Significance
The oncogenic retrovirus human T-cell leukemia virus type 1 (HTLV-1) encodes Tax, an activator of both viral replication and cellular oncogenic pathways. Despite the potent activities of Tax, its precise roles in pathogenesis remain unclear, since it is faintly expressed in vivo. This study shows that sporadic and transient Tax expression is observed in a small subpopulation of HTLV-1–induced leukemic cells. This limited Tax expression is critical for survival of the whole population through ignition of antiapoptotic signals. Tax is induced by various stresses, suggesting that Tax efficiently protects cells from apoptosis and reactivates virus from reservoirs under conditions of cellular stress. It is an elaborated strategy of HTLV-1 to evade host immunity and enable persistence in vivo.
Keywords: HTLV-1, Tax, HBZ, adult T-cell leukemia–lymphoma, computational simulation
Abstract
Viruses causing chronic infection artfully manipulate infected cells to enable viral persistence in vivo under the pressure of immunity. Human T-cell leukemia virus type 1 (HTLV-1) establishes persistent infection mainly in CD4+ T cells in vivo and induces leukemia in this subset. HTLV-1–encoded Tax is a critical transactivator of viral replication and a potent oncoprotein, but its significance in pathogenesis remains obscure due to its very low level of expression in vivo. Here, we show that Tax is expressed in a minor fraction of leukemic cells at any given time, and importantly, its expression spontaneously switches between on and off states. Live cell imaging revealed that the average duration of one episode of Tax expression is ∼19 hours. Knockdown of Tax rapidly induced apoptosis in most cells, indicating that Tax is critical for maintaining the population, even if its short-term expression is limited to a small subpopulation. Single-cell analysis and computational simulation suggest that transient Tax expression triggers antiapoptotic machinery, and this effect continues even after Tax expression is diminished; this activation of the antiapoptotic machinery is the critical event for maintaining the population. In addition, Tax is induced by various cytotoxic stresses and also promotes HTLV-1 replication. Thus, it seems that Tax protects infected cells from apoptosis and increases the chance of viral transmission at a critical moment. Keeping the expression of Tax minimal but inducible on demand is, therefore, a fundamental strategy of HTLV-1 to promote persistent infection and leukemogenesis.
Chronic viral infection is established when there is a metastable balance between host immunity and viral strategies for maintaining infected cells in infected individuals. Several human viruses cause persistent infection and are closely associated with inflammatory diseases and/or cancers (1). Human T-cell leukemia virus type 1 (HTLV-1) and human herpesviruses (HHVs), such as EBV (HHV-4) and Kaposi’s sarcoma-associated virus (KSHV; HHV-8), are representatives of this type of virus (2) and express a limited number/amount of viral products, enabling infected cells to survive in vivo by escaping from host immune surveillance. Reactivation of viral replication, such as in the lytic phase of EBV and KSHV infection, is then a critical step for facilitating viral transmission to new hosts. Human oncogenic viruses encode the regulatory factors, which are involved in viral replication and cellular oncogenesis (3); however, their expression dynamics and the roles in each phase are poorly understood.
HTLV-1 is a human retrovirus that chronically infects CD4+ T cells. Some infected individuals develop a malignant disease of CD4+CD25+ T cells, adult T-cell leukemia–lymphoma (ATL), and/or inflammatory diseases, such as HTLV-1–associated myelopathy/tropical spastic paraparesis and HTLV-1 uveitis (4, 5). Unlike that of HIV, the replication of HTLV-1 is at a very low level in vivo, and viral RNA is rarely detected in the plasma of infected individuals (6). Instead, this virus persists in the host by using two different strategies: cell-to-cell transmission of viral particles (de novo infection) and clonal proliferation of infected cells (mitotic expansion) (7, 8).
HTLV-1 encodes two oncogenic factors, Tax and human T-cell leukemia virus type 1 bZIP factor (HBZ), in the sense and antisense strands of provirus, respectively, and these two factors counteract each other in many signaling pathways (9). Tax is not only a potent oncoprotein (10) but also an efficient transactivator of viral replication, which means that Tax is required for de novo infection. However, Tax expression is generally suppressed in infected cells in vivo (11). In contrast, HBZ is constitutively expressed in infected cells and plays important roles in viral latency (8) and proliferation of infected cells (12). These facts suggest that HTLV-1 fine-tunes the expression and function of these counteracting viral factors to establish persistent infection in infected individuals. Since Tax is highly immunogenic (13–15), HTLV-1 seems to minimize Tax expression to escape from host immunity. Therefore, the significance of Tax in leukemogenesis has been an important unsolved issue.
In this study, we show that only a small fraction (0.05–3%) of cells transiently expresses Tax. However, knockdown (KD) of Tax induced apoptosis in the majority of cells, suggesting that Tax expressed in a minor subset is required for survival of the whole population. At a single-cell level, Tax-expressing cells highly expressed several antiapoptotic and NF-κB–related genes, and Tax-negative cells were divided into two subpopulations, which expressed medium or low levels of the antiapoptotic genes. Computational simulations support our hypothesis that transient Tax expression confers an antiapoptotic property on the expressing cell and that this effect lasts after Tax expression is diminished. This study shows the dynamics of Tax expression at a single-cell level and shows roles of Tax in establishing persistent infection by HTLV-1.
Results
A Small Fraction of MT-1 Cells Expresses Tax, and This Expression Is Critical for the Survival of the Whole Cell Population.
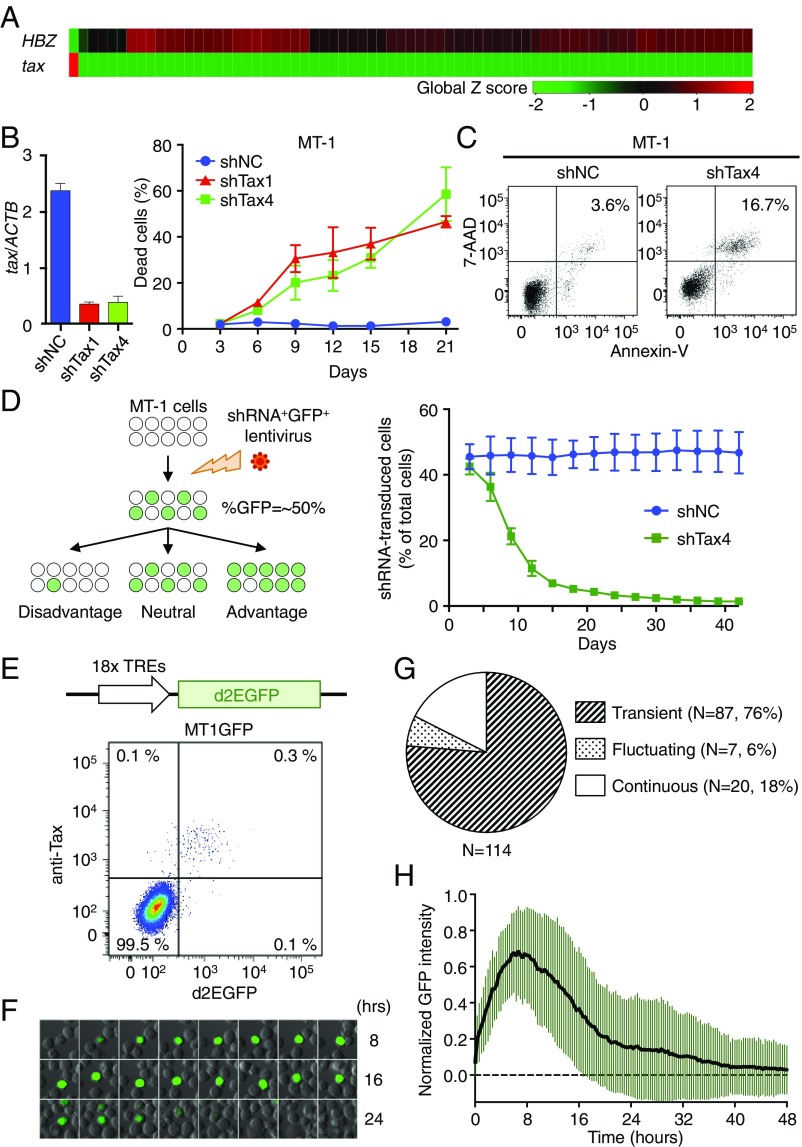
HTLV-1 tax is generally silenced or transcribed at quite low levels in ATL cells in vivo (16). An ATL cell line, MT-1, has an equivalent expression profile of viral genes to primary HTLV-1–infected cells (17). We carried out single-cell qRT-PCR to elucidate the expression levels of tax and HBZ in individual MT-1 cells. The initial experiment showed that only 1 in 71 cells expressed a high level of tax, while no detectable expression was observed in the remaining 70 cells (Fig. 1A). In contrast, HBZ was expressed in Tax-negative cells, while it was not detected in the Tax-expressing cell. These results suggest that the expression of tax and HBZ is strictly and reciprocally regulated in MT-1 cells.
