme activity, and uncoupling of NO synthase. Elevated cardiac tissue ROS not only increases polyol pathway flux, formation of AGEs, expression of the receptor for AGEs and its activating ligands, PKC signaling, and the hexosamine pathway, but also inhibits eNOS and prostacyclin synthase activity all of which contribute to the development of cardiomyopathy as previously reviewed.78.
Role of AGEs and RAGE in the pathophysiology of diabetic cardiomyopathy
Hyperglycemia increases AGE accumulation and induces myocardial structural alterations by increases in nonenzymatic glycation, oxidation of lipids and proteins, myocardial collagen and fibronectin production and connective tissue cross-linking and fibrosis (Fig. 1).13 Indeed, AGE-induced connective tissue cross-linking of ECM promotes myocardial fibrosis and impaired passive relaxation.13 AGEs may also bind to RAGE which further promotes maladaptive structural changes and impaired myocardial energetics.13 For example, interaction of AGEs with RAGE on cardiomyocyte surfaces induces maladaptive pro-inflammatory responses and increases matrix protein and connective tissue production, mediated through Janus kinase (JAK) and mitogen-activated protein kinase (MAPK) pathway activation.13 In addition, AGEs elevation increases ROS production and TGF-β1/SMAD pathway activation, and connective tissue production and fibrosis. In a prospective clinical study of 194 patients with acute coronary syndrome, AGEs were an independent risk factor in post-infarction heart failure.79 Further, increases in plasma AGEs independently predicted mortality and hospitalization for heart failure in a study of 580 diabetic patients.80
Impaired mitochondrial Ca2+ handling in diabetic cardiomyopathy
Cytosolic Ca2+ levels regulate cellular metabolism, muscle contraction, and cell signaling (Fig. 1). Typically, in cardiac excitation–contraction coupling, Ca2+ enters the cytoplasm through voltage sensitive L-type Ca2+ channels after depolarization of the sarcolemma and this triggers Ca2+-release from the sarcoplasmic reticulum. The Ca2+ binds troponin C to induce myofibrillar contraction.81, 82 During cardiac relaxation, Ca2+ is transported back into the sarcoplasmic reticulum and the remaining cardiomyocyte Ca2+ is pumped out by the sarcolemma Na+/Ca2+ exchanger and the plasma membrane Ca2+ pump.81, 82 However, in diabetic cardiomyopathy impaired Ca2+ handing by all of these transporters increases action potential duration and prolongs diastolic relaxation time.13 Elevated intracellular resting Ca2+, prolongation of intracellular Ca2+ decay, slowed Ca2+ transients, reduction of sarcoplasmic reticulum Ca2+ pumping, and impairment of sarcoplasmic reticulum Ca2+ reuptake have been documented in hearts of type 2 diabetic mice.83, 84 Similar changes are seen in type 1 diabetic rodent models.85, 86 The data suggest that impaired cardiomyocyte Ca2+ handling plays a key role in the development of the cardiac diastolic dysfunction characteristic of early diabetic cardiomyopathy.
Inflammation as an instigator of diabetic cardiomyopathy
A maladaptive pro-inflammatory response has been implicated in the development of diabetic cardiomyopathy. The innate immune system, i.e. neutrophils, mast cells, dendritic cells, macrophages, and eosinophils, is involved (Fig 1).13 Activation and expression of pro-inflammatory cytokines such as TNFα, interleukins (IL) 6 and 8, monocyte chemotactic protein 1 (MCP-1), adhesion molecule intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 all contribute to cardiac oxidative stress, remodeling and fibrosis, and diastolic dysfunction.13 Cytokine expression is regulated by the nuclear transcription factor, NF-κB.13 Finally, the Toll-like receptor-4 also plays an important role in triggering increased NF-κB, pro-inflammatory and innate immune system responses.87 These pro-inflammatory responses occur in different populations of cardiac cells including coronary endothelial and smooth muscle cells as well as fibroblasts and cardiomyocytes.
High FFA levels, impaired insulin metabolic signaling, and hyperglycemia activate the NLRP3 inflammasome, a novel molecular marker in diabetic cardiomyopathy.88 Separation from cytoplasmic chaperones and oligomerization follows NLRP3 activation, leading to recruitment of procaspase-1.88 Activated caspase-1 processes IL-1β and IL-18 precursors and serves as an enhancer of multiple pro-inflammatory pathways involving NF-κB, chemokines, and ROS. A NF-κB positive feedback loop further increases NLRP3 inflammasome assembly and pro-caspase-1 activation, and pro-IL-1β processing and maturation.88 Meanwhile, increased monocyte/macrophage migration through the coronary endothelium increases resident cardiac macrophages, which can be polarized into pro-inflammatory M1 phenotypes under conditions of increased ROS and reduced bioavailable NO.13, 17 In recent investigative work it has been observed that macrophage pro-inflammatory M1 polarization is upregulated whereas macrophage M2 anti-inflammatory response is repressed in diabetic heart tissues.13, 17
Activated RAAS in the genesis of diabetic cardiomyopathy
Increased activation of the systemic and tissue RAAS in states of hyperglycemia and insulin resistance plays an important role in the pathogenesis of diabetic cardiomyopathy and heart failure (Fig 1). Serum angiotensin II (Ang II) levels are significantly correlated with postprandial glucose concentrations in insulin resistance and type 2 diabetes.89 Further, the pro-inflammatory Ang II receptor 1 (AT-1R) is upregulated and the anti-inflammatory AT-2R is downregulated in early diabetes.90 Exercise induces a shift of the RAAS toward the angiotensin converting enzyme 2/Mas receptor axis in skeletal muscle, providing protection in obese rats.91 Conversely, inhibition of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice.92 In patients with type 1 diabetes, hyperglycemia-induced activation of systemic RAAS appears to play a role in the pathogenesis of diabetic cardiomyopathy.93,94 Experimental evidence also supports a role for increased mineralocorticoids in systemic and tissue insulin resistance.17 High plasma aldosterone and overexpression of the tissue mineralocorticoid receptors (MR) are associated with systemic insulin resistance, hyperglycemia and dyslipidemia.95 Large randomized controlled trials have shown that inhibition of the aldosterone/MR signaling pathway reduces morbidity and mortality in diabetic patients with both mild and moderately severe heart failure.62 RAAS activation impairs insulin metabolic signaling and induces systemic and cardiac insulin resistance, in part, through activation of the mTOR/S6K1 signaling pathway.96 Meanwhile, enhanced AT-1R and MR activation increases coronary artery endothelial leukocyte/monocyte adhesion, pro-inflammatory cytokine expression, macrophage infiltration and polarization resulting in an increase in the pro-inflammatory M1 phenotype in the myocardium. These abnormalities exacerbate the maladaptive cardiac remodeling, interstitial fibrosis and diastolic dysfunction seen in diabetic cardiomyopathy.17
Autonomic neuropathy in diabetic cardiomyopathy
Cardiac autonomic neuropathy is a secondary complication related to sustained hyperglycemia (Fig. 1) and includes abnormalities in heart rate control, vascular hemodynamics, and cardiac structure and function.13, 97, 98 An early characteristic of cardiac autonomic neuropathy is reduction of parasympathetic activity with an imbalance toward relatively higher SNS activity.99, 10001 In this regard, activation of the SNS enhances beta-1 adrenergic receptor (β1) signaling which promotes cardiac hypertrophy, interstitial fibrosis, cardiomyocyte apoptosis and impaired function.98
Endoplasmic reticulum stress and increased cell death in diabetic cariomyopathy
Cardiac oxidative stress, lipotoxicity, inflammation, and the accumulation of misfolded proteins impairs the function of cardiac endoplasmic reticulum and promotes endoplasmic reticulum stress, inducing the unfolded protein response (Fig. 1).13 Together, endoplasmic reticulum stress and the unfolded protein response inhibit cellular protein synthesis and degradation of misfolded or damaged proteins and ultimately increase cell apoptosis and autophagy.13 Increased cardiac apoptosis is a major risk factor for the development of diabetic cardiomyopathy; biopsied diabetic heart tissue expresses 85-fold more cardiomyocyte apoptosis than control non-diabetic hearts.101 Endoplasmic reticulum stress also induces autophagy through a Ca2+-dependent pathway, involving the inositol-requiring enzyme 1 (IRE1) and protein kinase RNA-like endoplasmic reticulum kinase (PERK) pathways.102 Typically, autophagy is regulated through the mTOR, AMPK, and silent information regulator (Sirt) pathways.13 mTORC1 has been proposed to regulate autophagy by repressing the autophagy related 1 (Atg1)–Atg13–Atg101/FIP200 (FAK family-interacting protein of 200 kDa) complex. Thus inhibition of mTORC1 facilitates the initiation of autophagy.102 The inositol requiring enzyme 1 arm of ER stress leads to JNK activation and increased phosphorylation of Bcl-2, which promotes its dissociation from Beclin-1.102 Thus, enhanced mTOR may serve as a convergence point for abnormalities involving the interplay between endoplasmic reticulum stress and autophagy in the hearts of diabetic individuals. However, in diabetic cardiomyopathy, dysregulation of autophagy impairs cardiomyocyte auto-phagosome and lysosome fusion.103 One study found that chronic metformin treatment activated AMPK activity, restored autophagic activity, and inhibited cardiomyocyte apoptosis by disrupting the Bcl-2 and Beclin1 complex in diabetic heart tissue.103 Therefore, the activation of the autophagic response is usually regarded as a compensatory feedback mechanism to protect against cell apoptosis and to maintain normal cellular function in conditions such as insulin resistance and type 2 diabetes.
