Significance
Multiple sclerosis (MS) is a neuroinflammatory disease characterized by demyelinating plaques in the brain and spinal cord, causing progressive loss of functions. While the causes of MS remain undefined, treatments that target immune cells or their functions greatly improve the clinical outcome. Our laboratory has previously shown that production of the inflammatory cytokine interleukin (IL)-1β by myeloid cells is required for the development of experimental autoimmune encephalomyelitis (EAE), an MS model. Here, we show that activation of central nervous system (CNS) endothelial cells by IL-1β enables inflammatory monocytes to enter the CNS and differentiate into antigen-presenting cells. Additionally, we demonstrate that factors released from the interaction between IL-1β–producing myeloid cells and autoreactive CD4+ T cells are toxic to neurons.
Keywords: autoimmunity, blood–brain barrier, experimental autoimmune encephalomyelitis, interleukin-1β, multiple sclerosis
Abstract
Molecular interventions that limit pathogenic CNS inflammation are used to treat autoimmune conditions such as multiple sclerosis (MS). Remarkably, IL-1β–knockout mice are highly resistant to experimental autoimmune encephalomyelitis (EAE), an animal model of MS. Here, we show that interfering with the IL-1β/IL-1R1 axis severely impairs the transmigration of myeloid cells across central nervous system (CNS) endothelial cells (ECs). Notably, we report that IL-1β expression by inflammatory CCR2hi monocytes favors their entry into the spinal cord before EAE onset. Following activation with IL-1β, CNS ECs release GM-CSF, which in turn converts monocytes into antigen-presenting cells (APCs). Accordingly, spinal cord-infiltrated monocyte-derived APCs are associated with dividing CD4+ T cells. Factors released from the interaction between IL-1β–competent myeloid cells and CD4+ T cells are highly toxic to neurons. Together, our results suggest that IL-1β signaling is an entry point for targeting both the initiation and exacerbation of neuroinflammation.
Multiple sclerosis (MS) is a neuroinflammatory disease characterized by episodes of inflammatory attacks and ultimately neurodegeneration of the central nervous system (CNS) (1). While numerous drugs that target immunological pathways have shown beneficial effects on patients with relapsing-remitting MS (2), there is currently no cure for this disease. The difficulties seen in treating people living with MS arise from the fact that to this day, the specific causes of the disease are still unknown. It has been proposed that both genetic and environmental factors have a significant role in the pathology (1). One of the tools used to understand the immunopathology of MS is its animal model experimental autoimmune encephalomyelitis (EAE). Although MS and EAE show important disparities, some key parallels have been identified over the recent years, one of which is the involvement of the interleukin-1 (IL-1) system (3).
The IL-1 pathway is composed of two cytokines, IL-1α and IL-1β, which bind the same receptor, IL-1R1 (4). Recently, our group showed that while IL-1α is dispensable for CNS autoimmunity, IL-1β production by neutrophils and monocyte-derived macrophages (MDMs) is crucial for EAE development (5). While the importance of neutrophils in MS and EAE remains the subject of debate (6), the role of inflammatory monocytes and their derivatives inside inflamed tissues—that is, macrophages and monocyte-derived dendritic cells (moDCs)—in the pathogenesis of EAE and MS has been much more clearly defined. Indeed, EAE development is severely impacted by the absence of Ly6Chi CCR2hi monocytes (7–10), while strong associations implicating monocytes have been reported in MS patients (11–13). Interestingly, Croxford et al. (10) recently identified CCR2hi monocytes as the target of the encephalitogenic cytokine GM-CSF, which ultimately leads to an increase in their production of IL-1β. Similarly, pertussis toxin, which potentiates EAE induction, has also been shown to induce the production of IL-1β by myeloid cells in secondary lymphoid organs (14–16). In addition, IL-1R1 was recently shown to be highly expressed on endothelial cells (ECs) lining leptomeningeal veins (5, 17).
Nevertheless, we and others have demonstrated that ECs are not the only relevant IL-1R1–expressing cells in EAE (5, 18). Indeed, CD4+ T cells are known to be highly responsive to IL-1β, with TH17 cells expressing significantly higher levels of IL-1R1 compared with other T-cell subsets (reviewed by ref. 3). One reported effect of IL-1β treatment on pathogenic T cells is the increased production of GM-CSF, which correlates with their encephalitogenecity (10, 15, 19). Interestingly, deletion of the Il1r1 gene from CD4+ T cells was shown to impact expansion but not generation of autoreactive TH17 cells, while only mildly affecting EAE development (16).
Here, we sought to investigate the IL-1β–mediated mechanisms that exacerbate EAE and found that they involve both myeloid and lymphoid cell populations. First, we discovered that myeloid cell transmigration is greatly affected by their lack of IL-1β. In particular, we report that IL-1β expression by CCR2hi monocytes is necessary for their transmigration across CNS blood vessels in vivo—a response that occurs before the onset of disease. Second, we detected a marked reduction in the activation of pathogenic CD4+ T cells when the Il1b gene is deleted in antigen-presenting cells (APCs) but not when deleted from CD4+ T cells. We also demonstrated that these effects are mediated directly by the action of APC-derived IL-1β on CD4+ T cells and not through their expression of CD80, CD86, and MHC class II (MHCII). Importantly, our data revealed that the production of IL-1β by APCs in the presence of myelin-reactive CD4+ T cells is absolutely critical to the release of factors that are highly toxic to neurons. Finally, we report that IL-1β–deficient mice that possess endogenous MOG35–55-specific T cells are completely protected from EAE and autoimmunity-induced death. Collectively, our data show that IL-1β potentiates the activation and response of autoreactive CD4+ T cells and is crucial for recruitment of CCR2hi inflammatory monocytes into the CNS during EAE. Our results suggest that the IL-1β/IL-1R1 axis is a key component in the initiation and exacerbation of neuroinflammation during EAE and MS. Consequently, it provides interesting ways to think about therapeutic avenues for neuroprotection in CNS autoimmune inflammatory diseases such as MS.
Results
IL-1β Deficiency Affects the Number of Circulating and Splenic Myeloid Cells After EAE Induction.