Fig. 1.
Significance and dynamics of Tax expression at a single-cell level in MT-1 cells. (A) Single-cell qPCR for tax and HBZ expression in MT-1 cells (n = 71). (B, Left) Efficiency of Tax-KD by shRNA. (B, Right) The percentage of cells that are dead after Tax-KD. MT-1 cells were infected with a bicistronic lentivirus vector expressing GFP and shRNA (shTax1, shTax4, or shNC). Cells were stained with LIVE/DEAD reagent, and the ratio of dead cells to shRNA-transduced (GFP+) cells was measured by flow cytometry. (C) AnnexinV/7-AAD double staining in shRNA-transduced MT-1 cells at day 15 posttransduction. (D, Left) Schematic depicting the concept behind the GFP competition assay. The ratio of GFP+ cells changes over time depending on the effect of shRNA on transduced cells. (D, Right) Serial measurements of the percentage of GFP+ cells among the whole MT-1 cell population after shNC or shTax4 transduction. For B and D, error bars show SDs for three experiments. (E, Upper) Scheme of Tax reporter cassette that expresses d2EGFP. MT1GFP is a stable subline of MT-1 transduced with this cassette. (E, Lower) Intracellular Tax staining of MT1GFP. (F) Live cell imaging of Tax expression in MT1GFP cells. This montage of time-lapse images shows changes in d2EGFP expression. (G) Expression pattern of Tax in MT1GFP. Cells were categorized based on their pattern of d2EGFP expression during the observation period. Continuous, cell continuously expressed d2EGFP until the end of observation period; fluctuating, cell has multiple episodes; transient, cell has a single spontaneous episode. (H) Single-cell dynamics of d2EGFP expression for 87 cells with transient expression. Mean ± SD is shown.
Our next question was the following: what is the role of Tax expression in a small number of MT-1 cells? To address this issue, we knocked down Tax with shRNA. Two different lentivirus vectors expressing shRNA targeting Tax (shTax1 and shTax4) were transduced into MT-1 cells, and each construct efficiently inhibited Tax expression to ∼15% of the level in control cells (Fig. 1B, Left). Intriguingly, progressive cell death was observed in MT-1 cells after Tax was knocked down (Fig. 1B, Right), and as a mechanism, we found that Tax-KD induced apoptosis of MT-1 cells but not Jurkat cells (Fig. 1C and Fig. S1). To compare population dynamics between Tax-KD cells and Tax-intact cells, a GFP competition assay was carried out (schema in Fig. 1D, Left). To our surprise, ∼90% of shTax-transduced cells were eliminated from culture by day 21 (Fig. 1D, Right), which was faster than we expected. These results indicated that Tax is indispensable for the survival of MT-1 cells, although only a small fraction of MT-1 cells expresses it. Since shTax-transduced cells were not rescued by the presence of nontransduced cells, it also seems that a cell’s survival may depend at least partly on Tax expression within that cell rather than depending on Tax expression by neighboring cells.
Tax Is Transiently Expressed in MT-1 Cells.
We wished to further distinguish between these two possible hypotheses: (i) that a small population of cells constantly expresses Tax and supports the survival of the whole population or (ii) that all cells transiently express Tax by turns. To monitor Tax expression, we established a reporter subline of MT-1, MT1GFP, which expresses destabilized EGFP (d2EGFP; the half-life of the modified EGFP is 2 h) under the control of 18 copies of the Tax-responsive element (schema in Fig. 1E, Upper) (18). It was confirmed that d2EGFP expression in MT1GFP cells was closely correlated with Tax expression by intracellular staining with anti-Tax antibody, and a very small population (∼0.5%) of MT1GFP cells expressed d2EGFP (plot in Fig. 1E, Lower). Several stable clones of MT1GFP were established, and all clones possessed a small Tax-expressing subset. Using one of the MT1GFP clones, we evaluated the dynamics of Tax expression in individual cells by time-lapse imaging. The result revealed that d2EGFP expression was transient in most d2EGFP-positive cells (Fig. 1F and Movie S1), indicating that Tax is expressed temporarily in MT-1 cells. We could also see cells with fluctuating and continuous patterns of d2EGFP expression (Fig. 1G has definitions of these terms), although the percentages of cells with those patterns were lower than cells with transient expression (6% for fluctuating and 18% for continuous cells) (Fig. 1G). Analysis of 87 cells with transient d2EGFP expression showed that the median and mean durations of Tax expression were ∼22 and 18 h, respectively (95% confidence interval, 19.3–24.7) (Fig. 1H).
It has been reported that two other ATL cell lines, KK-1 and SO-4, have expression levels of Tax and HBZ similar to those of fresh ATL cells (17). Small fractions of KK-1 and SO-4 cells each expressed Tax, and a subline of KK-1 reporting Tax expression, KK1GFP, was successfully established (Fig. S2 A and B). Since DNA hypermethylation in 5′ LTR is known to suppress tax transcription, we evaluated its methylation level and tax expression in fresh ATL cells and three ATL cell lines (MT-1, KK-1, and SO-4). In approximately one-half of ATL cases and all cell lines, DNA methylation level of 5′ LTR was low, and tax mRNA was detectable, suggesting that tax expression is inducible in these cells (Fig. S2C).
Computer Simulation Represents the Dynamics of Tax Expression.
It was an incomprehensible observation that KD of Tax in a minor fraction (∼0.5%) of MT-1 cells induced progressive apoptosis. To estimate the duration that all MT-1 cells experience Tax expression, we used a computational simulation based on a mathematical model. A previous study on HIV elegantly established a mathematical model of 5′ LTR activation by Tat in latently infected cells (19). We adapted this model to simulate the regulation of the HTLV-1 5′ LTR by Tax (details are in SI Materials and Methods, and Fig. S3A shows a schematic representation). We tested several parameters used in the HIV study and adjusted them to fit our experimental data of transient Tax expression (SI Materials and Methods, Fig. S3 B–D, and Table S1). The simulation could then successfully reproduce the dynamics of Tax expression in MT-1 cells, and the calculated mean duration of Tax expression was 13.6 h, consistent with experimental observations (Fig. S3C), suggesting that the parameters that we used were suitable for the model for activation of the HTLV-1 LTR. Using this model, we then estimated the distribution of the interval between two successive Tax expression episodes (Fig. S3E), and interestingly, our simulation reproduced the single-cell level expression pattern of Tax (Fig. S3F). Furthermore, we calculate that it takes ∼150 d for 90% of MT-1 cells to experience Tax expression (Fig. S3G). This result means that the loss of Tax-KD cells occurs much faster than the Tax expression (Fig. 1D). The details of our single cell-level computational simulations are described in SI Materials and Methods.
This simulation suggests a possible mechanism that short-term expression of Tax by a cell confers some effects on not only to the cell during its period of Tax expression but also, to the cell and its progeny, even after Tax expression is lost.
Distinct Transcriptional Profiles in Tax-Expressing Cells.
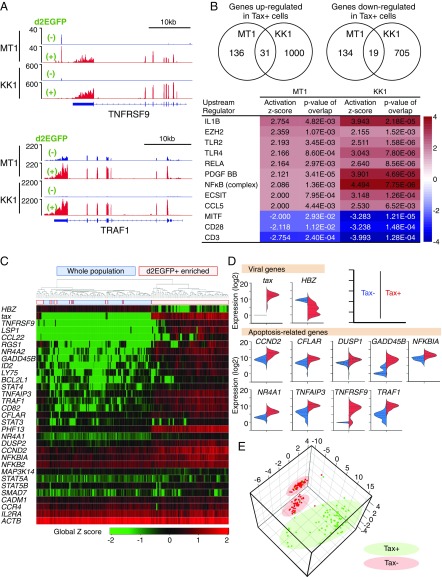
To clarify the effects of Tax on both Tax-expressing cells and nonexpressing cells, we analyzed the transcriptional profiles of each population. Tax-expressing MT1GFP or KK1GFP cells (Fig. 1E or Fig. S2B, respectively) were purified from bulk cells by a cell sorter using d2EGFP as a marker for Tax, and RNAs from each fraction were subjected to RNA-Seq. Many T-cell activation-associated genes were differentially regulated in Tax-expressing cells compared with nonexpressing cells (Fig. 2 A and B). Upstream regulator analysis was carried out using the Ingenuity Pathway Analysis program and revealed that NF-κB–related pathways were significantly affected by Tax in both cell lines (Fig. 2B).