Microvascular dysfunction in diabetic cardiomyopathy
Diabetic cardiomyopathy is classically defined in the context of absence of overt coronary artery disease. Nevertheless, diabetic cardiomyopathy may be associated with coronary microvascular dysfunction which impairs coronary blood flow and myocardial perfusion, ventricular function, and clinical outcomes including CVD.104 In a clinical study of 2783 consecutive patients, impaired coronary flow reserve was associated with an adjusted 3.2- and 4.9-fold increase in the rate of cardiac death for both diabetic and nondiabetic individuals, respectively, indicating that coronary microcirculation dysfunction is a powerful, independent correlate of cardiac mortality among both diabetics and nondiabetics.105 Treatment with an MR antagonist improved coronary microvascular function and prevented CVD in patients with type 2 diabetes suggesting an important role of cardiac MR activation in coronary microvascular dysfunction in diabetes.106 Structural abnormalities in the coronary microcirculation include luminal obstruction, inflammation infiltration, vascular remodeling, and perivascular fibrosis.107 Functional abnormalities in the coronary microcirculation include endothelial and smooth muscle cell dysfunction and impairment of vascular relaxation and constriction and ischemic reperfusion.107 The normal function of coronary arteries, and downstream microcirculatory vessels, is impaired in diabetic cardiomyopathy (Fig. 1). Physiologically, a number of vasoactive substances, including NO, prostacyclin (PGI2) and endothelium-derived hyperpolarizing factors (EDHF), released from endothelial cells lining the coronary microcirculation, play a beneficial vasodilatory role.42 Indeed, although NO-mediated vasodilation may be impaired, vascular function in the early stages of diabetes is often preserved through normal or even enhanced EDHF-induced vasodilation.42 However, both NO- and EDHF-induced vasodilation are eventually affected leading to significant dysfunction of the microcirculation in the later stages of diabetes.42 Recent investigation has also suggested that persistently high plasma endothelin-1 (ET-1) levels, along with reduced eNOS activity and NO production, are associated with the development of cardiac fibrosis and diastolic dysfunction in diabetic patients.108 In this regard, endothelial cell-specific ET-1 knockout prevented diabetes-induced cardiac fibrosis and exerted a beneficial effect in the prevention of diabetic cardiomyopathy.108
Molecular protein signature in diabetic cardiomyopathy
A number of molecular proteins and signaling pathways have been implicated as important contributors to the development of diabetic cardiomyopathy and heart failure. These include AMPK, PPARs, O-GlcNAc, SGLT2, PKC, MAPK, NFκB, nuclear factor erythroid 2-related factor 2 (Nrf2), cyclic adenosine 5′-monophosphate-responsive element modulator (CREM), microRNAs (miRNA), and exosomes as discussed below (Fig 2).
Impaired AMPK activation in diabetic cardiomyopathy
AMPK is a master regulator of cellular energy homeostasis.13 With cellular stress and/or an increased AMP/ATP ratio, AMPK activation enhances expression and translocation of GLUT4 and thus insulin-induced glucose uptake, and increases mitochondrial biogenesis leading to FFA oxidation and glycolysis.58 Specifically, in cardiomyocytes, activated AMPK positively stimulates glucose uptake, FFA oxidation, and glycolysis while negatively regulating mTOR signaling, gluconeogenesis, and lipid and protein synthesis.109 Therefore, AMPK activation plays a beneficial role in preventing the progression of diabetic cardiomyopathy. For this reason, AMPK has been considered as a promising target for drug discovery and development for the prevention and reversal of diabetic cardiomyopathy.
Alterations in activation of cardiac PPARs
Several different PPAR isoforms, namely α, β/δ and γ, are expressed in the heart and play a key role in myocardial glucose and lipid metabolism and energy homeostasis. Importantly, they also exert roles not directly related to metabolism such as inflammation and oxidative stress.13 PPAR-α is expressed at relatively high levels in the heart and its activation directly impacts FFA uptake and mitochondrial FFA oxidation.13 PPAR-α regulates lipoprotein assembly and transport and modulates both oxidant and antioxidant defenses.110 Overexpression of cardiomyocyte-specific PPARα causes decreased uptake of Ca2+ by the sarcoplasmic reticulum, LV hypertrophy, systolic dysfunction, and increased atrial natriuretic and B-type natriuretic peptide expression.59 Conversely, deletion of cardiac PPAR-α prevents fasting-induced expression of FFA metabolic genes and induces a switch from FFA to glucose utilization.111 With the development of diabetic cardiomyopathy, chronic exposure to elevated FFAs seems to reduce PPAR-α expression. In rodent cardiomyocytes, this was shown to further decrease cardiac function by inhibition of FFA oxidation and increased intracellular fat accumulation.112, 113 However, human studies suggest that PPAR-α expression is not significantly altered in the hearts of type 2 diabetes patients.72, 114 Activated PPAR-α-induced increases in FFA oxidation and utilization in the diabetic heart may thus initially serve as a compensatory mechanism to adjust substrate oxidation to available substrate supply. Further, reduced PPAR-α in advanced disease may have maladaptive consequences in terms of cardiac metabolism, including glucotoxicity and functional cardiac abnormalities. The role of PPAR-α in the development of cardiac dysfunction in diabetic cardiomyopathy has been inadequately evaluated and needs to be further investigated. Similar to PPAR-α, PPAR-β/δ isoforms are also expressed abundantly in heart tissue and regulate transcriptional gene expression and FFA metabolism.115 Enhanced PPAR-β/δ signaling promotes FFA utilization whereas deletion of PPAR- β/δ decreases FFA oxidative gene expression and FFA oxidation.115 In addition, PPAR-γ plays important roles in cardiac anti-hypertrophic and anti-inflammatory effects.13 PPAR-γ agonists enhance cardiomyocyte insulin sensitivity and improve cardiomyocyte glucose uptake.13 Thus, PPAR-γ may be beneficial in maintaining glucose and FFA metabolism and cardiac function. Conversely, a deficiency in PPAR-γ signaling may contribute to the development of diabetic cardiomyopathy.