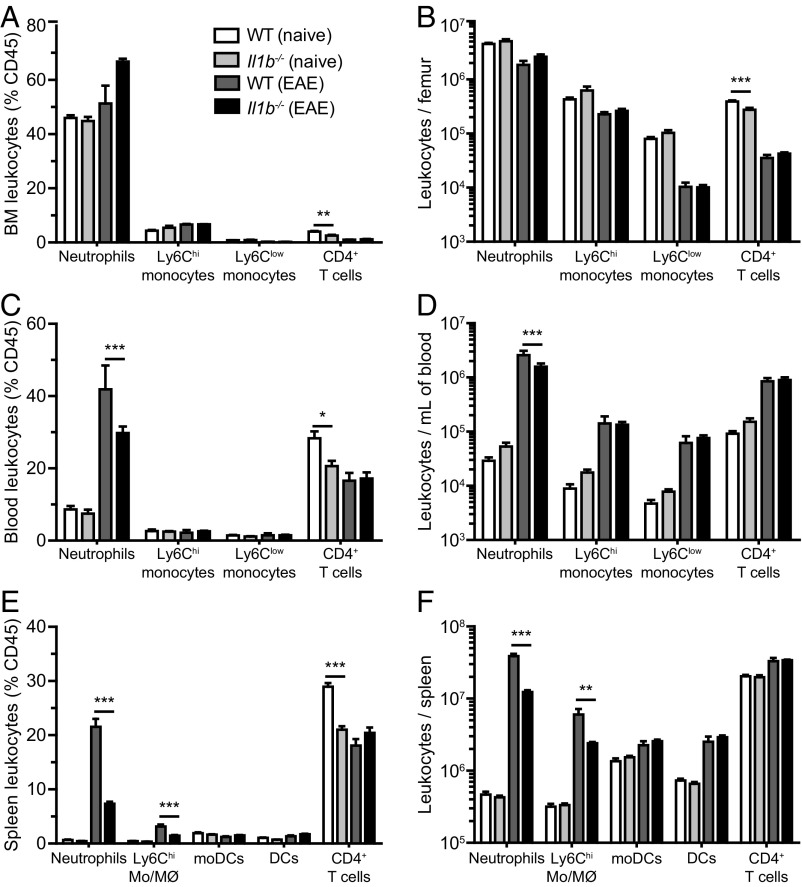
To understand how mice lacking IL-1β are protected from EAE, we first studied the composition and numbers of the various leukocyte populations distributed in the bone marrow, blood, and spleen of naïve or immunized WT or Il1b−/− mice. Comparable frequencies of neutrophils and both inflammatory (Ly6Chi) and patrolling (Ly6Clow) monocytes were observed in the bone marrow of naïve WT and Il1b−/− mice (Fig. 1 A and B). Although Il1b−/− mice showed significant reductions in the proportion and total number of CD4+ T cells, this difference was small (Fig. 1 A and B). At 7 d postimmunization (d.p.i.), all four of these cell populations were reduced in number in the bone marrow, suggesting immune cell egress from the bone marrow to the circulation and lymphoid organs (Fig. 1B). Accordingly, the total number of blood leukocytes drastically increased (>10-fold over naive) after immunization (Fig. 1D). Interestingly, immunized mice lacking IL-1β displayed a reduced number of blood neutrophils compared with WT mice (Fig. 1 C and D). A closer look at the spleen, an important secondary lymphoid organ and reservoir of myeloid cells, revealed an important mobilization of blood leukocytes, especially neutrophils and inflammatory monocytes/macrophages (Ly6Chi Mo/MØ), after immunization (Fig. 1F). However, the ratios and total numbers of splenic neutrophils and inflammatory monocytes/macrophages were significantly reduced in Il1b−/− mice compared with WT controls after immunization, results that are consistent with what was observed in the blood (Fig. 1 E and F). Together, these results show that the mobilization of neutrophils and inflammatory monocytes to the blood and spleen is strongly affected by the presence of IL-1β following EAE induction.
Fig. 1.
Myeloid cell egress from the bone marrow and homing to lymphoid organs during EAE are compromised in the absence of IL-1β. (A–D) Quantification of the number of neutrophils, CD4+ T cells, as well as Ly6Chi and Ly6Clow monocytes (CD115+) in the bone marrow (A and B) and blood (C and D) of unimmunized (naive) mice and immunized (EAE) mice killed at 7 d.p.i. (E and F) Quantification of the number of neutrophils, CD4+ T cells, Ly6Chi monocytes/macrophages (Ly6Chi Mo/MØ), moDCs, and DCs present in the spleen of WT and Il1b−/− mice at 7 d.p.i. Data are shown either as a percentage of CD45 leukocytes (A, C, and E) or as total number of cells per femur (B), milliliter of blood (D), or spleen (F). *P < 0.05, **P < 0.01, ***P < 0.001; two-way ANOVA followed by a Bonferroni post hoc test; data shown are mean ± SEM, n = 5–6 animals per group. Data are representative of at least two independent experiments.
Monocyte and Neutrophil Transmigration Across CNS ECs Is Severely Impaired When IL-1β Signaling Is Compromised.
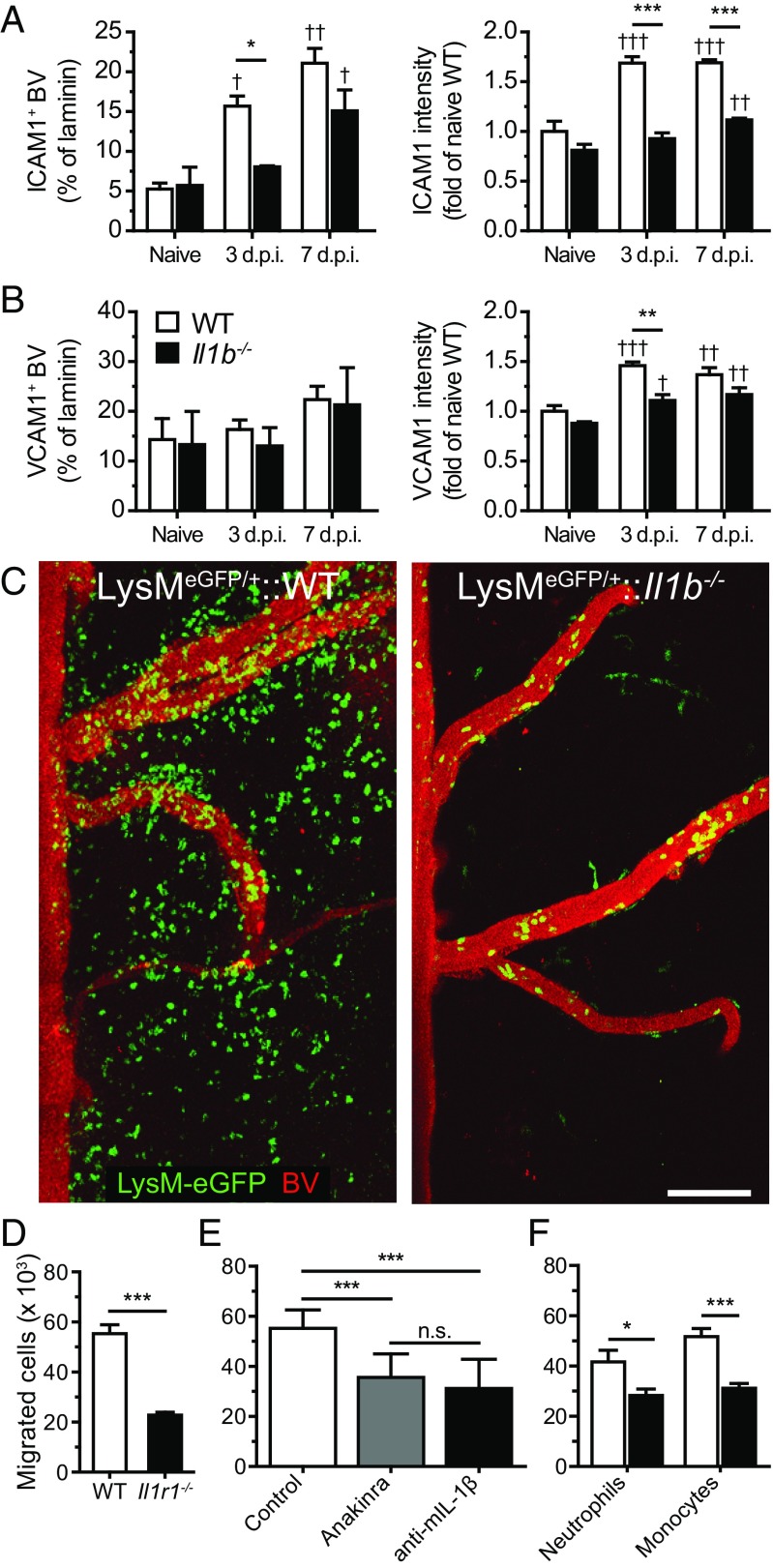
We next wanted to determine whether the reduced number of neutrophils in the bloodstream of Il1b−/− mice could explain their resistance to developing EAE. We therefore used a passive transfer strategy in which we collected neutrophils from the bone marrow of immunized WT mice and injected them into immunized Il1b−/− recipient mice (see SI Methods for details). Importantly, EAE susceptibility was not restored in Il1b−/− mice upon transfer of IL-1β–competent neutrophils (Table 1). Having previously demonstrated that IL-1β production is increased during the transmigration of myeloid cells across CNS ECs (5), we investigated the importance of IL-1R1 signaling for the migration of IL-1β–producing cells into the CNS. We found that ECs of the spinal cord of immunized WT mice up-regulated intercellular cell adhesion molecule (ICAM)-1 protein and, to a lesser degree, vascular cell adhesion molecule (VCAM)-1 protein during the preonset period (Fig. 2 A and B and Fig. S1 A–D). Similarly, human brain microvascular endothelial cells (BMECs) also displayed a higher expression of ICAM-1 compared with VCAM-1 following treatment with IL-1β (Fig. S1 E and F). Despite showing reduced ICAM-1 and VCAM-1 expression compared with WT controls, CNS ECs from Il1b−/− mice also displayed increased levels of both ICAM-1 and VCAM-1 after immunization. Accordingly, although fewer myeloid cells were found in the blood of Il1b−/− mice, these cells retain their ability to roll and adhere to spinal cord blood vessels before EAE onset (Fig. 2C and Movies S1 and S2). However, live imaging of the spinal cord of Il1b−/− mice at 12 d.p.i. revealed that neutrophils and monocytes were unable to reach the spinal cord parenchyma and that adherent cells eventually detached from the CNS vasculature (Fig. 2C and Movies S1 and S2). Considering that immune cell infiltration in the spinal cord of Il1b−/− mice is not observed until weeks after the onset of EAE in WT controls (5), we next investigated the involvement of IL-1β signaling in the transmigration events leading to the entry of myeloid cells in the CNS parenchyma. Using an in vitro transmigration assay, we evaluated the capability of IL-1β–producing myeloid cells (neutrophils and monocytes) to migrate across a monolayer of primary BMECs (see SI Methods for details). Significantly fewer myeloid cells migrated across Il1r1−/− BMECs as opposed to across WT BMECs (Fig. 2D). Similar results were obtained when the cells were cultured with the IL-1R1 antagonist anakinra or with an anti–IL-1β blocking antibody (Fig. 2E). Moreover, the transmigration of monocytes and neutrophils isolated from Il1b−/− mice was significantly reduced compared with those isolated from WT controls (Fig. 2F). Together, these results suggest that adhesion and transmigration events required for the recruitment of myeloid cells to the CNS are deeply affected by the lack of IL-1β.