Fig. 2.
Differences in gene expression between Tax-expressing and -nonexpressing MT1GFP cells. (A) RNA-Seq analysis comparing Tax+ and Tax− MT1GFP and KK1GFP cells. Read coverage plots for two Tax-associated genes: TNFRSF9 (Upper) and TRAF1 (Lower). (B) Ingenuity pathway analysis for dysregulated genes. Inclusion criteria are fragments per kilobase of transcript per million mapped reads (FPKM) ≥ 1 and greater than or equal to twofold expression change. (Upper) The number of dysregulated genes common to both MT1GFP and KK1GFP. (Lower) Top-scoring activated or inactivated upstream regulators. (C) Heat map of single-cell gene expression for viral and cellular genes in single MT1GFP cells. Two experiments were performed; either d2EGFP+-enriched or -unenriched (whole) populations were subjected to single-cell analysis. Ninety-four cells from each experiment were analyzed. (D) Violin plots comparing expression of viral- and apoptosis-related genes in the Tax+ vs. Tax− populations. Based on the apoptotic process gene list (GO:0006915; Gene Ontology database), nine apoptosis-related genes with expression levels that were significantly correlated with that of tax (r value > 0.5 by Pearson’s correlation test) were identified. (E) 3D PCA plot showing single-cell data clustering based on expression of apoptosis-related genes from D.
To analyze the correlation between Tax expression and that of other genes at a single-cell level, we chose ∼90 representative genes in addition to HBZ and compared their mRNA levels in sorted Tax-positive vs. Tax-negative MT-1 cells by single-cell qRT-PCR. As expected, clustering analysis could clearly separate Tax-positive cells from Tax-negative cells (Fig. 2C). Expression of NF-κB target genes and apoptosis-related genes was positively correlated with that of tax in each MT-1 cell (Fig. 2D and Fig. S4). Interestingly, violin plots of several antiapoptotic genes, such as CFLAR, GADD45B, TRAF1, and TNFAIP3, showed two peaks in Tax-negative cells: one with almost no expression and another with lower expression than Tax-positive cells (Fig. 2D). In addition, a 3D principal component analysis (PCA) plot generated by the expression levels of these genes revealed that there were two clusters in Tax-negative cells (Fig. 2E). These findings suggest that Tax influences the expression of antiapoptotic genes in Tax-negative cells: there are two subpopulations—those with medium and those with low levels of antiapoptotic factors—and thus, different degrees of sensitivity to apoptosis.
Tax Expression Is Induced by Cytotoxic Stresses and Contributes an Antiapoptotic Property to Cells.
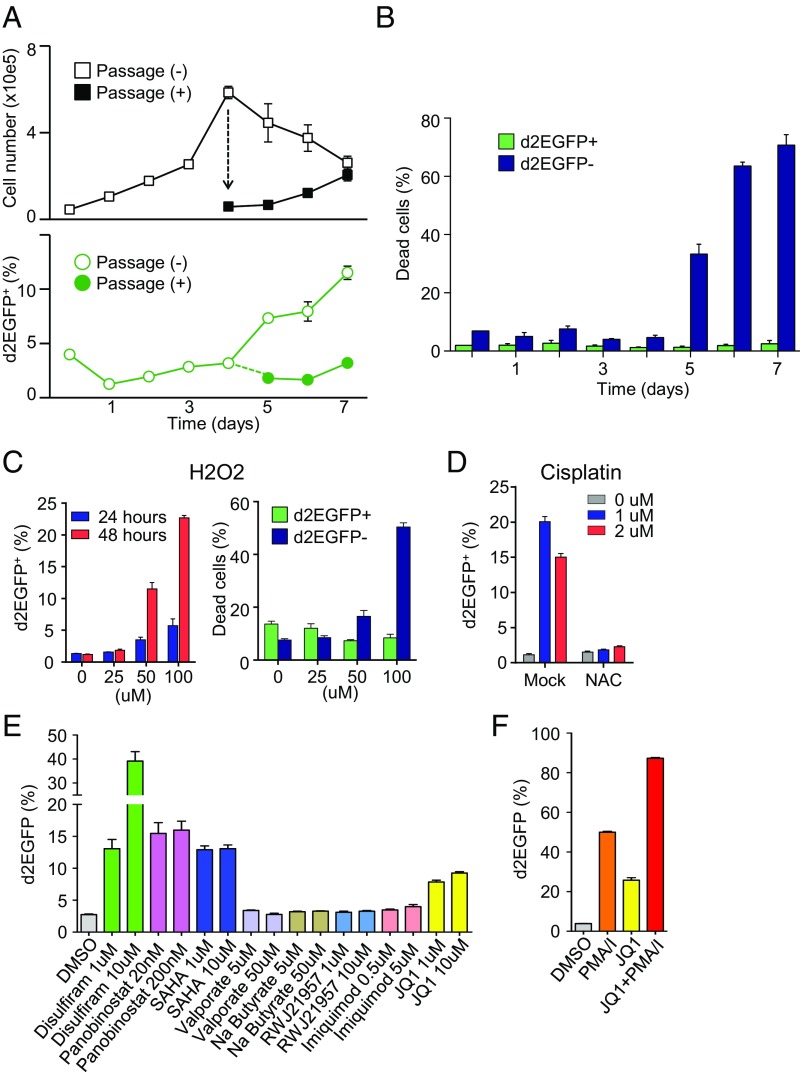
Since Tax expression was associated with the up-regulation of antiapoptotic genes, we hypothesized that Tax might be induced in response to cytotoxic stress. First, we evaluated the effect of the phases of cell growth on Tax expression. As shown in Fig. 3A, the percentage of d2EGFP-positive (Tax-expressing) cells increased stepwise from 1.2% at day 1 to 11.5% at day 7 if cells were cultured without passage. In contrast, the ratio of d2EGFP-expressing cells remained less than 3% when cells were properly passed, suggesting that stresses associated with the cell overgrowth triggered Tax expression. Importantly, under stressed conditions, the viability of Tax-expressing (d2EGFP+) cells was strikingly higher than that of Tax-negative (d2EGFP−) cells (Fig. 3B). These observations suggest that stress-induced Tax expression confers antiapoptotic capacities on MT-1 cells.
Fig. 3.
Induction of Tax in MT1GFP cells by cytotoxic stresses and HIV-reactivating reagents. (A) Effect of cellular overgrowth on Tax expression. On day 4, cells were either passaged or allowed to overgrow. Upper shows cell count, and Lower shows percentage of cells that are d2EGFP+ (Tax+). (B) Effect of cellular overgrowth on cell viability in d2EGFP+ (Tax+) and d2EGFP− (Tax−) cells. Viability is measured by LIVE/DEAD staining. (C) Induction of Tax expression by H2O2 treatment. (Left) Percentage of cells that are d2EGFP+ (Tax+); (Right) viability (LIVE/DEAD staining) of d2EGFP+ (Tax+) and d2EGFP− (Tax−) cells after 48 h. (D) Induction of Tax expression by cisplatin treatment is reversed by the antioxidant NAC. (E and F) Effect of HIV-reactivating reagents on Tax expression in MT1GFP cells. (E) The effect of HIV-reactivating reagents on Tax expression. MT1GFP cells were treated for 18 h with the indicated drugs that have been previously reported to reactivate HIV expression. (F) The effect of a combination of JQ-1 and PMA/I on Tax expression. The percentage of cells that are d2EGFP+ was measured by flow cytometry. Each figure is a representative of two independent experiments. Error bars show SDs for three replicates in one experiment.