Increased O-GlcNAc in promoting cardiac fibrosis in diabetic cardiomyopathy
Hyperglycemia is associated with increased O-GlcNAcylation, which causes posttranslational modification of cardiac proteins (Fig 2). Sustained O-GlcNAc signaling exists in the diabetic heart and can exert detrimental effects that include decreases in mitochondrial function and energy generation, and increases in cardiac dysfunction and heart failure.116 Under physiological conditions, the hexosamine biosynthesis pathway drives a component of fructose-6-phosphate metabolism from glycolysis to generate the O-GlcNAc moiety.117 Transient activation of O-GlcNAc signaling is normally cytoprotective and increases cell survival.118 In contrast to transient upregulation, sustained elevation of O-GlcNAc signaling in the diabetic heart mediates diabetes-induced impairment of insulin metabolic signaling, cardiomyocyte apoptosis, myocardial excitation-contraction coupling, and cardiac relaxation.119 Overexpression of O-GlcNAcase removes O-GlcNAc and restores normal cardiomyocyte Ca2+ handling and cardiac function,120 suggesting that targeting of hexosamine biosynthesis and O-GlcNAc may be a fruitful potential strategy for the prevention and therapy of diabetic cardiomyopathy.
SGLT2 abnormalities and potential cardiac benefits of inhibition of this transporter
Glucose is actively transported from the gut lumen into the gastrointestinal epithelium primarily by SGLT1, highly expressed in the brush-border membrane of enterocytes.121, 122 In hyperglycemia and insulin resistance, glucose and fructose absorption through the intestinal mucosa increases due to higher expression of SGLT1, GLUT2 and GLUT5 and increased brush border disaccharidases, sucrase, maltase and lactase activity. 121, 122 SGLT2 is exclusively expressed in the kidney and mostly localized in the brush border membrane of proximal tubule epithelial cells in the S1 segment of the proximal convoluted tubule.121, 122 SGLT2 expression is significantly increased in diabetic humans123, rats124, and db/db mice125 and this is correlated with glomerular hyperfiltration, increased glucose reabsorption and elevated plasma glucose.126 Conversely, SGLT2 inhibition leads to natriuresis, osmotic diuresis, plasma volume contraction, and reduction of blood pressure and arterial stiffness, all mechanisms that may mitigate diabetic cardiomyopathy and heart failure. Further, SGL2 inhibitor treatment can shift cell metabolism from glucose to FA oxidation. Thus, the cardiac beneficial effects of SGLT2 may include making more of the ketone body, B-hydroxybutyrate, a highly energy efficient substrate for cardiac metabolism. Targeting SGLT2 has been shown to improve cardiovascular outcomes and mortality in type 2 diabetic patients (Fig 2).21 Ongoing research is addressing the role of these agents for specific prevention and treatment of heart failure in diabetic individuals. 127
PKC activation promotes development of diabetic cardiomyopathy
PKC signaling pathways are activated in diabetic cardiomyopathy in response to hyperglycemia and insulin resistance (Fig 2). Oxidative stress, inflammation, and enhanced RAAS and SNS activity further promote PKC activation. To date, approximately 15 isoforms of PKC have been described in humans. These isoforms can be divided into 3 subfamilies based on second messenger signaling and particular mode of activation.128, 129 PKCα, β, ε, θ, and δ isoforms have been proposed to be involved in the development of diabetic cardiac hypertrophy.128, 129 For example, PKC β2 has been shown to mediate hyperglycemia-induced diastolic cardiac dysfunction in diabetic rats through alterations in caveolin-3 expression and insulin metabolic Akt/eNOS signaling.128 Further supporting its relevance, targeted inhibition of PKC β2 in a transgenic mouse model of diabetic cardiomyopathy has been reported to improve fractional shortening and reverse cardiac hypertrophy and fibrosis.130 Collectively, the data suggest that activation of PKC can induce cellular and functional changes that contribute to the development of diabetic cardiomyopathy and heart failure.
Role of MAPK and JNK activation in the genesis of diabetic cardiomyopathy
MAPK activation has also been implicated in the pathogenesis of diabetic cardiomyopathy and heart failure. Erk1/2, p38 MAPK and JNKs are three important MAPK subfamilies that regulate cardiac growth, hypertrophy, and remodeling.13 Enhanced myocardial phosphorylation of Erk 1/2 and activation of p38 MAPK occurs during ischemia in streptozotocin-induced diabetic models.131 Our research and that of others has demonstrated that obesity/insulin resistance-induced cardiac dysfunction is associated with enhanced S6K1 and Erk1/2 signaling.17,132 JNK can be activated by oxidative stress, inflammatory cytokines, and sphingolipid metabolites.133 In turn, enhanced JNK signaling in the diabetic heart contributes to oxidative stress, endoplasmic reticulum stress, and interstitial fibrosis.133 In contrast, inhibition of JNK phosphorylation by a curcumin analog prevents high glucose-induced inflammation and apoptosis in diabetic hearts.133 In addition to these observations, JNK may play an important role in cardiomyocyte apoptosis.134 As such, JNK activation was shown to cause increased cardiomyocyte apoptosis as early as days 3 and 7 in a type 1 diabetic rodent model.134 Collectively, activation of both MAPK and JNK signaling appears to contribute significantly to the development of diabetic cardiomyopathy.
Role of NF-κB activation in the genesis of diabetic cardiomyopathy
NF-κB is one of the key transcription factors that regulate the expression of pro-inflammatory cytokines, pro-fibrotic genes, and cell survival135 thus contributing to mitochondrial and cardiac dysfunction in diabetic hearts. NF-κB is found in the cytoplasm of non-stimulated cells. Upon stimulation, IκB is phosphorylated and its p50/p65 subunits translocate to the nucleus and bind κB nuclear elements.135 In diabetes, ROS, AGEs, and an activated cardiac tissue RAAS can directly induce NF-κB activation. This, in turn, promotes maladaptive immune responses and the release of pro-inflammatory cytokines, such as TNF-α, MCP-1, IL-6 and IL8.13 Activated NF-κB in diabetic mouse hearts has been shown to be associated with increased NADPH oxidase mediated generation of ROS, peroxynitrite and superoxide.136 These processes lead to a reduction in bioavailable NO. Inhibition of NF-κB with pyrrolidine dithiocarbamate improves mitochondrial structural integrity and inhibits oxidative stress, increasing ATP synthesis and NO bioavailability thereby restoring cardiac function in type 2 diabetes.136
Abnormalities of Nrf2 related antioxidant actions in diabetic cardiomyopathy
Nrf2 is a leucine zipper protein that promotes the expression of antioxidant proteins such as hemoxygenase in response to oxidative stress (Fig 2). Nrf2 is predominantly regulated by its binding to the inhibitor, Keap1, which targets Nrf2 for ubiquitination and degradation thus maintaining low cellular levels of Nrf2.137 Under oxidative stress, Nrf2 and its regulators undergo a variety of modifications that cause dissociation of Nrf2 from Keap1. Free Nrf2 can then bind to small Maf proteins in the nucleus to activate transcription.137 Hyperglycemia and insulin resistance repress Nrf2 expression and activity through an Erk 1/2 mediated pathway that contributes to oxidative stress and insulin resistance in cardiomyocytes.138 Restoration of Nrf2 activity prevents diabetes-induced lipid accumulation, inflammation, fibrosis, and associated cardiac dysfunction,139 providing another potential strategy for the prevention of diabetic cardiomyopathy.
Role of the transcription factor CREM in the genesis of diabetic cardiomyopathy
CREM is a transcription factor that regulates cAMP signaling and cardiac gene expression (Fig 2). Members of the CREM family may promote fibrosis of the heart, especially in response to hyperglycemia and elevated FFAs.140 For example, CREM expression is increased in cardiomyocytes of chronically hyperglycemic type 1 diabetic mice and this is accompanied by increased cardiac fibrosis.141 These adverse effects were associated with alterations in histone acetylation and alterations in miRNAs profiles suggesting that CREM activation may promote epigenetic as well as genetic modifications in cardiac proteins and mediate “glycemic memory” in on-going progression of diabetic cardiomyopathy.