Table 1.
Transfer of neutrophils to Il1b−/− mice does not restore EAE
| Group | Incidence | Mean day of onset (±SEM) | Max score (±SEM) |
| WT | 100% | 10.83 (0.48) | 2.42 (0.37) |
| Neutrophils → WT* | 67% | 10.25 (1.11) | 1.92 (0.61) |
| Neutrophils → Il1b−/−* | 0% | — | — |
n = 6 per group.
5 × 106 neutrophils transferred i.p. on day 3, 5, and 7. Animals were followed for 21 d.
Fig. 2.
Transmigration through the blood–CNS barrier is impaired when IL-1β signaling is disrupted. (A and B) Expression of ICAM1 (A) and VCAM1 (B) in laminin+ blood vessels (BVs) of the spinal cord of unimmunized (naive) and EAE mice (mean ± SEM). (C) 2P-IVM of the spinal cord of LysM-eGFP (Left) and LysM-eGFP::Il1b−/− (Right) mice at 12 d.p.i., which corresponds to the onset of EAE clinical symptoms in LysM-eGFP mice. Mature cells of the granulo-myelomonocytic lineage express the eGFP reporter protein (green), whereas BVs were labeled with a solution of Qdot 705 (red). (D) Quantification of the number of bone marrow Gr1+ cells (neutrophils and monocytes) that transmigrated across primary BMECs isolated from WT or IL-1R1–knockout mice (n = 13, representative of two independent experiments). (E) Quantification of the number of transmigrated Gr1+ cells following treatment with an IL-1R1 antagonist (anakinra) or an anti–IL-1β blocking antibody (n = 8–9). (F) Transmigration assays performed using either neutrophils (Ly6G+) or monocytes (Ly6GnegGr1+) harvested from the bone marrow of WT and IL-1β–knockout mice (mean ± SEM, n = 6–7). *P < 0.05, **P < 0.01, ***P < 0.001; †P < 0.05, ††P < 0.01, †††P < 0.001, compared with the naive group of the same strain; n.s., not significant; two-way ANOVA followed by a Bonferroni post hoc test. [Scale bar, (C) 100 µm.]
IL-1β Expression Favors the Migration of Inflammatory Monocytes, but Not Neutrophils, into the Spinal Cord Before EAE Onset.
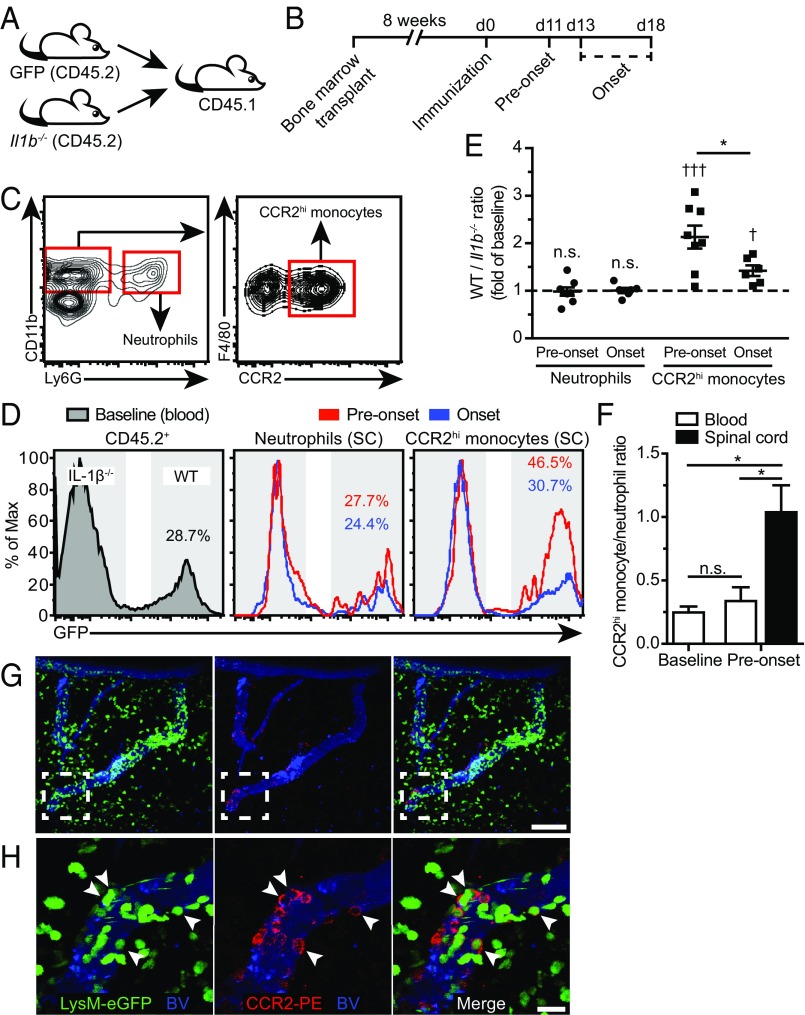
To analyze the effect of IL-1β on the recruitment of myeloid cells to the spinal cord, we generated mixed bone marrow chimeras using a 50/50 mix of WT (β-actin–GFP) and Il1b−/− bone marrow transferred to congenic CD45.1+ recipients (Fig. 3A). We next immunized BM chimeric mice, collecting peripheral blood and spinal cord-infiltrating mononuclear cells at two different time points: before onset of paralysis (“preonset”; EAE score of 0, 11 d.p.i.) and at disease onset (“onset”; EAE score of 0.5–1, 13–18 d.p.i.) (Fig. 3B). The percentage of WT cells (GFP+) in the blood accounted for 25% of all CD45.2+ leukocytes under baseline conditions. This ratio did not significantly change in the blood from baseline to preonset or onset. Notably, the ratio of WT versus Il1b−/− neutrophils (CD45.2+CD11b+Ly6G+) in the spinal cord was comparable to the baseline ratio in the blood at both preonset and onset, suggesting that neutrophils reach the CNS in an IL-1β–independent fashion. In contrast, the proportion of WT inflammatory monocytes (CD45.2+CD11b+F4/80+CCR2hi) measured in the spinal cord was significantly higher than baseline both at preonset and at onset of EAE (Fig. 3 D and E). After EAE onset, the WT-to-Il1b−/− ratio of CCR2+ monocytes decreased significantly, a result that suggests that IL-1β expression is mostly important for the first wave of infiltration (Fig. 3 D and E). While neutrophil numbers in the blood normally exceed those of CCR2hi monocytes by a ratio of 4:1, the situation drastically changed in the CNS tissue to reach a 1:1 ratio, suggesting that recruitment cues favor monocytes over neutrophils just before EAE onset (Fig. 3F). Using two-photon intravital microscopy (2P-IVM) (SI Methods), we confirmed that the recruitment of CCR2hi monocytes to the spinal cord occurred within leptomeningeal vessels (Fig. 3 G and H), blood vessels that express high levels of IL-1R1 (5). Together, these results suggest that the expression of IL-1β by pioneering CCR2hi monocytes is required for their migration to the spinal cord parenchyma.