Similarly, an inducer of oxidative stress, H2O2, induced Tax (Fig. 3C, Left), and the viability of Tax-expressing cells was significantly higher than that of nonexpressing cells (Fig. 3C, Right). It has been reported that one of the platinum-containing anticancer drugs, cisplatin, induces production of reactive oxygen species (ROS) in cancer cells and contributes to cytotoxicity (20). Cisplatin induced Tax in MT1GFP cells, while an inhibitor of ROS, N-acetyl-l-cysteine (NAC), completely canceled its effect (Fig. 3D). These results suggest that Tax functions as a safeguard against apoptosis induced by various cytotoxic stresses.
Similar Mechanisms for Latency of HTLV-1 and HIV.
It has been known that cytotoxic stresses, such as DNA damage and oxidative stress, activate the HIV LTR (21, 22). Several HIV reactivating reagents (disulfiram, panobinostat, SAHA, and JQ-1) could also induce Tax expression in MT1GFP cells (Fig. 3E). JQ-1 is an inhibitor of bromodomain-containing 4 (BRD4) and robustly reactivates HIV transcription when it is used with phorbol 12-myristate 13-acetate (PMA) and ionomycin (23). A combination of JQ-1 and PMA/ionomycin exhibited potent activity on Tax induction in MT1GFP cells (Fig. 3F), suggesting overlapping mechanisms for latency of HTLV-1 and HIV.
It has been reported that minimum feedback circuit between HIV Tat and the HIV 5′ LTR is sufficient to establish HIV latency (19). When a lentivirus vector expressing Tat through the HIV 5′ LTR was transduced into Jurkat cells, gene expression from the 5′ LTR was highly transactivated in most cells, while a small population of infected cells became latent (24). Since our results suggested that Tax has roles similar to Tat in latency, it was tested if the HTLV-1 LTR–Tax circuit can generate latency in T cells. We found that, in Jurkat cells expressing Tax and d2EGFP through the HTLV-1 5′ LTR (Jurkat/LTRd2EGFP Tax cells), a very small population expressed d2EGFP—just as was the case for MT1GFP cells (Fig. S5A, Right). In contrast, a similar construct containing HIV Tat and HIV LTRs induced continuous expression of Tat and d2EGFP in more than 95% of cells, while a remaining subset was dormant (Fig. S5B); this observation was compatible with the previous report (24). Treatment by JQ-1, PMA, and ionomycin together could activate expression of d2EGFP in all Jurkat/LTRd2EGFP Tax clones (Fig. S5C), and time-lapse imaging revealed transient expression of d2EGFP in some untreated Jurkat/LTRd2EGFP Tax cells (Fig. S5D). These findings suggest that the Tax–5′ LTR circuit is a basic unit for modulating Tax expression flexibly in response to various stimulations.
Tax Suppresses the Cell Cycle Transition from S to G2/M.
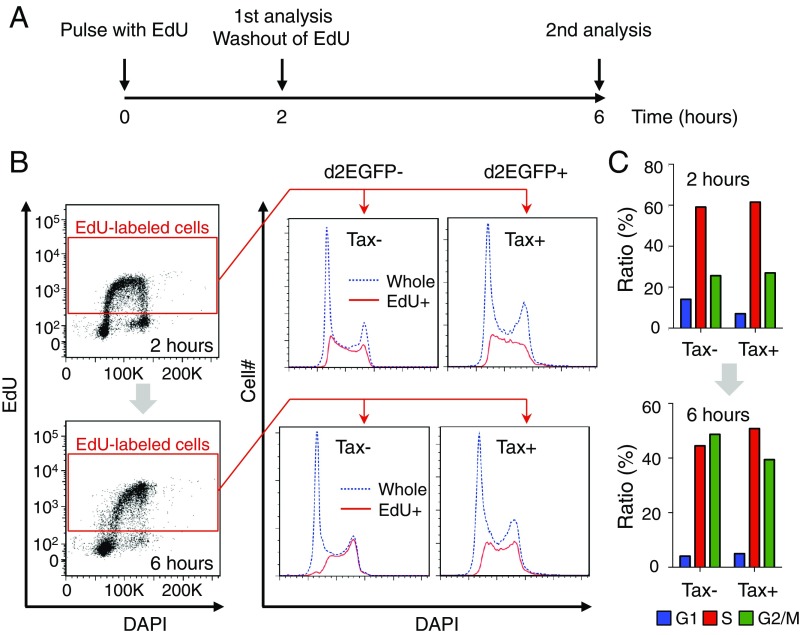
A possible alternate explanation for the big impact of Tax-KD on MT-1 cell survival was that Tax-expressing cells might proliferate faster than nonexpressing cells. To test this hypothesis, the effect of Tax on the cell cycle was analyzed using a method combining DAPI staining and 5-ethynyl-2′-deoxyuridine (EdU) incorporation (Fig. 4A)—a method by which we can evaluate temporal cell cycle progression without synchronization (25). We found that the proportions of cells in S and G2/M phases 2 h after the start of an EdU pulse were comparable between Tax-positive and Tax-negative cells. However, at 6 h, the proportion of G2/M cells within the Tax-positive population was only ∼80% of that within the Tax-negative population (G2/M cells made up 39.4 and 48.7% of Tax-positive and -negative cells, respectively), suggesting that, if anything, Tax retarded, rather than sped up, the G2/M transition of MT-1 cells (Fig. 4 B and C). This result implies that Tax does not promote faster proliferation of expressing cells; rather, the antiapoptotic property of Tax is responsible for the survival of MT-1 cells.
Fig. 4.
Cell cycle transition in d2EGFP+ (Tax+) and d2EGFP− (Tax−) cells. (A) Scheme for analysis of cell cycle transition without cell synchronization. (B and C) Cell cycle phase distributions for EdU+ cells among d2EGFP+ (Tax+) and d2EGFP− (Tax−) cells at 2 and 6 h. (B, Left) Gating strategy for EdU+ cells; (B, Right) linear flow cytometric analysis of cell cycle with DAPI staining. Distribution of EdU+ cells is shown in red, and that of whole cells is blue. (C) Bar charts showing the percentage of EdU+ cells in the indicated phase.
The Carryover of Antiapoptotic Factors into the Tax-Negative Interval Is Important for Maintaining the MT-1 Cell Population.