Role of miRNAs in promotion of diabetic cardiomyopathy
Diabetic cardiomyopathy is associated with increased expression of miRNAs – a group of short single-stranded non-coding RNA molecules with an average length of 22 nucleotides. Importantly, miRNAs control the expression of transcriptional and post-transcriptional target genes through binding to the 3′-untranslated region and regulate mitochondrial function, ROS production, Ca2 + handling, apoptosis, autophagy, and fibrosis, all of which are regarded as important mechanisms in diabetes-induced cardiac hypertrophy, remodeling and fibrosis, as well as heart failure progression.142, 143 miR-15a, -21, -24, -29, -30d, -103, -126, -146a, -150, -191, -223, -320, -375, and -486 have all been reported to be increased in type 2 diabetic individuals.142, 143 In cardiac tissue of a type 1 diabetic rodent model, expression of miR-21, -24, -142-3p, -195, -199a-3p, -700, -705, -208, -221 and 499-3p was upregulated whereas expression of miR-1, -20a, -29a, -143, -220b, and -373 was downregulated.144 miR103, 107, -143 and -181 play a role in insulin sensitivity and systemic glucose metabolism.145, 146 Increased expression of miR-454, 500,-142-3p/5p and 1246 has been identified in cardiac diastolic dysfunction.147 Other miRNAs, such as miR-113a, -133a,-150 have been found to be involved in the regulation of cardiomyocyte hypertrophy and interstitial fibrosis.148
Abnormalities of Exosomes in diabetic cardiomyopathy
There is a close relationship between nutrient metabolism and exosome release from cells such as cardiomyocytes. Exosomes are extracellular vesicles with a diameter ranging from 30 to 90 nm and are regarded as important mediators of cell- to-cell communication.24 They contain a variety of biological components including lipids, miRNAs, proteins and transcription factors that regulate both normal physiological and pathophysiological effects.24 Exosomes that function with glucose transporters and glycolytic enzymes to increase glucose uptake, glycolysis, and pyruvate production in ECs were released from cardiomyocytes after glucose deprivation.149 In type 2 diabetic hearts, exosomes containing high levels of miR-320 are released from cardiomyocytes and transported to coronary endothelial cells, resulting in reduced NO production and inhibition of angiogenesis via decreases heat shock protein 20 (Hsp20).150 However, HSP20-engineered exosomes have a beneficial role in the regulation of cardiomyocyte exosome secretion and restoration of hyperglycemia-induced cardiac dysfunction.151 Therefore, exosomes may not only act as biomarkers, but targeting exosomes may also be a potential therapeutic strategy in the prevention and or progression of diabetic cardiomyopathy.
Conclusion and future perspectives
The cardinal features of diabetic cardiomyopathy include cardiac stiffness, myocardial fibrosis and hypertrophy with cardiac diastolic dysfunction and subsequent progression to both systolic dysfunction and clinical heart failure. Importantly, hyperglycemia and systemic and cardiac insulin resistance are independently associated with the development and progression of cardiac dysfunction and heart failure in diabetes. From a mechanistic point of view, mitochondrial dysfunction, oxidative stress, increased formation and deposition of AGEs, impaired mitochondrial Ca2+ handling and function, inflammation, activation of RAAS and SNS, cardiac autonomic neuropathy, endoplasmic reticulum stress, microvascular dysfunction, and cardiac metabolic disorders are involved in the pathophysiological process. Underlying these pathophysiological events, evidence supports a role for a number of proteins and signaling pathways including AMPK, PPARs, O-GlcNAc, SGLT2, PKC, MAPK, NFκB, Nrf2, miRNA and exosomes. Although diabetic cardiomyopathy appears to have an extensive preclinical course and the pathophysiological changes appear to be induced by the metabolic alterations in diabetes mellitus, a formal definition for diabetic cardiomyopathy as a distinct clinical entity remains vague due to a lack of accepted diagnostic criteria and information on subclinical CVD in the early stages of diabetes. Currently, there is not a specific histological property, biochemical marker, or clinical manifestation for the definitive diagnosis of diabetic cardiomyopathy. Also, there are no prospective clinical trials to support that hyperglycemia or hyperinsulinemia independently increase the risk for development of diabetic cardiomyopathy in the absence of other risk factors such as obesity, coronary heart disease, and hypertension. Recent scientific evidence for the potential use of exosome and circulating miRNAs as biomarkers for detection of diabetic cardiomyopathy highlights emerging methods for the diagnosis and prevention of diabetes and cardiomyopathy. The American Diabetes Association recommends screening patients with type 2 diabetes for the prevention of diabetic cardiomyopathy but the results of the recently published Detection of Ischemia in Asymptomatic Diabetics (DIAD) trial indicate that screening of asymptomatic patients with nuclear imaging does not improve cardiac event rates.152 Unfortunately, screening approaches including B-type natriuretic peptide, exercise stress testing, and echocardiographic assessment do not appear to be sufficiently sensitive to identify subclinical dysfunction in diabetic patients.153 Therefore, further studies are vital to the understanding of the precise mechanisms involved in the initiation and progression of diabetic cardiomyopathy, and to the development of novel strategies to reduce the risk of heart failure in diabetic patients.
Acknowledgments
Sources of Funding
JRS received funding from NIH (R01 HL73101-01A and R01 HL107910-01) and the Veterans Affairs Merit System (0018). Dr. Jia received funding from American Diabetes Association (Innovative Basic Science Award #1-17-IBS-201). Dr. Hill received funding from NIH (RO1HL085119).
Nonstandard Abbreviations and Acronyms
- ACCF
American College of Cardiology Foundation
- AGE
Advanced glycation end products
- AHA
American Heart Association
- Akt
Protein kinase B
- AMPK
AMP-activated protein kinase
- Ang II
Angiotensin II
- AT-1R
Ang II receptor 1
- ATP
Adenosine triphosphate
- Ca2+
Calcium
- CD 36
Cluster of Differentiation 36
- CGI-58
Comparative gene identification 58
- CHS
Cardiovascular Health Study
- CoA
Coenzyme A
- CREM
Cyclic adenosine 5′-monophosphate-responsive element modulator
- CVD
Cardiovascular disease
- DAGs
Diacylglycerols
- DIAD
Detection of Ischemia in Asymptomatic Diabetics
- EASD
European Association for the Study of Diabetes
- ECM
Extracellular matrix
- EDHF
Endothelium-derived hyperpolarizing factors
- eNOS
Endothelial NO synthase
- Erk1/2
Extracellular signal-regulated kinase 1/2
- ESC
European Society of Cardiology
- ET-1
Endothelin-1
- FFA
Free fatty acid
- FoxO1
Forkhead box-containing protein, O subfamily
- GLP-1
Glucagon-like peptide 1
- GLUT4
Glucose transporter type 4
- HbA1c
Hemoglobin A(1c)
- HFpEF
Heart failure with normal ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- Hsp20
Heat shock protein 20
- IGF-1
Insulin-like growth factor 1
- IL
Interleukins
- IRE1
Inositol-requiring enzyme 1
- IRS
Insulin receptor substrate
- JAK
Janus kinase
- JNK
c-Jun N terminal kinase
- LV
Left ventricular
- MAPK
Mitogen-activated protein kinase
- MCP-1
Monocyte chemotactic protein 1
- MESA
Multi-Ethnic Study of Atherosclerosis
- MG53
Mitsugumin 53
- miRNA
MicroRNAs
- MR
Mineralocorticoid receptors
- mTOR
Rapamycin
- NADH
Nicotinamide adenine dinucleotide
- NADPH
Nicotinamide-adenine dinucleotide phosphate
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
Nitric oxide
- Nrf2
Nuclear factor erythroid 2-related factor 2
- O-GlcNAc
O-linked N-acetylglucosamine
- PERK
Protein kinase RNA-like endoplasmic reticulum kinase
- PGI2
Prostacyclin
- PI3K