Fig. 3.
IL-1β expression favors the migration of CCR2+ inflammatory monocytes into the CNS before EAE onset. (A) Experimental strategy used to generate mixed bone marrow chimeras using lethally irradiated CD45.1 recipient mice reconstituted with a 50/50 mix of bone marrow from β-actin–GFP (GFP) donors (CD45.2) and Il1b−/− donors (CD45.2). (B) Schematic representation of the timeline of the experiment showing when the bone marrow transplant, immunization, and tissue collection were performed. (C) Gating strategy used to identify neutrophils (CD45.2+ CD11b+ Ly6G+) and inflammatory monocytes (CD45.2+ CD11b+ Ly6Gneg F4/80+ CCR2hi). (D) Representative histograms depicting the expression of GFP by blood CD45.2+ leukocytes (Left) and spinal cord neutrophils (Center) and inflammatory monocytes (Right). GFP expression was used to discriminate between WT (GFP+, colored numbers) and Il1b−/− (GFPneg) leukocytes before onset of EAE clinical signs (preonset, red line) or at EAE onset (blue line). (E) Quantification of the WT-to-Il1b−/− ratio of neutrophils and inflammatory monocytes that have infiltrated the spinal cord at preonset and onset of EAE. Data are represented as a fold of baseline levels established using blood leukocytes before immunization (mean ± SEM). (F) Histogram showing the ratio between the number of CCR2hi monocytes and neutrophils in the blood and spinal cord under baseline conditions (unimmunized) and before EAE onset (mean ± SEM). (G and H) Projection images obtained from the living spinal cord of a LysM-eGFP mouse using 2P-IVM showing the presence of several CCR2-expressing inflammatory monocytes (eGFP+ and red membrane labeling) firmly adhered to blood vessels (blue) at EAE onset. Shown in H are zoomed images corresponding to the dashed-line white box seen in G. *P < 0.05, preonset vs. onset; †P < 0.05, †††P < 0.001, compared with baseline; two-way ANOVA followed by a Bonferroni post hoc test; n = 4–8 animals per group. [Scale bars, (G) 100 µm and (H) 20 µm.]
Spinal Cord-Infiltrated Monocytes Display an APC Phenotype and Are Associated with Dividing CD4+ T Cells.
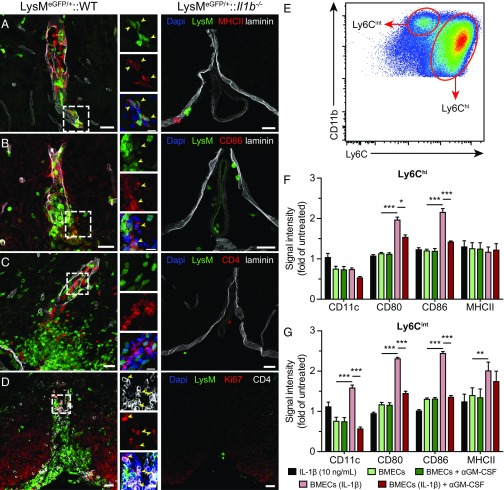
We next sought to characterize the phenotype of IL-1β–producing cells that entered the CNS. In the context of adaptive immunity, monocytes play a crucial role in both priming and propagating tissue-specific CD4+ T-cell responses. They up-regulate MHCII molecules, which present cognate antigen to T cells, as well as T-cell costimulatory ligands, such as CD86, which license full T-cell responses. Further, both MHCII and CD86 have been associated with the inflammatory (M1) macrophage phenotype in the spinal cord (20). Using the LysM-eGFP reporter mouse line that expresses eGFP in mature cells of the granulomyelomonocytic lineage, which mainly includes neutrophils and MDMs, we observed that blood-derived myeloid cells express MHCII and CD86 (Fig. 4 A and B). Using an antibody directed against Iba1, a microglia and macrophage marker, we were able to confirm that most of the MHCII-expressing blood-derived myeloid cells (LysM+) were MDMs (Fig. S2A). Such cells were not present in the spinal cord parenchyma of IL-1β–deficient mice. Accordingly, a high density of CD4+ T cells was found in close proximity to infiltrating myeloid cells in WT but not in Il1b−/− mice (Fig. 4C). Furthermore, the presence of the cell division marker Ki67 in CD4+ T cells indicated the occurrence of T-cell reactivation in the spinal cord of WT mice with EAE but not in immunized Il1b−/− mice (Fig. 4D). We have previously shown that IL-1β–activated CNS ECs express several inflammatory cytokines that are required for EAE development, including GM-CSF (5). To investigate whether cytokines secreted by IL-1β–activated BMECs are sufficient to induce an APC phenotype on monocytes, we treated primary mouse monocytes with conditioned media derived from BMECs cultured in the presence or absence of IL-1β and assessed the expression of APC-associated markers in Ly6Chi and Ly6Cint monocytes by flow cytometry (Fig. 4E). Media from IL-1β–treated BMECs significantly increased the expression of costimulatory molecules CD80 and CD86 on Ly6Chi monocytes. However, expression of MHCII and the activation marker CD11c were unchanged in Ly6Chi cells upon treatment (Fig. 4F and Fig. S2B). Ly6Cint monocytes showed a marked up-regulation of CD11c, CD80, CD86, and MHCII when cultured with conditioned media from IL-1β–stimulated BMECs (Fig. 4G and Fig. S2C). Given their intermediate expression of Ly6C and high levels of CD11c, the immunophenotype adopted by Ly6Cint monocytes was reminiscent of that of splenic moDCs (Fig. 1E). Interestingly, the neutralization of GM-CSF in the media largely abolished the up-regulation of most APC markers in both monocyte populations, suggesting that IL-1β–induced GM-CSF release was mainly responsible for the acquisition of the APC phenotype (Fig. 4 F and G). IL-1β alone did not impact the expression of the four markers (Fig. 4 F and G), indicating that adoption of an APC-like phenotype by Ly6Cint/hi monocytes was the result of IL-1β–induced alterations to BMECs and not merely to residual IL-1β in the conditioned media. Together, these results suggest that blood-derived monocytes that migrate across the blood–spinal cord barrier (BSCB) adopt an APC phenotype under the influence of GM-CSF released by IL-1β–activated CNS ECs and that this response contributes to the local reactivation of autoreactive CD4+ T cells.
Fig. 4.