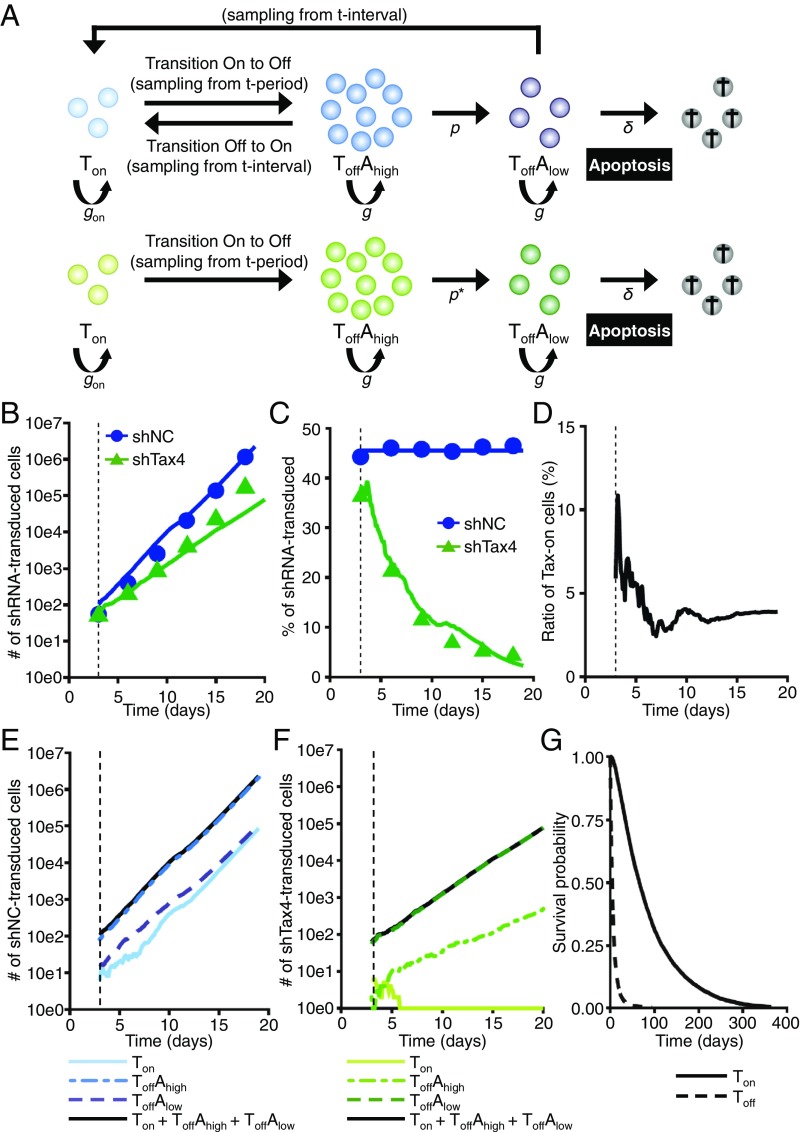
Based on the results of the Tax-KD and single-cell analysis experiments, we hypothesized that transient Tax expression triggers antiapoptotic genes in a small number of MT-1 cells, that this effect is carried over in a significant number of these cells when they enter the Tax-negative phase, and that such carryover is critical for the survival and expansion of the cell population. To check this hypothesis, we constructed a population-level agent-based model (ABM) that can represent our experimental observations. More precisely, the ABM simulates the population dynamics of MT-1 cells transduced with shNC or shTax4 as an inhomogeneous (birth–death) Poisson process with a stage transition (Fig. 5A shows a schematic representation, and SI Materials and Methods has details). We carried out ABM simulations and confirmed that the time course of experimental data for the number and fraction of shNC and shTax4 cells was well-reproduced in Fig. 5 B and C. Based on our ABM simulations, we reconstructed the time course of the frequency of Tax-positive cells under normal conditions. As shown in Fig. 5D, our simulation predicted that a small number of MT-1 cells would express Tax (i.e., around 3% at the steady state). In addition, we calculated the predicted subpopulation dynamics of shNC and shTax4 cells (Fig. 5 E and F, respectively). The numbers of cells that are Tax positive and antiapoptotic gene positive (i.e., ), Tax negative and antiapoptotic gene high (i.e., ), and Tax negative and antiapoptotic gene low (i.e., ) are shown. Under normal conditions (Fig. 5E), the fraction of cells that are remains small; however, the number of cells in each subpopulation increases, and the total cell population expands, since MT-1 cells are able to express Tax. This expression protects them from apoptosis. In fact, our simulation predicts that the major subpopulation will be , which is consistent with our detection of the expression of apoptosis-related genes in Fig. 2D. However, under Tax-KD conditions (Fig. 5F), the fraction of cells that are decreases until there are almost none left, because there is no additional transition of cells from Tax negative to Tax positive. The collapse of the subpopulation leads to a corresponding decrease in antiapoptotic gene expression, renders more cells susceptible to apoptosis, and decreases the degree to which the total cell population expands.
Fig. 5.
Agent-based simulations of cell population dynamics under normal and Tax-KD conditions. (A) Scheme for modeling of cell population dynamics as a birth–death (Poisson) process with a stage transition. The ABM simulates the population dynamics of MT-1 cells transduced with shNC or shTax4. (Upper) Normal conditions; (Lower) Tax-KD conditions. (B and C) The time course of the absolute number (B) and fraction (C) of shNC (control) and shTax4 (Tax-KD) cells in a simulation resembling the experiment in Fig. 1D. In this simulated experiment, some MT-1 cells are shRNA+, and some are shRNA−. The simulated population dynamics of the shRNA+ cells in the mixed population are shown. The blue and green lines give the best fit solutions for the agent-based simulations based on Tax period sampling from experimental values (i.e., gray bars in Fig. S3C) and Tax interval sampling from simulated values (i.e., Fig. S3G). All data were fitted simultaneously. (D) The simulated dynamics of the frequency of Tax-expressing cells in the normal MT-1 cell population. A steady state is reached, in which about 3% of cells are Tax+. (E and F) The simulated dynamics of subpopulations of cells transduced with shNC (E, blue lines) or shTax4 (F, green lines). (G) The survival probabilities for subpopulations of shNC cells that have expressed Tax at least once and that have never expressed Tax are described by solid and dashed lines, respectively.
To examine whether having experienced Tax expression prolongs cell survival time, we also calculated the survival probabilities of the subpopulations of shNC cells in our ABM simulations (details are in SI Materials and Methods). For this calculation, we defined the subpopulations of shNC cells based on whether Tax had been expressed at least once or had never been expressed (solid and dashed lines in Fig. 5G, respectively). Interestingly, in this simulation, Tax expression significantly increases the average lifespan of the cell.
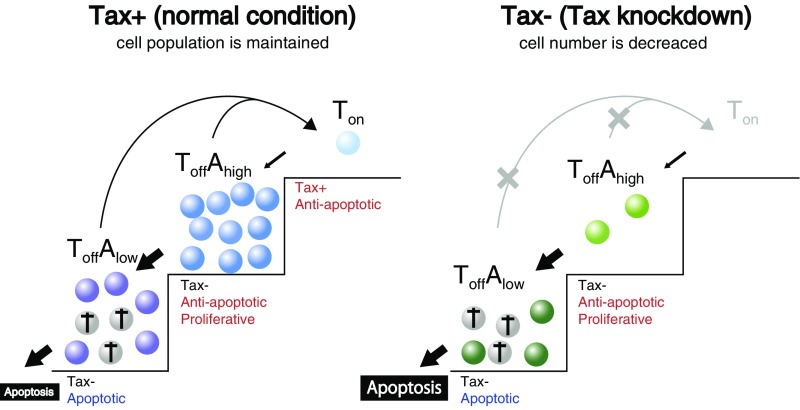
Taken together, these simulations support a model for ATL persistence: transient expression of Tax in cells is responsible for cell population-level maintenance and expansion (Fig. 6). In a steady state (Fig. 6, Left), cells turn successively into and then, cells. Although cells are sensitive to apoptotic signals and gradually die in culture, Tax-negative cells proliferate more rapidly than Tax-positive cells. In response to cytotoxic stresses, Tax can be induced in some Tax-negative cells, and thus, cells are replenished, a process that is critical for the persistence of the population. Tax-KD (Fig. 6, Right) causes a decrease in cells, resulting in a shortage of apoptosis-resistant cells and the induction of massive apoptosis. This model enables us to explain how such a small number of Tax-expressing cells has a big impact on the dynamics of the whole population.
Fig. 6.
Proposed model of the dynamics and significance of Tax expression. (Left) Under normal conditions; (Right) under Tax-KD conditions.
Discussion
Persistent viruses have evolved shrewd strategies to propagate in vivo while evading host immune surveillance. Here, we show that HTLV-1 utilizes a unique way to enhance survival and proliferation of infected cells: the transient expression of Tax confers an antiapoptotic property to cells and maintains the whole population. Since it has been reported that continuous expression of Tax induces DNA damage and senescence in the expressing cells (26, 27), it is suggested that short-term expression of Tax is beneficial to cells. Interestingly, a similar phenomenon of cell survival being promoted by transient gene expression was reported in mouse ES cells (mESCs). Zscan4, which functions in the maintenance of telomeres, is transiently expressed in only ∼5% of mESCs at any given time. KD of Zscan4 induces massive cell death before all cells experience its expression (28). Moreover, expression of Zscan4 is linked to activation of an endogenous retrovirus, MERVL (29). Those studies and our observations suggest an association between the transient activation of retroviral LTRs and the maintenance of cell populations.
Tax expression is essential for de novo infection by HTLV-1, since viral transcription depends on Tax (30, 31). However, Tax expression strongly induces expression of viral proteins, including Tax, Env, and Gag, resulting in attacks by cytotoxic T lymphocytes (CTLs). Therefore, intermittent Tax expression is a clever strategy of HTLV-1 to evade the host immune response most of the time, but it maintains the ability to cause de novo infection under certain conditions. A recent study has reported that tax is induced by hypoxia (32); it is compatible with the previous observation that high tax expression was detected in the bone marrow, which is physiologically hypoxic (33). As another example, HTLV-1 can be transmitted through breastfeeding—a process in which HTLV-1–infected cells have to pass through the alimentary tract with acidic conditions and bile acids. Stress-induced Tax expression would be beneficial for de novo infection in these conditions. It is known that low pH and hypoxia in the physiological environment suppress adaptive immunity (34, 35), suggesting that infected cells may be able to “get away with” expressing Tax for a limited time in such immunological niches. To clarify the in vivo dynamics of Tax expression in immunocompetent hosts, additional studies using animal models will be required.