Phosphatidylinositol 3-kinase
- PKC
Protein kinase C
- PPAR
Peroxisome proliferator-activated receptor
- RAAS
Renin-angiotensin-aldosterone system
- RAGE
Cell surface receptor for AGE
- ROS
Reactive oxygen species
- S6K1
S6 kinase 1
- SHS
Strong Heart Study
- SGLT
Sodium–glucose cotransporter
- SNS
Sympathetic nervous system
- TGF-β1
Transforming growth factor beta 1
- TNF-α
Tumor necrosis factor-alpha
Footnotes
Disclosures
None
References
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology F, American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Authors/Task Force M; Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG Guidelines ESCCfP. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD) Eur Heart J. 2013;34:3035–87. doi: 10.1093/eurheartj/eht108. [DOI] [PubMed] [Google Scholar]
- 5.Ryden L, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA. Efficacy and safety of high-dose lisinopril in chronic heart failure patients at high cardiovascular risk, including those with diabetes mellitus. Results from the ATLAS trial. Eur Heart J. 2000;21:1967–78. doi: 10.1053/euhj.2000.2311. [DOI] [PubMed] [Google Scholar]
- 6.Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, Pinkett T, Ghali JK, Wilson AC. Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77:1017–20. doi: 10.1016/s0002-9149(97)89163-1. [DOI] [PubMed] [Google Scholar]
- 7.Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Ryden L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28:612–6. doi: 10.2337/diacare.28.3.612. [DOI] [PubMed] [Google Scholar]
- 8.Aronow WS, Ahn C. Incidence of heart failure in 2,737 older persons with and without diabetes mellitus. Chest. 1999;115:867–8. doi: 10.1378/chest.115.3.867. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, Fabsitz RR, Robbins D, Rhoades ER, Howard BV. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101:2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 10.Bertoni AG, Goff DC, Jr, D’Agostino RB, Jr, Liu K, Hundley WG, Lima JA, Polak JF, Saad MF, Szklo M, Tracy RP, Siscovick DS. Diabetic cardiomyopathy and subclinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2006;29:588–94. doi: 10.2337/diacare.29.03.06.dc05-1501. [DOI] [PubMed] [Google Scholar]
- 11.Lind M, Bounias I, Olsson M, Gudbjornsdottir S, Svensson AM, Rosengren A. Glycaemic control and incidence of heart failure in 20,985 patients with type 1 diabetes: an observational study. Lancet. 2011;378:140–6. doi: 10.1016/S0140-6736(11)60471-6. [DOI] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12:144–53. doi: 10.1038/nrendo.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itzhaki Ben Zadok O, Kornowski R, Goldenberg I, Klempfner R, Toledano Y, Biton Y, Fisman EZ, Tenenbaum A, Golovchiner G, Kadmon E, Omelchenko A, Gal TB, Barsheshet A. Admission blood glucose and 10-year mortality among patients with or without pre-existing diabetes mellitus hospitalized with heart failure. Cardiovasc Diabetol. 2017;16:102. doi: 10.1186/s12933-017-0582-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, Szklo M, Lima JA. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 16.Mellor KM, Bell JR, Young MJ, Ritchie RH, Delbridge LM. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol. 2011;50:1035–43. doi: 10.1016/j.yjmcc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Barrett Mueller K, Jaffe IZ, Sowers JR. Endothelial Mineralocorticoid Receptor Deletion Prevents Diet-Induced Cardiac Diastolic Dysfunction in Females. Hypertension. 2015;66:1159–67. doi: 10.1161/HYPERTENSIONAHA.115.06015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M, Gardin JM, Lynch JC, Smith VE, Tracy RP, Savage PJ, Szklo M, Ward BJ. Diabetes mellitus and echocardiographic left ventricular function in free-living elderly men and women: The Cardiovascular Health Study. Am Heart J. 1997;133:36–43. doi: 10.1016/s0002-8703(97)70245-x. [DOI] [PubMed] [Google Scholar]
- 19.Tate M, Deo M, Cao AH, Hood SG, Huynh K, Kiriazis H, Du XJ, Julius TL, Figtree GA, Dusting GJ, Kaye DM, Ritchie RH. Insulin replacement limits progression of diabetic cardiomyopathy in the low-dose streptozotocin-induced diabetic rat. Diab Vasc Dis Res. 2017;14:423–433. doi: 10.1177/1479164117710390. [DOI] [PubMed] [Google Scholar]
- 20.Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, Lange T, Abildstrom SZ, Schramm TK, Vaag A, Kober L, Torp-Pedersen C, Gislason GH. Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia. 2010;53:2546–53. doi: 10.1007/s00125-010-1906-6. [DOI] [PubMed] [Google Scholar]
- 21.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE Investigators E-RO. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 22.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T Investigators S. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 23.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, Committee LS Investigators LT. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westermeier F, Riquelme JA, Pavez M, Garrido V, Diaz A, Verdejo HE, Castro PF, Garcia L, Lavandero S. New Molecular Insights of Insulin in Diabetic Cardiomyopathy. Front Physiol. 2016;7:125. doi: 10.3389/fphys.2016.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, Zweier JL. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1426–34. doi: 10.1152/ajpheart.01016.2007. [DOI] [PubMed] [Google Scholar]
- 26.Pollack PS, Malhotra A, Fein FS, Scheuer J. Effects of diabetes on cardiac contractile proteins in rabbits and reversal with insulin. Am J Physiol. 1986;251:H448–54. doi: 10.1152/ajpheart.1986.251.2.H448. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra A, Sanghi V. Regulation of contractile proteins in diabetic heart. Cardiovasc Res. 1997;34:34–40. doi: 10.1016/s0008-6363(97)00059-x. [DOI] [PubMed] [Google Scholar]
- 28.Kanamori H, Takemura G, Goto K, Tsujimoto A, Mikami A, Ogino A, Watanabe T, Morishita K, Okada H, Kawasaki M, Seishima M, Minatoguchi S. Autophagic adaptations in diabetic cardiomyopathy differ between type 1 and type 2 diabetes. Autophagy. 2015;11:1146–60. doi: 10.1080/15548627.2015.1051295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holscher ME, Bode C, Bugger H. Diabetic Cardiomyopathy: Does the Type of Diabetes Matter? Int J Mol Sci. 2016:17. doi: 10.3390/ijms17122136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, Yun UJ, McQueen AP, Wayment B, Litwin SE, Abel ED. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57:2924–32. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenkranz AC, Hood SG, Woods RL, Dusting GJ, Ritchie RH. B-type natriuretic peptide prevents acute hypertrophic responses in the diabetic rat heart: importance of cyclic GMP. Diabetes. 2003;52:2389–95. doi: 10.2337/diabetes.52.9.2389. [DOI] [PubMed] [Google Scholar]
- 32.Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1481–9. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev Diabet Stud. 2006;3:108–17. doi: 10.1900/RDS.2006.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mytas DZ, Stougiannos PN, Zairis MN, Foussas SG, Pyrgakis VN, Kyriazis IA. Diabetic myocardial disease: pathophysiology, early diagnosis and therapeutic options. J Diabetes Complications. 2009;23:273–82. doi: 10.1016/j.jdiacomp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Bando YK, Murohara T. Diabetes-related heart failure. Circ J. 2014;78:576–83. doi: 10.1253/circj.cj-13-1564. [DOI] [PubMed] [Google Scholar]