Newly infiltrated monocytes in the spinal cord of WT mice display an APC phenotype and are associated with dividing CD4+ T cells. (A–C) Representative confocal images showing blood-derived myeloid cells (LysM-eGFP, green) expressing APC markers MHCII (A, red) and CD86 (B, red) and typically found in close association with CD4+ T cells (C, red). The interaction between myeloid cells and T cells is often observed in the vicinity of spinal cord blood vessels, within the basement membranes (laminin, gray). Yellow arrowheads in Insets (A and C) point to examples of colocalization between eGFP+ cells and APC markers MHCII and CD86. No immune cell infiltration nor MHCII or CD86 expression by myeloid cells was observed in LysM-eGFP::Il1b−/− mice (Right). (D) CD4+ T cells expressing the cell division marker Ki67 (red) are predominantly found in areas rich in myeloid cells and are absent in LysM-eGFP::Il1b−/− mice. Yellow arrowheads in Insets indicate CD4 and Ki67 double-positive cells. All animals were killed 15 d after EAE induction, and DAPI (blue) was used as a nuclear counterstaining. (E) Representative dot plot of the Ly6Chi and Ly6Cint monocyte populations in culture. Cells were gated on living CD45+CD11b+ cells. (F and G) Relative expression of the APC-associated markers CD11c, CD80, CD86, and MHCII in Ly6Chi (F) and Ly6Cint (G) monocytes treated with IL-1β alone (black bars) or with conditioned media from untreated (light green) or IL-1β–stimulated (light red) BMECs (mean ± SEM). A GM-CSF neutralizing antibody (αGM-CSF) was added to the medium where indicated (dark green and red). Data show the mean fluorescence intensity of each marker expressed as a fold of untreated monocytes. *P < 0.05, **P < 0.01, ***P < 0.001; two-way repeated measure ANOVA followed by a Bonferroni post hoc test; n = 4 per condition. [Scale bars, (A–D) 25 µm and (Insets) 10 µm.] Data are representative of two independent experiments.
APCs from Il1b−/− Mice Are Less Efficient at Activating Autoreactive CD4+ T Cells.
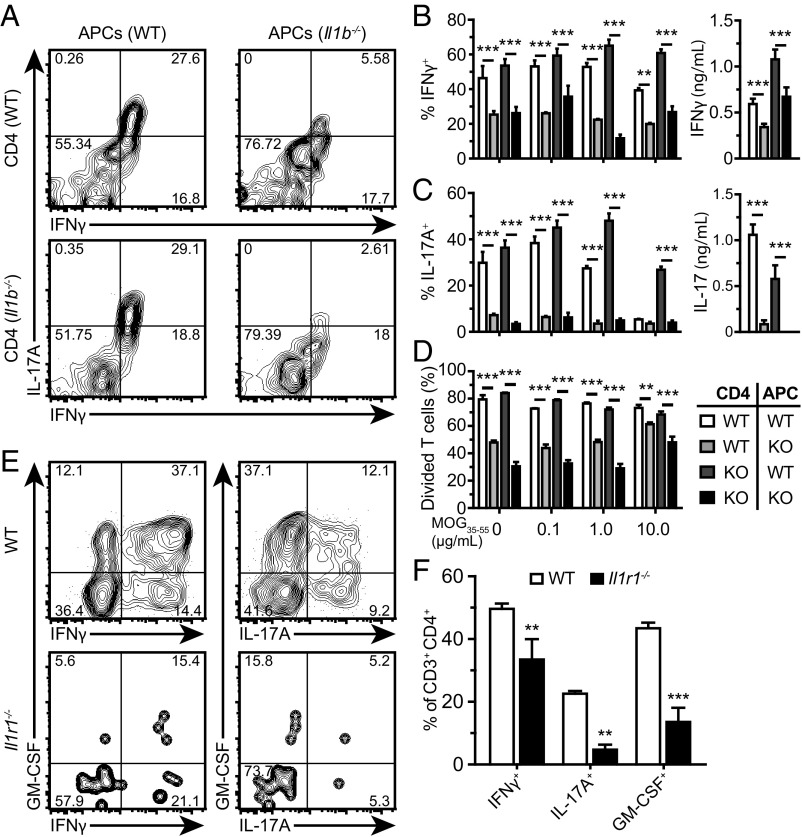
Given that high concentrations of bioactive IL-1β are detected in the spinal cord of MOG35–55–immunized mice at both EAE onset and disease peak (5), we next investigated the effects of myeloid cell-derived IL-1β on the antigen-specific activation of CD4+ T cells. We cocultured naïve 2D2 transgenic CD4+ T cells, which specifically recognize MOG35–55, with MOG35–55–pulsed APCs harvested from WT or Il1b−/− mice 7 d after EAE induction (SI Methods). To assess whether T cell-intrinsic production of IL-1β affected T-cell priming, we additionally tested CD4+ T cells from 2D2+/−::Il1b−/− mice. We observed that intracellular levels of IFNγ and IL-17A in reactivated CD4+ T cells were significantly lower when APCs were derived from Il1b−/− mice compared with WT controls (Fig. 5 A–C). Extracellular levels of both cytokines, detectable only at the highest MOG35–55 concentration, were also deeply affected by the absence of IL-1β in APCs (Fig. 5 B and C). Similarly, a greater percentage of T cells that went through at least one cell division was observed when cocultured with APCs that can produce IL-1β (Fig. 5D and Fig. S3A). While T cell-intrinsic production of IL-1β was recently found to impact EAE development (21), we observed no evidence that IL-1β deficiency in CD4+ T cells impacted IFNγ or IL-17A production, or T-cell division (Fig. 5 A–D). To confirm these findings in vivo, we purified CD4+ T cells from the spleen of WT and Il1b−/− mice at 7 d.p.i. and assessed mRNA expression of multiple markers of IFNγ+ TH1 and IL-17+ TH17 responses (Fig. S3B). In agreement with our in vitro data, mRNA levels for Ifng and Il17a were consistently lower in CD4+ T cells from immunized IL-1β–deficient mice, as were levels of the TH1 master regulator T-bet (Tbx21) and RORγt (Rorc) (Fig. S3C). Il1b−/− T cells additionally displayed a reduction in mRNA levels of the T-cell inflammatory cytokine GM-CSF (Csf2), IL-1R1 (Il1r1), and IL-23R (Il23r), the receptor for the TH17-skewing cytokine IL-23. Interestingly, mRNA levels of Il1r1 and Il23r were unaffected by the Il1b gene deletion in mice treated with adjuvants only (CFA + PTX), suggesting that their expression correlates with IL-1β–dependent and antigen-specific responses (Fig. S3D). Since the milieu of the spleen and CNS are likely to be different, we next measured intracellular production of TH1- and TH17-associated cytokines by CD3+ CD4+ T cells that infiltrated the spinal cord of Il1r1−/− and WT mice at 24 d.p.i. (Fig. 5 E and F). Mice lacking the Il1r1 gene had significantly reduced numbers and frequencies of IFNγ+, IL-17A+, and GM-CSF+ T cells in their spinal cord compared with WT mice. Together, these results indicate that the expression of IL-1β by APCs severely impacts the activation state of autoreactive CD4+ T cells and their skewing toward a TH1/TH17 profile.
Fig. 5.
IL-1β production by APCs is required for the reactivation of autoreactive CD4+ T cells and their coproduction of IL-17A and IFNγ. (A) Representative intracellular staining for IFNγ and IL-17A in 2D2 CD4+ T cells, either WT or Il1b−/−, after 4 d in coculture with splenic APCs isolated from WT or Il1b−/− mice. The number in each quadrant represents the frequency of each cell population. (B and C, Left) Quantification of the results shown in A. Concentrations of IFNγ and IL-17A proteins in the conditioned medium of cultures treated with 10 µg/mL of MOG35–55 are shown in the Right graphs (mean ± SEM). (D) Quantification of the percentage of CD4+ T cells that have divided at least once over 4 d in culture. (E) Representative intracellular cytokine staining plots for IFNγ, IL-17A, and GM-CSF in CD3+CD4+ T cells isolated from the spinal cord of either Il1r1−/− or WT mice at 24 d postimmunization. The number in each quadrant represents the frequency of each cell population. (F) Quantification of the percentage of CD3+CD4+ T cells expressing IFNγ, IL-17A, and GM-CSF (mean ± SEM, n = 7 mice per group). **P < 0.01; ***P < 0.001; two-way ANOVA followed by a Bonferroni post hoc test.