Tax expression is suppressed by genetic/epigenetic mechanisms in 50% of ATL cases (16, 36). The other one-half of ATL cases have the potential to express Tax, and indeed, it is known that nearly 50% of ATL cases express viral antigens after ex vivo culture (37). In this study, we show that survival of MT-1 cells, which express a level of tax similar to that of primary ATL cells (17), depends on Tax, suggesting that clinical ATL cases are divided into at least two classes: Tax-dependent and -independent ATL. A previous finding that the arsenic/IFN treatment induced apoptosis of several Tax-expressing cell lines, including MT-1, through degradation of Tax protein implies that those established cell lines still possess the characteristics of Tax-dependent ATL (38). It is known that the activity of Tax-specific CTLs is an important factor in inhibiting the onset of ATL (15). A recent study provided more evidence that Tax plays an important role in ATL: a clinical trial of a dendritic cell vaccine targeting Tax was efficacious (39). Tax expression was faint in fresh ATL cells of the enrolled patients, but it was inducible by ex vivo culture. These reports support our hypothesis that transiently expressed Tax plays critical roles in the development and maintenance of ATL, even if its expression is limited to a small fraction of leukemic cells. Recent comprehensive genomic studies of clinical ATL cases showed that genetic/epigenetic alterations accumulate in Tax-associated genes (40, 41), suggesting that the effects of these changes can substitute for Tax functions in leukemogenesis.
It is noteworthy that expression of tax was contrary to that of HBZ, even at the single-cell level (r = −0.8, P < 0.0001) (Fig. 2D). A similar observation in HTLV-1–infected T-cell clones was published while our study was under consideration for publication (42). That study showed the heterogeneity in the expression levels of tax and HBZ mRNA in fixed cells, whereas we here show the dynamics of Tax expression using live cell imaging. Tax and HBZ have opposing functions in many signaling pathways (9); however, the significance of their contrary activities has not been clarified. In this study, we found that Tax induces antiapoptotic genes, such as CFLAR, TNFRSF9, TRAF1, and TNFAIP3 (43–45). In contrast, pathways associated with cell proliferation, such as CD3, CD28, and melanogenesis-associated transcription factor (46, 47), are significantly activated in Tax-negative cells that express HBZ (Fig. 2B). This finding is compatible with the previous studies that showed that HBZ promotes T-cell growth by enhancing signals through CD3 and that KD of HBZ suppresses the proliferation of ATL cells, including MT-1 cells (12, 48, 49). The alternation of expression of Tax and HBZ seems to execute cooperative programs for survival and proliferation rather than cause them to interfere with each other. It has been reported that hyperactivation of the NF-κB pathway by Tax triggers senescence in HeLa cells, while HBZ could cancel this suppressive effect (27), suggesting that the collaboration of Tax and HBZ is important for the expansion of HTLV-1–infected cells. Transgenic mouse models have shown that both Tax and HBZ are oncogenic (50); however, their roles in HTLV-1–infected individuals are thought to be more complicated due to immune surveillance and the fact that human cells are more resistant to malignant transformation than rodent cells (51, 52). HBZ plays important roles in determining the immunophenotype of HTLV-1–infected cells, and HBZ itself has low immunogenicity (53, 54). It has been reported that the expression level of HBZ is higher in ATL cells than in nonleukemic infected cells, implying that infected clones with higher HBZ expression are selected during leukemogenesis (17). These findings suggest that constant expression of HBZ drives proliferation of cells with this specific immunophenotype, while transient Tax expression engages to inhibit cell death caused by various stresses. The different functions and expression patterns of Tax vs. HBZ are thought to be important in the malignant transformation of human T cells.
Recent studies show that many pathogens can persist in their reservoirs during both acute and chronic infections and reemerge in the host under stressful conditions (55). In this study, we show that the HTLV-1 5′ LTR is activated by various cytotoxic stresses and several reagents that reactivate latent HIV (Fig. 3). These results suggest that HTLV-1–infected cells utilize mechanisms analogous to those of latent HIV-infected cells to act as reservoirs. One example is the antagonism of Tax and Tat to BRD4. BRD4 is a negative regulator of positive transcription elongation factor b (P-TEFb). It has been reported that both Tax and Tat compete with BRD4 for binding to P-TEFb (56, 57), and indeed, we found that a BRD4 inhibitor, JQ-1, efficiently reactivated the HTLV-1 5′ LTR (Fig. 3 E and F and Fig. S5C). These findings suggested that BRD4 can interrupt the positive feedback loop generated by the 5′ LTR and Tax/Tat and allow the establishment of latent infection by HTLV-1/HIV. Interestingly, many other viruses, including KSHV and human papilloma virus, also use BRD4 and/or P-TEFb to regulate viral lifecycles and pathogenesis (58–60). Identification of the cellular factors acted on by diverse viruses will contribute to exploring common mechanisms of viral latency and reactivation. In the case of HTLV-1, several repressors of viral replication, such as p30, Rex, and HBZ, are encoded in the provirus (9). These viral factors may be able to regulate the duration and/or timing of Tax expression in infected cells more elaborately. Additional investigation will be required to understand the precise roles of each protein in HTLV-1 latency.
Since the discovery of HTLV-1, a number of studies on its pathogenesis have been conducted; however, the prevention and treatment of HTLV-1–induced diseases are still unsatisfactory (61). In this study, we showed that sporadic and transient Tax expression in a small subset of cells has significant influence on gene expression of their progeny cells and consequently, maintains the whole population of ATL cells. This is a mechanism of the retrovirus for persistence and latency in vivo, and elucidation of this mechanism can contribute to a better understanding of viral pathogenesis and the development of strategies for treatment and prophylaxis of viral-associated diseases.
Materials and Methods
Cells.
An IL-2–independent ATL cell line MT-1 (62), two IL-2–dependent ATL cell lines KK-1 and SO-4 (63), and an HTLV-1–negative T-cell line Jurkat were used in this study. MT-1 and Jurkat cells were maintained in RPMI supplemented with 10% (vol/vol) FBS. The MT1GFP cell line was maintained as MT-1 cells were, with the addition of 500 μg/mL G418 (Nacalai). KK-1 and SO-4 cell lines were maintained in RPMI supplemented with 10% FBS and IL-2 (100 U/mL; PeproTech).
Clinical Samples.
Fresh ATL cells were obtained from 20 aggressive-type ATL cases and used for extraction of genomic DNA and total RNA. Use of the clinical samples in this research was approved by the Ethics Committee of Kyoto University (approval no. G204). Written consent was obtained from the patients. Using genomic DNAs from primary ATL cells and ATL cell lines, DNA methylation level of 5′ LTR was analyzed by the Combined Bisulfite Restriction Analysis method as previously described (36). Expression level of tax in fresh ATL cells was analyzed by a conventional qRT-PCR (16).
GFP Competition Assay.
The GFP competition assay (64) was carried out to observe the long-term effect of Tax-KD on MT-1 or Jurkat cells transduced with pLKO-GFP lentivirus expressing shNC, shTax1, or shTax4. Cells were infected with concentrated lentivirus at a multiplicity of infection (MOI) of 0.5 to adjust the ratio of transduced cells to around 50%. The effect of shRNAs on target cells was evaluated by measuring the percentage of GFP+ cells using a FACSverse flow cytometer (BD Biosciences).
Single-Cell qPCR.
The C1 Single-Cell Auto Prep Array for PreAmp (Fluidigm) was used for harvest of RNA, cDNA synthesis, and preamplification of cDNA (18 cycles of PCR for the target genes) from single cells according to the manufacturer’s instructions. After loading cells onto an integrated fluidic circuit, we checked all 96 chambers by microscope to verify capture of a single cell. Thereafter, preamplified cDNA was harvested and subjected to qPCR. The Biomark HD system (Fluidigm) combined with EvaGreen chemistry (Bio-Rad) was used for the qPCR assay. To increase specificity, we used nested primers (one pair for the preamplification step and another pair for the subsequent qPCR of 30 cycles). The sequences of the primers used in this study are indicated in Table S3. Raw data were processed by Fluidigm Real-Time PCR analysis software, and the melting curve was used to determine the pass/failure call of qPCR. Data were analyzed with the R program using the Singular Analysis Toolset package (Fluidigm). Any chamber that contained more than one cell was excluded from analysis; outlier cells that had low global expression were also excluded. The level of detection value was set to 24 cycles according to the manufacturer’s recommendation.