- 36.Passino C, Barison A, Vergaro G, Gabutti A, Borrelli C, Emdin M, Clerico A. Markers of fibrosis, inflammation, and remodeling pathways in heart failure. Clin Chim Acta. 2015;443:29–38. doi: 10.1016/j.cca.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Bugger H, Riehle C, Jaishy B, Wende AR, Tuinei J, Chen D, Soto J, Pires KM, Boudina S, Theobald HA, Luptak I, Wayment B, Wang X, Litwin SE, Weimer BC, Abel ED. Genetic loss of insulin receptors worsens cardiac efficiency in diabetes. J Mol Cell Cardiol. 2012;52:1019–26. doi: 10.1016/j.yjmcc.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi Y, Xu Z, Zhu Q, Thomas C, Kumar R, Feng H, Dostal DE, White MF, Baker KM, Guo S. Myocardial loss of IRS1 and IRS2 causes heart failure and is controlled by p38alpha MAPK during insulin resistance. Diabetes. 2013;62:3887–900. doi: 10.2337/db13-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, Punjabi P, Camici PG. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2010;31:100–11. doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song R, Peng W, Zhang Y, Lv F, Wu HK, Guo J, Cao Y, Pi Y, Zhang X, Jin L, Zhang M, Jiang P, Liu F, Meng S, Zhang X, Jiang P, Cao CM, Xiao RP. Central role of E3 ubiquitin ligase MG53 in insulin resistance and metabolic disorders. Nature. 2013;494:375–9. doi: 10.1038/nature11834. [DOI] [PubMed] [Google Scholar]
- 41.Liu F, Song R, Feng Y, Guo J, Chen Y, Zhang Y, Chen T, Wang Y, Huang Y, Li CY, Cao C, Zhang Y, Hu X, Xiao RP. Upregulation of MG53 induces diabetic cardiomyopathy through transcriptional activation of peroxisome proliferation-activated receptor alpha. Circulation. 2015;131:795–804. doi: 10.1161/CIRCULATIONAHA.114.012285. [DOI] [PubMed] [Google Scholar]
- 42.Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes. 2004;53:1418–23. doi: 10.2337/diabetes.53.6.1418. [DOI] [PubMed] [Google Scholar]
- 43.Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab. 2006;290:E1191–7. doi: 10.1152/ajpendo.00497.2005. [DOI] [PubMed] [Google Scholar]
- 44.Vollus GC, Bradley EA, Roberts MK, Newman JM, Richards SM, Rattigan S, Barrett EJ, Clark MG. Graded occlusion of perfused rat muscle vasculature decreases insulin action. Clin Sci (Lond) 2007;112:457–66. doi: 10.1042/CS20060311. [DOI] [PubMed] [Google Scholar]
- 45.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest. 2012;122:1109–18. doi: 10.1172/JCI60329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi Y, Zhu Q, Zhang K, Thomas C, Wu Y, Kumar R, Baker KM, Xu Z, Chen S, Guo S. Activation of Foxo1 by insulin resistance promotes cardiac dysfunction and beta-myosin heavy chain gene expression. Circ Heart Fail. 2015;8:198–208. doi: 10.1161/CIRCHEARTFAILURE.114.001457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mellor KM, Bell JR, Ritchie RH, Delbridge LM. Myocardial insulin resistance, metabolic stress and autophagy in diabetes. Clin Exp Pharmacol Physiol. 2013;40:56–61. doi: 10.1111/j.1440-1681.2012.05738.x. [DOI] [PubMed] [Google Scholar]
- 49.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22:60–5. doi: 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RJ, Sanchez-Lozada LG, Nakagawa T. The effect of fructose on renal biology and disease. J Am Soc Nephrol. 2010;21:2036–9. doi: 10.1681/ASN.2010050506. [DOI] [PubMed] [Google Scholar]
- 51.Delbridge LM, Benson VL, Ritchie RH, Mellor KM. Diabetic Cardiomyopathy: The Case for a Role of Fructose in Disease Etiology. Diabetes. 2016;65:3521–3528. doi: 10.2337/db16-0682. [DOI] [PubMed] [Google Scholar]
- 52.Vega RB, Horton JL, Kelly DP. Maintaining ancient organelles: mitochondrial biogenesis and maturation. Circ Res. 2015;116:1820–34. doi: 10.1161/CIRCRESAHA.116.305420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo CA, Guo S. Insulin receptor substrate signaling controls cardiac energy metabolism and heart failure. J Endocrinol. 2017;233:R131–R143. doi: 10.1530/JOE-16-0679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee TW, Bai KJ, Lee TI, Chao TF, Kao YH, Chen YJ. PPARs modulate cardiac metabolism and mitochondrial function in diabetes. J Biomed Sci. 2017;24:5. doi: 10.1186/s12929-016-0309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. AMPK-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal CD36. Biochem Biophys Res Commun. 2007;355:204–10. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–17. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka T, Nakata T, Oka T, Ogawa T, Okamoto F, Kusaka Y, Sohmiya K, Shimamoto K, Itakura K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J Lipid Res. 2001;42:751–9. [PubMed] [Google Scholar]
- 58.Zou MH, Xie Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 2013;9:624–5. doi: 10.4161/auto.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuel VT, Shulman GI. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, Jiang DC, Zhang D, Lee HY, Samuel VT, Shulman GI. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A. 2011;108:5748–52. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Atkinson LL, Kozak R, Kelly SE, Onay Besikci A, Russell JC, Lopaschuk GD. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am J Physiol Endocrinol Metab. 2003;284:E923–30. doi: 10.1152/ajpendo.00360.2002. [DOI] [PubMed] [Google Scholar]
- 63.Cantley JL, Yoshimura T, Camporez JP, Zhang D, Jornayvaz FR, Kumashiro N, Guebre-Egziabher F, Jurczak MJ, Kahn M, Guigni BA, Serr J, Hankin J, Murphy RC, Cline GW, Bhanot S, Manchem VP, Brown JM, Samuel VT, Shulman GI. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc Natl Acad Sci U S A. 2013;110:1869–74. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kruljac I, Cacic M, Cacic P, Ostojic V, Stefanovic M, Sikic A, Vrkljan M. Diabetic ketosis during hyperglycemic crisis is associated with decreased all-cause mortality in patients with type 2 diabetes mellitus. Endocrine. 2017;55:139–143. doi: 10.1007/s12020-016-1082-7. [DOI] [PubMed] [Google Scholar]
- 65.Pawlak M, Bauge E, Lalloyer F, Lefebvre P, Staels B. Ketone Body Therapy Protects From Lipotoxicity and Acute Liver Failure Upon Pparalpha Deficiency. Mol Endocrinol. 2015;29:1134–43. doi: 10.1210/me.2014-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mudaliar S, Alloju S, Henry RR. Can a Shift in Fuel Energetics Explain the Beneficial Cardiorenal Outcomes in the EMPA-REG OUTCOME Study? A Unifying Hypothesis. Diabetes Care. 2016;39:1115–22. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 67.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–91. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J. 1998;329(Pt 1):191–6. doi: 10.1042/bj3290191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–9. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 70.McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–70. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- 71.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008;102:401–14. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54:1891–8. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol. 2011;300:H118–24. doi: 10.1152/ajpheart.00932.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teshima Y, Takahashi N, Nishio S, Saito S, Kondo H, Fukui A, Aoki K, Yufu K, Nakagawa M, Saikawa T. Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Circ J. 2014;78:300–6. doi: 10.1253/circj.cj-13-1187. [DOI] [PubMed] [Google Scholar]
- 75.Jia G, Habibi J, Aroor AR, Hill MA, DeMarco VG, Lee LE, Ma L, Barron BJ, Whaley-Connell A, Sowers JR. Enhanced endothelium epithelial sodium channel signaling prompts left ventricular diastolic dysfunction in obese female mice. Metabolism. 2017;78:69–79. doi: 10.1016/j.metabol.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, Shah AM. Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. Journal of the American College of Cardiology. 2014;63:2734–41. doi: 10.1016/j.jacc.2014.02.572. [DOI] [PubMed] [Google Scholar]