Deficits in IL-1β Signaling, but Not in Antigen Presentation, Are Responsible for the Reduced T-Cell Activation in Il1b−/− Mice.
We next wanted to determine whether APC-derived IL-1β enhanced T-cell activation by acting on T cells or via autocrine signaling on APCs themselves. Using the same coculture system as in Fig. 5, cells were treated with the IL-1R1 antagonist anakinra or IL-1β blocking antibody. As shown in Fig. 6A, interfering with the IL-1β/IL-1R1 pathway significantly reduced the production of IFNγ and IL-17, thus suggesting that IL-1β/IL-1R1 signaling plays a key role in CD4+ T-cell activation. Taking advantage of the pIL-1β–DsRed reporter mouse line, we confirmed that splenic DCs, moDCs, and neutrophils produced significant levels of IL-1β (Fig. S4). We next investigated the effects of IL-1β deficiency on the expression of antigen presentation-associated molecules by APCs at 7 d.p.i., a time at which they are producing IL-1β (Fig. S4). Cell-surface expression of CD80 (B7.1), CD86 (B7.2), and MHCII was comparable between WT and Il1b−/− mice in all three cell populations (Fig. 6 B–E). Although surface levels of CD80 and MHCII were respectively higher and lower in moDCs and macrophages of WT compared with Il1b−/− mice, those differences were small. Collectively, these results suggest that the reduced encephalitogenic CD4+ T-cell response observed in IL-1β–deficient mice is caused directly by the lack of IL-1β/IL-1R1 signaling on CD4+ T cells rather than a reduction in antigen presentation capability of myeloid cells.
Fig. 6.
Deficits in IL-1β signaling, but not with antigen presentation, are responsible for the reduced T-cell activation in Il1b−/− mice. (A) Quantification of the production of IFNγ and IL-17A by CD4+ T cells cocultured for 4 d with WT APCs in the presence of the indicated inhibitors of IL-1β signaling (mean ± SEM). Cells were either treated with saline (control), anakinra, or an anti–IL-1β blocking antibody. (B) Gating strategy used to differentiate between DCs (CD45hiCD11bhiCD11chiLy6Clow), moDCs (CD45hiCD11bhiCD11cintLy6Cint), and Ly6Chi monocytes/macrophages (Ly6Chi Mo/MØ; CD45hiCD11bhiCD11clowLy6Chi) isolated from the spleen at 7 d.p.i. (C–E) Representative histograms (Left) of the expression of APC markers (CD80, CD86, MHCII) in DCs (C), moDCs (D), and Ly6Chi Mo/MØ (E) isolated from the spleen of immunized WT (blue) and Il1b−/− (red) mice. The fluorescence-minus-one control (Control) is depicted as a gray-filled histogram. Shown on the Right are the quantifications of results displayed on the Left (mean ± SEM). (A, D, and E) *P < 0.05; **P < 0.01; ***P < 0.001; (A) two-way ANOVA followed by a Bonferroni post hoc test (n = 3); (C–E) Student’s t test; n = 4 mice per group.
Factors Released from the Interaction Between IL-1β–Competent Myeloid Cells and Myelin-Reactive CD4+ T Cells Are Highly Toxic to Neurons.
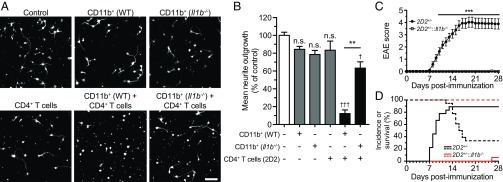
Having demonstrated that myeloid cell-derived IL-1β actively participates in activation of peripheral CD4+ T cells, we next sought to assess the effects of this activation on neuronal growth. CD11b+ cells isolated from the spleen of either WT or Il1b−/− EAE mice (7 d.p.i.) were placed in coculture with CD4+ T cells from 2D2 mice in the presence of MOG35–55. After 4 d, the media was collected and placed in the presence of freshly isolated cortical neurons (SI Methods). The mean neurite outgrowth was calculated after 24 h, and a conditioned media without any cells was used as a control (average neurite length of 36 ± 13 µm per neuron). The conditioned media derived from CD11b+ cells or CD4+ T cells alone did not significantly impact neurite outgrowth compared with control levels. However, the medium generated from CD4+ T cells cocultured with WT CD11b+ cells severely reduced neurite outgrowth (Fig. 7 A and B). This neurotoxicity was significantly undermined by the absence of IL-1β production from CD11b+ cells. Interestingly, for CD4+ T cells to reach their neurotoxic potential, a higher number of CD11b+ cells from Il1b−/− EAE mice was required, compared with WT cells (Fig. 7 A and B and Fig. S5). These data suggest that the activation threshold of encephalitogenic CD4+ T cells is lower when myeloid cells produce IL-1β. An alternative explanation could be that factors that caused neuronal toxicity were released by IL-1β–competent myeloid cells themselves when cultured in the presence of CD4+ T cells. To investigate this issue in vivo, we crossed mice harboring endogenous MOG35–55–reactive T cells (2D2+/−) with IL-1β–deficient animals, thus bypassing the generation of autoreactive CD4+ T cells. As expected, the active immunization of 2D2 mice led to very severe paralysis accompanied by a high mortality rate (>60%), a phenomenon that was not observed in 2D2+/−::Il1b−/− mice (Fig. 7 C and D). Taken together, these results suggest that IL-1β signaling is essential to the proper activation of MOG-reactive T cells as well as their encephalitogenic effector functions.
Fig. 7.
Coculture of IL-1β–competent myeloid cells and myelin-reactive CD4+ T cells triggers the release of neurotoxic factors. (A) Representative images of primary cortical neurons treated for 24 h with the indicated conditioned media and then stained against β-tubulin (white signal). (B) Quantification of the average neurite length normalized to the control condition. Cocultures were performed using a 1:1 ratio of CD11b+ and CD4+ cells. (C) Clinical EAE scores of immunized 2D2+/− and 2D2+/−::Il1b−/− mice. (D) EAE incidence (full lines) and survival (doted lines) of immunized 2D2+/− (black) and 2D2+/−::Il1b−/− (red) mice (mean ± SEM). Data in C and D are representative of two independent experiments. **P < 0.01, ***P < 0.001; †P < 0.05, †††P < 0.001 versus control; (B) one-way ANOVA followed by a Bonferroni post hoc test (n = 3–7); (D) two-way repeated-measures ANOVA followed by a Bonferroni post hoc test (n = 17–18). [Scale bar, (A) 50 µm.]
Discussion
Previous research found that the development of EAE requires IL-1β signaling through IL-1R1 in both ECs and T cells. Here, we provide evidence that inflammatory CCR2hi monocytes are particularly dependent on IL-1β to cross the blood–CNS barrier before EAE onset. Inside the CNS, blood-derived monocytes adopt an APC phenotype under the influence of IL-1β–induced secretion of GM-CSF by ECs and then associate with proliferating CD4+ T cells. Myeloid cell-derived IL-1β is directly responsible for the reactivation of MOG35–55–specific CD4+ T cells and their acquisition of an encephalitogenic phenotype. A critical finding of our study is that only the combined presence of IL-1β–competent myeloid cells and autoreactive CD4+ T cells is associated with neuronal toxicity in culture and EAE pathogenesis in vivo.