Time-Lapse Imaging.
For live cell imaging, 8 × 104 MT1GFP cells were seeded in a 5-mm glass-bottom dish (Matsunami) precoated with poly-d-lysine (Sigma) and incubated at 37 °C in 5% CO2. Images in the differential interference contrast (DIC) and GFP channels were captured with an LCV110 microscope (Olympus) every 20 min for 96 h. Semiautomated cell tracking was done by Fiji software with the Trackmate plugin (65). Cells, which had already expressed d2EGFP at the beginning of the observation, were excluded from analysis, because the starting point for expression was unknown. To analyze single-cell dynamics of d2EGFP expression, normalized fluorescence intensities are plotted against time. The starting time (t = 0) is the time at which the cell started expressing d2EGFP above background level.
RNA-Seq.
MT1GFP or KK1GFP cells were sorted into d2EGFP+ and d2EGFP− populations with a FACSAria III (BD Biosciences), and RNA was then extracted using the RNeasy mini kit (Qiagen). Single-end RNA sequencing was performed (BGI). A quality check was done with FastQC, and then, Tuxedo pipeline was used for RNA quantification (66). Upstream regulator analysis was carried out by Ingenuity Pathway Analysis (Qiagen).
Cell Cycle Analysis.
To measure the cell cycle dynamics of MT1GFP cells without cell synchronization, a method combining EdU incorporation and DAPI staining was carried out as previously described (25) using the Click-iT Plus EdU Alexa Fluor 647 kit (Thermo Fisher). Initially, 1 × 107 cells were cultured in complete RPMI medium containing 10 µM EdU for 2 h and washed thereafter. At that point (time = 2 h), one-half of the cells were fixed and stained for EdU and DAPI according to the manufacturer’s protocol. The other one-half of the cells were recultured again for 4 h without adding EdU. At the end of the assay (time = 6 h), those cells were washed, fixed, and stained. Cell cycle status in the d2EGFP+ or d2EGFP− population was analyzed using a FACSVerse based on the levels of EdU and DAPI.
Statistical Analysis.
Statistical analysis was done using Microsoft Excel, GraphPad Prism, R, or Python. Data obtained by flow cytometry were analyzed with FlowJo. Two-sided t test was used to compare between two different groups.
Computational Simulations.
A simple deterministic two-state model of Tax-positive feedback was developed (the chemical reaction scheme is shown in Fig. S3A) (19) and simulated by the Gillespie algorithm (67) to investigate the stochastic dynamics of Tax expression, especially to estimate the length of Tax expression () and the interval between Tax expression episodes (). In addition, an ABM of cell population dynamics (birth–death Poisson process) with stage transitions as described in Fig. 5A was constructed based on the individual-based Gillespie algorithm (67) to confirm the experiment-based hypothesis that the transient expression of Tax is a critical event for the persistence of the MT-1 cell population. Additional details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Mitsuaki Yoshida (The Cancer Institute, Japanese Foundation for Cancer Research) for helpful discussion, Dr. Chou-Zen Giam (Uniformed Services University of the Health Sciences) for providing PB-18X21-RFP and PB-Tase, Drs. Hiroo Hasegawa and Yasuaki Yamada (Nagasaki University) for KK-1 and SO-4, and Dr. Linda Kingsbury for proofreading. This study was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grants JP16H05336 (to M. Matsuoka), 26460554 and JP17K07166 (to J.-i.Y.), and JP15H05707 and JP16K05265 (to S.N.); Project for Cancer Research and Therapeutic Evolution Grant 17cm0106306h0002 (to J.-i.Y. and M. Matsuoka); Research Program on Emerging and Re-Emerging Infectious Diseases Grant 17fk0108227h0002 (to J.-i.Y. and M. Matsuoka) and Japanese Initiative for Progress of Research on Infectious Disease for Global Epidemics (J-PRIDE) Grants 17fm0208006h0001 (to S.I.), 17fm0208019h0101 (to S.I.), and 17fm0208014h0001 (to S.I.) from the Japan Agency for Medical Research and Development; a grant from the Princess Takamatsu Cancer Research Fund (to J.-i.Y.); a grant from The Yasuda Medical Foundation (to J.-i.Y.); the Japan Science and Technology Agency (JST) Precursory Research for Embryonic Science and Technology (PRESTO) [S.I., and Grant JPMJPR16E9 (to S.N.)] and Core Research for Evolutional Science and Technology (CREST) Programs (S.I.); and Grants-in-Aid for Scientific Research on Innovative Areas 16H06429 (S.I.), 16K21723 (S.I.), and 17H05819 (to S.I.) from Ministry of Education, Culture, Sports, Science, and Technology (MEXT). This study was also supported in part by the JSPS Core-to-Core Program A, Advanced Research Networks.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-Seq data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE108601). C++ source code is freely available at https://github.com/petadimension/Tax_dynamics/. R source code is available at https://github.com/petadimension/Tax_dynamics/tree/master/ABM.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715724115/-/DCSupplemental.
References
- 1.Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 2.Traylen CM, et al. Virus reactivation: A panoramic view in human infections. Future Virol. 2011;6:451–463. doi: 10.2217/fvl.11.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe. 2014;15:266–282. doi: 10.1016/j.chom.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagaya Y, Gallo RC. The exceptional oncogenicity of HTLV-1. Front Microbiol. 2017;8:1425. doi: 10.3389/fmicb.2017.01425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 6.Demontis MA, Sadiq MT, Golz S, Taylor GP. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J Med Virol. 2015;87:2130–2134. doi: 10.1002/jmv.24264. [DOI] [PubMed] [Google Scholar]
- 7.Melamed A, et al. Genome-wide determinants of proviral targeting, clonal abundance and expression in natural HTLV-1 infection. PLoS Pathog. 2013;9:e1003271. doi: 10.1371/journal.ppat.1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philip S, Zahoor MA, Zhi H, Ho YK, Giam CZ. Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex. PLoS Pathog. 2014;10:e1004040. doi: 10.1371/journal.ppat.1004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuoka M, Yasunaga J. Human T-cell leukemia virus type 1: Replication, proliferation and propagation by tax and HTLV-1 bZIP factor. Curr Opin Virol. 2013;3:684–691. doi: 10.1016/j.coviro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Boxus M, et al. The HTLV-1 tax interactome. Retrovirology. 2008;5:76. doi: 10.1186/1742-4690-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanon E, et al. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood. 2000;95:1386–1392. [PubMed] [Google Scholar]
- 12.Satou Y, Yasunaga J, Yoshida M, Matsuoka M. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci USA. 2006;103:720–725. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 14.Kannagi M, et al. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol. 1991;3:761–767. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- 15.Kannagi M, Hasegawa A, Takamori A, Kinpara S, Utsunomiya A. The roles of acquired and innate immunity in human T-cell leukemia virus type 1-mediated diseases. Front Microbiol. 2012;3:323. doi: 10.3389/fmicb.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takeda S, et al. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int J Cancer. 2004;109:559–567. doi: 10.1002/ijc.20007. [DOI] [PubMed] [Google Scholar]
- 17.Usui T, et al. Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells. Retrovirology. 2008;5:34. doi: 10.1186/1742-4690-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Liu M, Merling R, Giam CZ. Versatile reporter systems show that transactivation by human T-cell leukemia virus type 1 Tax occurs independently of chromatin remodeling factor BRG1. J Virol. 2006;80:7459–7468. doi: 10.1128/JVI.00130-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Razooky BS, Pai A, Aull K, Rouzine IM, Weinberger LS. A hardwired HIV latency program. Cell. 2015;160:990–1001. doi: 10.1016/j.cell.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marullo R, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8:e81162. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valerie K, et al. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature. 1988;333:78–81. doi: 10.1038/333078a0. [DOI] [PubMed] [Google Scholar]