- 77.Lee SJ, Kang JG, Ryu OH, Kim CS, Ihm SH, Choi MG, Yoo HJ, Kim DS, Kim TW. Effects of alpha-lipoic acid on transforming growth factor beta1-p38 mitogen-activated protein kinase-fibronectin pathway in diabetic nephropathy. Metabolism. 2009;58:616–23. doi: 10.1016/j.metabol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 78.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raposeiras-Roubin S, Rodino-Janeiro BK, Paradela-Dobarro B, Grigorian-Shamagian L, Garcia-Lacuna JM, Aguiar-Souto P, Jacquet-Hervet M, Reino-Maceiras MV, Alvarez E, Gonzalez-Juanatey JR. Predictive value of advanced glycation end products for the development of post-infarction heart failure: a preliminary report. Cardiovasc Diabetol. 2012;11:102. doi: 10.1186/1475-2840-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willemsen S, Hartog JW, van Veldhuisen DJ, van der Meer P, Roze JF, Jaarsma T, Schalkwijk C, van der Horst IC, Hillege HL, Voors AA. The role of advanced glycation end-products and their receptor on outcome in heart failure patients with preserved and reduced ejection fraction. Am Heart J. 2012;164:742–749. e3. doi: 10.1016/j.ahj.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 81.Liu W, Chen P, Deng J, Lv J, Liu J. Resveratrol and polydatin as modulators of Ca2+ mobilization in the cardiovascular system. Ann N Y Acad Sci. 2017;1403:82–91. doi: 10.1111/nyas.13386. [DOI] [PubMed] [Google Scholar]
- 82.Kanaporis G, Blatter LA. Membrane potential determines calcium alternans through modulation of SR Ca2+ load and L-type Ca2+ current. J Mol Cell Cardiol. 2017;105:49–58. doi: 10.1016/j.yjmcc.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, Wuytack F, Holvoet P, Flameng W, Balligand JL, Herijgers P. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–9. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 84.Belke DD, Swanson EA, Dillmann WH. Decreased sarcoplasmic reticulum activity and contractility in diabetic db/db mouse heart. Diabetes. 2004;53:3201–8. doi: 10.2337/diabetes.53.12.3201. [DOI] [PubMed] [Google Scholar]
- 85.Ye G, Metreveli NS, Ren J, Epstein PN. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–83. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- 86.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–43. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 87.Uchimura K, Hayata M, Mizumoto T, Miyasato Y, Kakizoe Y, Morinaga J, Onoue T, Yamazoe R, Ueda M, Adachi M, Miyoshi T, Shiraishi N, Ogawa W, Fukuda K, Kondo T, Matsumura T, Araki E, Tomita K, Kitamura K. The serine protease prostasin regulates hepatic insulin sensitivity by modulating TLR4 signalling. Nat Commun. 2014;5:3428. doi: 10.1038/ncomms4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose Reductase Mediates NLRP3 Inflammasome-Initiated Innate Immune Response in Hyperglycemia-Induced Thp1 Monocytes and Male Mice. Endocrinology. 2017;158:3661–3675. doi: 10.1210/en.2017-00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu YZ, Zhang X, Wang L, Zhang F, Qiu Q, Liu ML, Zhang GR, Wu XL. An increased circulating angiotensin II concentration is associated with hypoadiponectinemia and postprandial hyperglycemia in men with nonalcoholic fatty liver disease. Intern Med. 2013;52:855–61. doi: 10.2169/internalmedicine.52.8839. [DOI] [PubMed] [Google Scholar]
- 90.Giacchetti G, Sechi LA, Rilli S, Carey RM. The renin-angiotensin-aldosterone system, glucose metabolism and diabetes. Trends Endocrinol Metab. 2005;16:120–6. doi: 10.1016/j.tem.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 91.Frantz EDC, Giori IG, Machado MV, Magliano DC, Freitas FM, Andrade MSB, Vieira AB, Nobrega ACL, Tibirica E. High, but not low, exercise volume shifts the balance of renin-angiotensin system toward ACE2/Mas receptor axis in skeletal muscle in obese rats. Am J Physiol Endocrinol Metab. 2017;313:E473–E482. doi: 10.1152/ajpendo.00078.2017. [DOI] [PubMed] [Google Scholar]
- 92.Munoz MC, Burghi V, Miquet JG, Cervino IA, Quiroga DT, Mazziotta L, Dominici FP. Chronic blockade of the AT2 receptor with PD123319 impairs insulin signaling in C57BL/6 mice. Peptides. 2017;88:37–45. doi: 10.1016/j.peptides.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Miller JA, Floras JS, Zinman B, Skorecki KL, Logan AG. Effect of hyperglycaemia on arterial pressure, plasma renin activity and renal function in early diabetes. Clin Sci (Lond) 1996;90:189–95. doi: 10.1042/cs0900189. [DOI] [PubMed] [Google Scholar]
- 94.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10:1778–85. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- 95.Baudrand R, Gupta N, Garza AE, Vaidya A, Leopold JA, Hopkins PN, Jeunemaitre X, Ferri C, Romero JR, Williams J, Loscalzo J, Adler GK, Williams GH, Pojoga LH. Caveolin 1 Modulates Aldosterone-Mediated Pathways of Glucose and Lipid Homeostasis. J Am Heart Assoc. 2016:5. doi: 10.1161/JAHA.116.003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab. 2012;302:E201–8. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pappachan JM, Sebastian J, Bino BC, Jayaprakash K, Vijayakumar K, Sujathan P, Adinegara LA. Cardiac autonomic neuropathy in diabetes mellitus: prevalence, risk factors and utility of corrected QT interval in the ECG for its diagnosis. Postgrad Med J. 2008;84:205–10. doi: 10.1136/pgmj.2007.064048. [DOI] [PubMed] [Google Scholar]
- 98.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, Roden R, Asano K, Blaxall BC, Wu SC, Communal C, Singh K, Colucci W, Bristow MR, Port DJ. Myocardial-directed overexpression of the human beta(1)-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817–30. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 99.Axelrod S, Lishner M, Oz O, Bernheim J, Ravid M. Spectral analysis of fluctuations in heart rate: an objective evaluation of autonomic nervous control in chronic renal failure. Nephron. 1987;45:202–6. doi: 10.1159/000184117. [DOI] [PubMed] [Google Scholar]
- 100.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–71. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 101.Kuethe F, Sigusch HH, Bornstein SR, Hilbig K, Kamvissi V, Figulla HR. Apoptosis in patients with dilated cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy? Horm Metab Res. 2007;39:672–6. doi: 10.1055/s-2007-985823. [DOI] [PubMed] [Google Scholar]
- 102.Yang L, Zhao D, Ren J, Yang J. Endoplasmic reticulum stress and protein quality control in diabetic cardiomyopathy. Biochim Biophys Acta. 2015;1852:209–18. doi: 10.1016/j.bbadis.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 103.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–8. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sandesara PB, O’Neal WT, Kelli HM, Samman-Tahhan A, Hammadah M, Quyyumi AA, Sperling LS. The Prognostic Significance of Diabetes and Microvascular Complications in Patients With Heart Failure With Preserved Ejection Fraction. Diabetes Care. 2018;41:150–155. doi: 10.2337/dc17-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murthy VL, Naya M, Foster CR, Gaber M, Hainer J, Klein J, Dorbala S, Blankstein R, Di Carli MF. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garg R, Rao AD, Baimas-George M, Hurwitz S, Foster C, Shah RV, Jerosch-Herold M, Kwong RY, Di Carli MF, Adler GK. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes. 2015;64:236–42. doi: 10.2337/db14-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shome JS, Perera D, Plein S, Chiribiri A. Current perspectives in coronary microvascular dysfunction. Microcirculation. 2017:24. doi: 10.1111/micc.12340. [DOI] [PubMed] [Google Scholar]
- 108.Widyantoro B, Emoto N, Nakayama K, Anggrahini DW, Adiarto S, Iwasa N, Yagi K, Miyagawa K, Rikitake Y, Suzuki T, Kisanuki YY, Yanagisawa M, Hirata K. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–18. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 109.Abdel Malik R, Zippel N, Fromel T, Heidler J, Zukunft S, Walzog B, Ansari N, Pampaloni F, Wingert S, Rieger MA, Wittig I, Fisslthaler B, Fleming I. AMP-Activated Protein Kinase alpha2 in Neutrophils Regulates Vascular Repair via Hypoxia-Inducible Factor-1alpha and a Network of Proteins Affecting Metabolism and Apoptosis. Circ Res. 2017;120:99–109. doi: 10.1161/CIRCRESAHA.116.309937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee TI, Kao YH, Chen YC, Huang JH, Hsiao FC, Chen YJ. Peroxisome proliferator-activated receptors modulate cardiac dysfunction in diabetic cardiomyopathy. Diabetes Res Clin Pract. 2013;100:330–9. doi: 10.1016/j.diabres.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 111.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci U S A. 1999;96:7473–8. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Young ME, Patil S, Ying J, Depre C, Ahuja HS, Shipley GL, Stepkowski SM, Davies PJ, Taegtmeyer H. Uncoupling protein 3 transcription is regulated by peroxisome proliferator-activated receptor (alpha) in the adult rodent heart. FASEB J. 2001;15:833–45. doi: 10.1096/fj.00-0351com. [DOI] [PubMed] [Google Scholar]