The bone marrow and spleen are major reservoirs of neutrophils and monocytes that contribute to myeloid cell recruitment at sites of inflammation (22, 23). In this study, we demonstrate that the bone marrow and spleen encounter important immune cell movements after EAE induction. Although both WT and Il1b−/− mice exhibited a marked reduction in total cell numbers for all bone marrow leukocyte populations after immunization, inflammatory monocyte and neutrophil cell numbers were increased to a significantly lesser degree in the blood and spleen of mice lacking IL-1β. A possible explanation for this difference may come from the fact that IL-1β signaling was recently shown to promote expansion of granulocyte–macrophage progenitors (GMPs) and pregranulocyte cell populations in the bone marrow (24–26). Additionally, splenic monocytopoiesis is also regulated by IL-1β during acute inflammation, as suggested by Leuschner et al. using an animal model of acute myocardial infarction (27). Thus, it is important to take into account that although Il1b−/− mice have normal white blood cell counts, these animals have a slightly reduced ability to generate myeloid cells under inflammatory conditions compared with WT mice.
We found that inflammatory monocytes, but not neutrophils, are recruited into the spinal cord of EAE mice via an IL-1β–dependent mechanism. Interestingly, a study by Lalor et al. (28) reported that mice treated with a caspase-1 inhibitor display a reduced number of macrophages, but not neutrophils, in their CNS. We previously reported that neutrophils and MDMs produce pro–IL-1β upon migration across the BSCB during EAE (5). We now extend this observation by showing that the transmigration of CCR2hiLy6Chi monocytes across the BSCB before EAE onset is compromised in the absence of IL-1β. This key finding is in line with recently published work by the group of Steffen Jung that showed that the migration of inflammatory monocytes in the spinal cord is accompanied by a marked up-regulation of IL-1β mRNA (29). Interestingly, IL-1β was shown earlier to activate a nonclassical MyD88–ARNO–ARF6 pathway downstream of IL-1R1 that leads to the permeability of dermal ECs in vitro (30). The data presented in this study may explain why Il1b−/− neutrophils can adhere to inflamed spinal cord blood vessels of EAE mice to a similar degree than WT neutrophils (5). Because neutrophil infiltration in the spinal cord of Il1b−/− mice is not observed until weeks after immunization, we speculate that IL-1β–competent CCR2hiLy6Chi monocytes may be needed to disrupt vascular permeability and allow neutrophil entry. Also supporting this hypothesis is the fact that the passive transfer of IL-1β–producing cells on the spinal cord of immunized Il1b−/− mice was sufficient to induce the recruitment of peripheral leukocytes and provoke paralysis (5).
That inflammatory monocytes are key players in CNS autoimmunity is a concept that is rapidly evolving. One of the most recent discoveries related to this concept is the demonstration that mice lacking the receptor for GM-CSF specifically in CCR2+ cells fail to develop EAE (10). This study further revealed that CNS-infiltrating moDCs, the derivatives of inflammatory monocytes inside inflamed tissues, require responsiveness to GM-CSF to be able to up-regulate IL-1β expression. GM-CSF is a cytokine shown to license autoimmunity and drive tissue damage in the CNS (31). This once again reinforces the existence of an inflammatory amplification loop between IL-1β and GM-CSF, as we recently suggested (3). These findings implicate a role for IL-1β in the transmigration of GM-CSF–activated CCR2hiLy6Chi monocytes inside the CNS.
Our data demonstrate that inflammatory monocytes need to produce IL-1β to cross the blood–CNS barrier and in return that activation of IL-1R1 signaling in CNS ECs dictates the differentiation of Ly6C+ monocytes toward professional APCs. Work with primary brain ECs treated with IL-1β indicates that GM-CSF is the main cytokine responsible for the induction of this cell differentiation process. That monocytes can mature into cells with antigen-presenting capacity under the influence of GM-CSF secreted by CNS ECs following their transmigration across the inflamed blood–CNS barrier was initially proposed by the A. Prat laboratory (32). However, the present study shows that secretion of GM-CSF by CNS ECs occurs as a result of the activation of endothelial IL-1R1 by IL-1β secreted by the infiltrating monocytes themselves.
While IL-1β has been known to be a potent lymphocyte-activating factor for almost 40 y, it is only recently that its role as a T cell-polarizing factor in EAE has been recognized (for reviews, see refs. 3 and 33). In this study, we report that IL-1β produced by myeloid cells in secondary lymphoid organs directly contributes to the activation of autoreactive CD4+ T cells, as demonstrated by their release of IFNγ and IL-17. Moreover, flow cytometric and histological analysis of the EAE mouse spinal cord suggests that local T-cell reactivation by infiltrating inflammatory monocytes occurs at the level of the blood–CNS barrier after disease onset. Interestingly, the conditioned media derived from brain microvascular ECs stimulated with IL-1β was sufficient to induce an APC phenotype in primary monocytes, an effect that depended mainly on the production of GM-CSF. This phenotype was more pronounced in Ly6Cint monocytes, which have been shown to produce more IL-1β than Ly6Chi macrophages in the CNS (34). In addition, we have demonstrated that MOG-reactive CD4+ T cells display a more activated and aggressive phenotype when cocultured with IL-1β–competent myeloid cells. While we were unable to detect GM-CSF in our in vitro coculture experiments, we have established that Csf2 mRNA levels were significantly reduced in CD4+ T cells isolated from mice lacking IL-1β, a phenotype that strongly correlated with their low expression of both Ifng and Il17a.
Interestingly, we have shown that MOG-reactive T cells interacting with IL-1β–competent myeloid cells acquire a neurotoxic phenotype that almost completely prevented neurite growth. While the encephalitogenic phenotype of T cells is required for normal EAE development, recent work has established that it can be bypassed by genetically increasing GM-CSF levels in polyclonal TH cells or myeloid cells alone (35). Considering that the main targets of GM-CSF are inflammatory monocytes and that the neurological deficits acquired by GM-CSF–overexpressing mice were completely independent of antigen specificity, these results suggest that demyelination and neuronal death are mainly caused by myeloid cells (10, 35). Hence, it is possible that the soluble factor(s) causing neurotoxicity in our coculture experiment could be released by myeloid cells themselves, especially by activated monocytes. Using live imaging in the spinal cord, the laboratories of Misgeld and Kerschensteiner have found that axonal degeneration is initiated in close proximity to macrophages rather than T cells in acute EAE lesions (36). Moreover, a recent study by the group of Ransohoff presented strong evidence that monocyte-derived cells are responsible for the initial CNS demyelination (37). From all this, we can speculate that during EAE and possibly MS, both T cells and CNS ECs are acting as major sources of GM-CSF, which acts as a key regulator of inflammatory monocyte trafficking and effector function inside the CNS parenchyma. We further propose that this mobilization of CCR2hiLy6Chi monocytes is responsible for the permeabilization of the blood–CNS barrier and subsequent entry of additional effector cells in the CNS, a response that is strongly dependent on IL-1β/IL-1R1 signaling.
The results obtained from the immunization of 2D2::Il1b−/− mice also suggest that even in the presence of endogenous myelin-reactive T cells, the development of autoimmunity requires the recruitment of CCR2hi monocytes to the CNS, at least in part via their expression of IL-1β. This affirmation is supported by several facts. First, CCR2-knockout mice are refractory to the development of EAE (7–10). Second, induction of GM-CSF production leads to neuroinflammation characterized mainly by myeloid cell infiltrates and CNS tissue damage independent of T-cell receptor specificity (35). Notably, splenic inflammatory monocytes, but not neutrophils, increased their IL-1β production in mice overexpressing GM-CSF (35). Third, the artificial transfer of IL-1β–competent Gr1+ cells to the CNS of Il1b−/− mice—but not, as shown here, the transfer of IL-1β–competent neutrophils i.p.—is sufficient to restore EAE weeks before that normally seen in these animals (5). Thus, it appears that the pathological role of IL-1β during EAE mainly occurs at the level of the blood–CNS barrier. This could explain why mice with specific deletion of the Il1r1 gene in CD4+ T cells are only partially resistant to EAE induction (16). We propose that the major detrimental effect of IL-1β in EAE and MS is to provoke the initial disruption of the cerebrovascular stability and allow subsequent entry of a toxic mix of leukocytes into the CNS.