- 22.Legrand-Poels S, Vaira D, Pincemail J, van de Vorst A, Piette J. Activation of human immunodeficiency virus type 1 by oxidative stress. AIDS Res Hum Retroviruses. 1990;6:1389–1397. doi: 10.1089/aid.1990.6.1389. [DOI] [PubMed] [Google Scholar]
- 23.Zhu J, et al. Reactivation of latent HIV-1 by inhibition of BRD4. Cell Rep. 2012;2:807–816. doi: 10.1016/j.celrep.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberger LS, Burnett JC, Toettcher JE, Arkin AP, Schaffer DV. Stochastic gene expression in a lentiviral positive-feedback loop: HIV-1 Tat fluctuations drive phenotypic diversity. Cell. 2005;122:169–182. doi: 10.1016/j.cell.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Fleisig H, Wong J. Measuring cell cycle progression kinetics with metabolic labeling and flow cytometry. J Vis Exp. 2012:e4045. doi: 10.3791/4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinjo T, Ham-Terhune J, Peloponese JM, Jr, Jeang KT. Induction of reactive oxygen species by human T-cell leukemia virus type 1 tax correlates with DNA damage and expression of cellular senescence marker. J Virol. 2010;84:5431–5437. doi: 10.1128/JVI.02460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhi H, et al. NF-κB hyper-activation by HTLV-1 tax induces cellular senescence, but can be alleviated by the viral anti-sense protein HBZ. PLoS Pathog. 2011;7:e1002025. doi: 10.1371/journal.ppat.1002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zalzman M, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–863. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckersley-Maslin MA, et al. MERVL/Zscan4 network activation results in transient genome-wide DNA demethylation of mESCs. Cell Rep. 2016;17:179–192. doi: 10.1016/j.celrep.2016.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cann AJ, Rosenblatt JD, Wachsman W, Shah NP, Chen IS. Identification of the gene responsible for human T-cell leukaemia virus transcriptional regulation. Nature. 1985;318:571–574. doi: 10.1038/318571a0. [DOI] [PubMed] [Google Scholar]
- 31.Felber BK, Paskalis H, Kleinman-Ewing C, Wong-Staal F, Pavlakis GN. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985;229:675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni A, et al. Glucose metabolism and oxygen availability govern reactivation of the latent human retrovirus HTLV-1. Cell Chem Biol. 2017;24:1377–1387.e3. doi: 10.1016/j.chembiol.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin MC, et al. Extensive latent retroviral infection in bone marrow of patients with HTLV-I-associated neurologic disease. Blood. 1997;89:346–348. [PubMed] [Google Scholar]
- 34.Kareva I, Hahnfeldt P. The emerging “hallmarks” of metabolic reprogramming and immune evasion: Distinct or linked? Cancer Res. 2013;73:2737–2742. doi: 10.1158/0008-5472.CAN-12-3696. [DOI] [PubMed] [Google Scholar]
- 35.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi Y, et al. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology. 2005;2:64. doi: 10.1186/1742-4690-2-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurihara K, et al. Potential immunogenicity of adult T cell leukemia cells in vivo. Int J Cancer. 2005;114:257–267. doi: 10.1002/ijc.20737. [DOI] [PubMed] [Google Scholar]
- 38.Dassouki Z, et al. ATL response to arsenic/interferon therapy is triggered by SUMO/PML/RNF4-dependent Tax degradation. Blood. 2015;125:474–482. [Google Scholar]
- 39.Suehiro Y, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. Br J Haematol. 2015;169:356–367. doi: 10.1111/bjh.13302. [DOI] [PubMed] [Google Scholar]
- 40.Kataoka K, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304–1315. doi: 10.1038/ng.3415. [DOI] [PubMed] [Google Scholar]
- 41.Fujikawa D, et al. Polycomb-dependent epigenetic landscape in adult T-cell leukemia. Blood. 2016;127:1790–1802. doi: 10.1182/blood-2015-08-662593. [DOI] [PubMed] [Google Scholar]
- 42.Billman MR, Rueda D, Bangham CRM. Single-cell heterogeneity and cell-cycle-related viral gene bursts in the human leukaemia virus HTLV-1. Wellcome Open Res. 2017;2:87. doi: 10.12688/wellcomeopenres.12469.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okamoto K, Fujisawa J, Reth M, Yonehara S. Human T-cell leukemia virus type-I oncoprotein tax inhibits Fas-mediated apoptosis by inducing cellular FLIP through activation of NF-kappaB. Genes Cells. 2006;11:177–191. doi: 10.1111/j.1365-2443.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 44.Saitoh Y, et al. A20 targets caspase-8 and FADD to protect HTLV-I-infected cells. Leukemia. 2016;30:716–727. doi: 10.1038/leu.2015.267. [DOI] [PubMed] [Google Scholar]
- 45.Sabbagh L, et al. Leukocyte-specific protein 1 links TNF receptor-associated factor 1 to survival signaling downstream of 4-1BB in T cells. J Leukoc Biol. 2013;93:713–721. doi: 10.1189/jlb.1112579. [DOI] [PubMed] [Google Scholar]
- 46.Acuto O, Michel F. CD28-mediated co-stimulation: A quantitative support for TCR signalling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 47.Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: Targeted strategies for melanoma. Nat Rev Cancer. 2012;12:349–361. doi: 10.1038/nrc3218. [DOI] [PubMed] [Google Scholar]
- 48.Arnold J, Zimmerman B, Li M, Lairmore MD, Green PL. Human T-cell leukemia virus type-1 antisense-encoded gene, Hbz, promotes T-lymphocyte proliferation. Blood. 2008;112:3788–3797. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinosada H, et al. HTLV-1 bZIP factor enhances T-cell proliferation by impeding the suppressive signaling of co-inhibitory receptors. PLoS Pathog. 2017;13:e1006120. doi: 10.1371/journal.ppat.1006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yasunaga J, Matsuoka M. Molecular mechanisms of HTLV-1 infection and pathogenesis. Int J Hematol. 2011;94:435–442. doi: 10.1007/s12185-011-0937-1. [DOI] [PubMed] [Google Scholar]
- 51.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 52.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24:5976–5985. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 53.Bangham CRM, Matsuoka M. Human T-cell leukaemia virus type 1: Parasitism and pathogenesis. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160272. doi: 10.1098/rstb.2016.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma G, Yasunaga J, Matsuoka M. Multifaceted functions and roles of HBZ in HTLV-1 pathogenesis. Retrovirology. 2016;13:16. doi: 10.1186/s12977-016-0249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anonymous Lessons from reservoirs. Nat Med. 2017;23:899. doi: 10.1038/nm.4387. [DOI] [PubMed] [Google Scholar]
- 56.Bisgrove DA, Mahmoudi T, Henklein P, Verdin E. Conserved P-TEFb-interacting domain of BRD4 inhibits HIV transcription. Proc Natl Acad Sci USA. 2007;104:13690–13695. doi: 10.1073/pnas.0705053104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cho WK, et al. Modulation of the Brd4/P-TEFb interaction by the human T-lymphotropic virus type 1 tax protein. J Virol. 2007;81:11179–11186. doi: 10.1128/JVI.00408-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen HS, et al. BET-inhibitors disrupt Rad21-dependent conformational control of KSHV latency. PLoS Pathog. 2017;13:e1006100. doi: 10.1371/journal.ppat.1006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jang MK, Shen K, McBride AA. Papillomavirus genomes associate with BRD4 to replicate at fragile sites in the host genome. PLoS Pathog. 2014;10:e1004117. doi: 10.1371/journal.ppat.1004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaborowska J, Isa NF, Murphy S. P-TEFb goes viral. Inside Cell. 2016;1:106–116. doi: 10.1002/icl3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willems L, et al. Reducing the global burden of HTLV-1 infection: An agenda for research and action. Antiviral Res. 2017;137:41–48. doi: 10.1016/j.antiviral.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 62.Miyoshi I, et al. A novel T-cell line derived from adult T-cell leukemia. Gan. 1980;71:155–156. [PubMed] [Google Scholar]
- 63.Yamada Y, et al. Interleukin-15 (IL-15) can replace the IL-2 signal in IL-2-dependent adult T-cell leukemia (ATL) cell lines: Expression of IL-15 receptor alpha on ATL cells. Blood. 1998;91:4265–4272. [PubMed] [Google Scholar]
- 64.Eekels JJ, et al. A competitive cell growth assay for the detection of subtle effects of gene transduction on cell proliferation. Gene Ther. 2012;19:1058–1064. doi: 10.1038/gt.2011.191. [DOI] [PubMed] [Google Scholar]
- 65.Jaqaman K, et al. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillespie DT. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J Comput Phys. 1976;22:403–434. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.