- 113.Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106:407–11. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 115.Cheng L, Ding G, Qin Q, Huang Y, Lewis W, He N, Evans RM, Schneider MD, Brako FA, Xiao Y, Chen YE, Yang Q. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 116.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142:62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol Ther. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 118.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–22. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 119.Makino A, Dai A, Han Y, Youssef KD, Wang W, Donthamsetty R, Scott BT, Wang H, Dillmann WH. O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. Am J Physiol Cell Physiol. 2015;309:C593–9. doi: 10.1152/ajpcell.00069.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–13. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 121.Lehrke M, Marx N. Diabetes Mellitus and Heart Failure. Am J Cardiol. 2017;120:S37–S47. doi: 10.1016/j.amjcard.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Pham D, Albuquerque Rocha N, McGuire DK, Neeland IJ. Impact of empagliflozin in patients with diabetes and heart failure. Trends Cardiovasc Med. 2017;27:144–151. doi: 10.1016/j.tcm.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.von Lewinski D, Rainer PP, Gasser R, Huber MS, Khafaga M, Wilhelm B, Haas T, Machler H, Rossl U, Pieske B. Glucose-transporter-mediated positive inotropic effects in human myocardium of diabetic and nondiabetic patients. Metabolism. 2010;59:1020–8. doi: 10.1016/j.metabol.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 124.Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol. 2012;302:C1174–88. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, Sharma K, Thomson SC, Rieg T. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304:F156–67. doi: 10.1152/ajprenal.00409.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017;136:1643–1658. doi: 10.1161/CIRCULATIONAHA.117.030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Raz I, Cahn A. Heart failure: SGLT2 inhibitors and heart failure -- clinical implications. Nat Rev Cardiol. 2016;13:185–6. doi: 10.1038/nrcardio.2016.35. [DOI] [PubMed] [Google Scholar]
- 128.Lei S, Li H, Xu J, Liu Y, Gao X, Wang J, Ng KF, Lau WB, Ma XL, Rodrigues B, Irwin MG, Xia Z. Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling. Diabetes. 2013;62:2318–28. doi: 10.2337/db12-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Z, Abdullah CS, Jin ZQ. Inhibition of PKC-theta preserves cardiac function and reduces fibrosis in streptozotocin-induced diabetic cardiomyopathy. Br J Pharmacol. 2014;171:2913–24. doi: 10.1111/bph.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, Walsh RA, King GL. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–5. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Strniskova M, Barancik M, Neckar J, Ravingerova T. Mitogen-activated protein kinases in the acute diabetic myocardium. Mol Cell Biochem. 2003;249:59–65. [PubMed] [Google Scholar]
- 132.Zhang H, Shi X, Hampong M, Blanis L, Pelech S. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J Biol Chem. 2001;276:6905–8. doi: 10.1074/jbc.C000917200. [DOI] [PubMed] [Google Scholar]
- 133.Wang Y, Zhou S, Sun W, McClung K, Pan Y, Liang G, Tan Y, Zhao Y, Liu Q, Sun J, Cai L. Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. Am J Physiol Endocrinol Metab. 2014;306:E1239–47. doi: 10.1152/ajpendo.00629.2013. [DOI] [PubMed] [Google Scholar]
- 134.Gurusamy N, Watanabe K, Ma M, Zhang S, Muslin AJ, Kodama M, Aizawa Y. Dominant negative 14-3-3 promotes cardiomyocyte apoptosis in early stage of type I diabetes mellitus through activation of JNK. Biochem Biophys Res Commun. 2004;320:773–80. doi: 10.1016/j.bbrc.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 135.Yang J, Park Y, Zhang H, Xu X, Laine GA, Dellsperger KC, Zhang C. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. 2009;296:H1850–8. doi: 10.1152/ajpheart.01199.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mariappan N, Elks CM, Sriramula S, Guggilam A, Liu Z, Borkhsenious O, Francis J. NF-kappaB-induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473–83. doi: 10.1093/cvr/cvp305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Niture SK, Khatri R, Jaiswal AK. Regulation of Nrf2-an update. Free Radic Biol Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tan Y, Ichikawa T, Li J, Si Q, Yang H, Chen X, Goldblatt CS, Meyer CJ, Li X, Cai L, Cui T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes. 2011;60:625–33. doi: 10.2337/db10-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, Mellen N, Kong M, Gu J, Tan Y, Zheng Y, Cai L. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. 2014;77:42–52. doi: 10.1016/j.yjmcc.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 140.Zhou YP, Marlen K, Palma JF, Schweitzer A, Reilly L, Gregoire FM, Xu GG, Blume JE, Johnson JD. Overexpression of repressive cAMP response element modulators in high glucose and fatty acid-treated rat islets. A common mechanism for glucose toxicity and lipotoxicity? J Biol Chem. 2003;278:51316–23. doi: 10.1074/jbc.M307972200. [DOI] [PubMed] [Google Scholar]
- 141.Barbati SA, Colussi C, Bacci L, Aiello A, Re A, Stigliano E, Isidori AM, Grassi C, Pontecorvi A, Farsetti A, Gaetano C, Nanni S. Transcription Factor CREM Mediates High Glucose Response in Cardiomyocytes and in a Male Mouse Model of Prolonged Hyperglycemia. Endocrinology. 2017;158:2391–2405. doi: 10.1210/en.2016-1960. [DOI] [PubMed] [Google Scholar]
- 142.Barwari T, Joshi A, Mayr M. MicroRNAs in Cardiovascular Disease. J Am Coll Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 143.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–7. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 144.Diao X, Shen E, Wang X, Hu B. Differentially expressed microRNAs and their target genes in the hearts of streptozotocin-induced diabetic mice. Mol Med Rep. 2011;4:633–40. doi: 10.3892/mmr.2011.489. [DOI] [PubMed] [Google Scholar]
- 145.Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Bottger T, Braun T, Seibler J, Bruning JC. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13:434–46. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 146.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–53. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 147.Nair N, Kumar S, Gongora E, Gupta S. Circulating miRNA as novel markers for diastolic dysfunction. Mol Cell Biochem. 2013;376:33–40. doi: 10.1007/s11010-012-1546-x. [DOI] [PubMed] [Google Scholar]
- 148.Liu X, Liu S. Role of microRNAs in the pathogenesis of diabetic cardiomyopathy. Biomed Rep. 2017;6:140–145. doi: 10.3892/br.2017.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res. 2016;109:397–408. doi: 10.1093/cvr/cvv260. [DOI] [PubMed] [Google Scholar]
- 150.Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol. 2014;74:139–50. doi: 10.1016/j.yjmcc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang X, Gu H, Huang W, Peng J, Li Y, Yang L, Qin D, Essandoh K, Wang Y, Peng T, Fan GC. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes. 2016;65:3111–28. doi: 10.2337/db15-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE Investigators D. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–55. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149:349–54. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]