In summary, we present new evidence that IL-1β triggers CNS autoimmunity by acting on several fronts. In addition to its demonstrated role in the expansion of encephalitogenic GM-CSF–producing TH17 cells, we now report that pioneer CCR2+ monocytes must express IL-1β to be able to cross the blood–CNS barrier and initiate neuroinflammation in EAE. Furthermore, we show that IL-1β is a critical regulatory cytokine for GM-CSF production by CNS ECs, which in turn induces the differentiation of monocytes into APCs within the perivascular space of CNS blood vessels. We speculate that this inflammatory feedback loop triggers the local reactivation of autoreactive T cells. The data presented in this study also reveal that direct activation of myelin-reactive T cells by myeloid cell-derived IL-1β leads to the production of neurotoxic factors. Thus, IL-1β and GM-CSF are regulating each other at many levels, including at the level of the blood–CNS barrier. Based on our work and that of others, we propose that the interactions between these two cytokine systems are at the center of a vicious feedback loop of CNS inflammation that ultimately leads to myelin and neuronal damage.
Methods
All animal experiments were approved by the Animal Welfare Committee of Université Laval in accordance with the Canadian Council on Animal Care policy. Primary cultures of human BMECs were prepared from temporal lobe tissue obtained during surgical resection in patients suffering from epilepsy, as previously described (5). Informed consent and ethical approval were obtained before surgery (ethical approval number BH07.001 to A. Prat). See SI Methods for detailed information regarding animals, production of mixed bone marrow chimeras, EAE induction and clinical scoring, biological sample collection and processing for cytometry, neutrophil transfer, flow cytometry, quantitative real-time RT-PCR, stimulation of MOG-reactive CD4+ T cells, immunostaining and histology, mouse BMEC and monocyte cultures and myeloid cell transmigration assay, human BMEC culture, 2P-IVM, primary cortical neuron culture, and statistical analysis.
Supplementary Material
Acknowledgments
We thank Nadia Fortin, Martine Lessard, and Nicolas Vallières for their invaluable technical assistance. We also thank Dr. Hermann Gram at Novartis Institutes for Biomedical Research for the generous gift of the anti–IL-1β neutralizing antibody. This study was supported by an operating grant from the Multiple Sclerosis Society of Canada (MSSC) awarded to S.L. Salary support was provided by the MSSC (to A. Paré and S.A.L.) and the Fonds de recherche du Québec en Santé (to M.-A.L. and A. Prat).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1714948115/-/DCSupplemental.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Comi G, Radaelli M, Soelberg Sørensen P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet. 2017;389:1347–1356. doi: 10.1016/S0140-6736(16)32388-1. [DOI] [PubMed] [Google Scholar]
- 3.Paré A, Mailhot B, Lévesque SA, Lacroix S. Involvement of the IL-1 system in experimental autoimmune encephalomyelitis and multiple sclerosis: Breaking the vicious cycle between IL-1β and GM-CSF. Brain Behav Immun. 2017;62:1–8. doi: 10.1016/j.bbi.2016.07.146. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 5.Lévesque SA, et al. Myeloid cell transmigration across the CNS vasculature triggers IL-1β-driven neuroinflammation during autoimmune encephalomyelitis in mice. J Exp Med. 2016;213:929–949. doi: 10.1084/jem.20151437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierson ER, Wagner CA, Goverman JM. The contribution of neutrophils to CNS autoimmunity. Clin Immunol. 2016:S1521-6616(16)30143-7. doi: 10.1016/j.clim.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mildner A, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- 8.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- 9.Ko HJ, et al. GM-CSF-responsive monocyte-derived dendritic cells are pivotal in Th17 pathogenesis. J Immunol. 2014;192:2202–2209. doi: 10.4049/jimmunol.1302040. [DOI] [PubMed] [Google Scholar]
- 10.Croxford AL, et al. The cytokine GM-CSF drives the inflammatory signature of CCR2+ monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Kouwenhoven M, Teleshova N, Ozenci V, Press R, Link H. Monocytes in multiple sclerosis: Phenotype and cytokine profile. J Neuroimmunol. 2001;112:197–205. doi: 10.1016/s0165-5728(00)00396-9. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Or A, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]
- 13.Waschbisch A, et al. Pivotal role for CD16+ monocytes in immune surveillance of the central nervous system. J Immunol. 2016;196:1558–1567. doi: 10.4049/jimmunol.1501960. [DOI] [PubMed] [Google Scholar]
- 14.Dumas A, et al. The inflammasome pyrin contributes to pertussis toxin-induced IL-1β synthesis, neutrophil intravascular crawling and autoimmune encephalomyelitis. PLoS Pathog. 2014;10:e1004150. doi: 10.1371/journal.ppat.1004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ronchi F, et al. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat Commun. 2016;7:11541. doi: 10.1038/ncomms11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mufazalov IA, et al. IL-1 signaling is critical for expansion but not generation of autoreactive GM-CSF+ Th17 cells. EMBO J. 2017;36:102–115. doi: 10.15252/embj.201694615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aubé B, et al. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. J Immunol. 2014;193:2438–2454. doi: 10.4049/jimmunol.1400401. [DOI] [PubMed] [Google Scholar]
- 18.McCandless EE, et al. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J Immunol. 2009;183:613–620. doi: 10.4049/jimmunol.0802258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Behi M, et al. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin BN, et al. T cell-intrinsic ASC critically promotes T(H)17-mediated experimental autoimmune encephalomyelitis. Nat Immunol. 2016;17:583–592. doi: 10.1038/ni.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 23.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu LC, et al. IL-1β-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKβ. Nat Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietras EM, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey A, et al. Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 2017;18:3204–3218. doi: 10.1016/j.celrep.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leuschner F, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalor SJ, et al. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- 29.Wolf Y, et al. Autonomous TNF is critical for in vivo monocyte survival in steady state and inflammation. J Exp Med. 2017;214:905–917. doi: 10.1084/jem.20160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu W, et al. Interleukin receptor activates a MYD88-ARNO-ARF6 cascade to disrupt vascular stability. Nature. 2012;492:252–255. doi: 10.1038/nature11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Croxford AL, Spath S, Becher B. GM-CSF in neuroinflammation: Licensing myeloid cells for tissue damage. Trends Immunol. 2015;36:651–662. doi: 10.1016/j.it.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Ifergan I, et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 33.Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T helper immune responses. Front Immunol. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vainchtein ID, et al. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia. 2014;62:1724–1735. doi: 10.1002/glia.22711. [DOI] [PubMed] [Google Scholar]
- 35.Spath S, et al. Dysregulation of the cytokine GM-CSF induces spontaneous phagocyte invasion and immunopathology in the central nervous system. Immunity. 2017;46:245–260. doi: 10.1016/j.immuni.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Nikić I, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–499. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki R, et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 39.Boivin N, Baillargeon J, Doss PM, Roy AP, Rangachari M. Interferon-β suppresses murine Th1 cell function in the absence of antigen-presenting cells. PLoS One. 2015;10:e0124802. doi: 10.1371/journal.pone.0124802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC. Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. J Histochem Cytochem. 2011;59:813–825. doi: 10.1369/0022155411416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soulet D, Paré A, Coste J, Lacroix S. Automated filtering of intrinsic movement artifacts during two-photon intravital microscopy. PLoS One. 2013;8:e53942. doi: 10.1371/journal.pone.0053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.