Significance
Association of the adaptor protein STAC3 to the calcium channel was recently identified as essential for skeletal muscle contraction. While STAC3 is expressed exclusively in skeletal muscle, the other two isoforms, STAC1 and STAC2, are found in the brain, but their function has not yet been investigated. Recently we identified the C1 domain of STAC as crucial for its association with calcium channels. However, the corresponding binding domain remained unknown. Here, we demonstrate that STAC proteins associate with the C terminus of CaV1.2 at the IQ domain, and that this interaction results in the inhibition of calcium-dependent inactivation. Taken together, our data identify a functionally important STAC interaction domain and suggest that STAC proteins modulate calcium entry through CaV1 channels.
Keywords: STAC, calcium-dependent inactivation, voltage-gated calcium channels, calmodulin
Abstract
The adaptor proteins STAC1, STAC2, and STAC3 represent a newly identified family of regulators of voltage-gated calcium channel (CaV) trafficking and function. The skeletal muscle isoform STAC3 is essential for excitation–contraction coupling and its mutation causes severe muscle disease. Recently, two distinct molecular domains in STAC3 were identified, necessary for its functional interaction with CaV1.1: the C1 domain, which recruits STAC proteins to the calcium channel complex in skeletal muscle triads, and the SH3-1 domain, involved in excitation–contraction coupling. These interaction sites are conserved in the three STAC proteins. However, the molecular domain in CaV1 channels interacting with the STAC C1 domain and the possible role of this interaction in neuronal CaV1 channels remained unknown. Using CaV1.2/2.1 chimeras expressed in dysgenic (CaV1.1−/−) myotubes, we identified the amino acids 1,641–1,668 in the C terminus of CaV1.2 as necessary for association of STAC proteins. This sequence contains the IQ domain and alanine mutagenesis revealed that the amino acids important for STAC association overlap with those making contacts with the C-lobe of calcium-calmodulin (Ca/CaM) and mediating calcium-dependent inactivation of CaV1.2. Indeed, patch-clamp analysis demonstrated that coexpression of either one of the three STAC proteins with CaV1.2 opposed calcium-dependent inactivation, although to different degrees, and that substitution of the CaV1.2 IQ domain with that of CaV2.1, which does not interact with STAC, abolished this effect. These results suggest that STAC proteins associate with the CaV1.2 C terminus at the IQ domain and thus inhibit calcium-dependent feedback regulation of CaV1.2 currents.
Recently STAC3 (SH3 and cysteine-rich containing protein 3) has been identified as an essential regulator of calcium channel trafficking and function in skeletal muscle excitation–contraction (EC) coupling and a mutation in STAC3 has been linked to the severe muscle disease Native American myopathy (NAM) (1, 2). Expression of STAC3 is restricted to skeletal muscle, where STAC3 associates with the voltage-gated calcium channel CaV1.1 and is involved in mediating voltage-induced calcium release from the sarcoplasmic reticulum (3, 4). In nonmuscle cells, STAC3 was shown to facilitate functional membrane expression of CaV1.1 and alter the current properties of CaV1.2 (5), suggesting a role of STAC proteins as an L-type calcium channel (CaV1) regulator. STAC3 belongs to a family of adaptor proteins that comprises two additional isoforms, STAC1 and STAC2, which are highly expressed in the brain (1).
All three STAC proteins contain one C1 domain and two SH3 protein interaction domains (6). We previously demonstrated that the stable association of STAC3 to the CaV1 channel complex in the triads of skeletal muscle relies on the C1 domain (7). In contrast, the NAM mutation, which impairs EC coupling and is located in the SH3-1 domain of STAC3 (2–4), did not abolish the interaction with the calcium channel (2, 4, 7). We therefore proposed that STAC3 establishes multiple interactions: one through the C1 domain, responsible for the stable anchoring of STAC3 to the channel, and another one through the SH3-1 domain, involved in EC coupling (7). Using X-ray crystallography, we recently identified the II-III loop of CaV1 channels as the SH3-1 binding domain. Mutation of the three amino acids of CaV1.1 critical for this interaction resulted in impaired EC coupling, similarly to the effects of introducing the NAM mutation in the SH3-1 domain (6). However, the molecular domain of CaV1 channels responsible for the interaction with the C1 domain of STAC proteins remained to be discovered.
Here, we used a systematic domain swapping and mutagenesis approach to identify this STAC interaction site in CaV1.2. Surprisingly the IQ domain in the C terminus of CaV1.2 turned out to be essential for the association of STAC proteins, and the critical residues for STAC association overlap with those known to interact with the C-lobe of calcium-calmodulin (Ca/CaM) and to mediate calcium-dependent inactivation (CDI). Accordingly, our electrophysiological analysis of inactivation properties showed that STAC proteins inhibit CDI of CaV1.2, but not of CaV2.1 or of a CaV1.2 chimera containing the IQ domain of CaV2.1. These results suggest that neuronal STAC proteins represent a class of L-type calcium channel (CaV1) regulators, which modulate negative feedback regulation of L-type calcium channels by interfering with the functional interaction of CaM with the IQ domain in the C terminus of CaV1.2.
Results
The Proximal C Terminus of CaV1.2 Is Crucial for the Interaction with STAC Proteins.
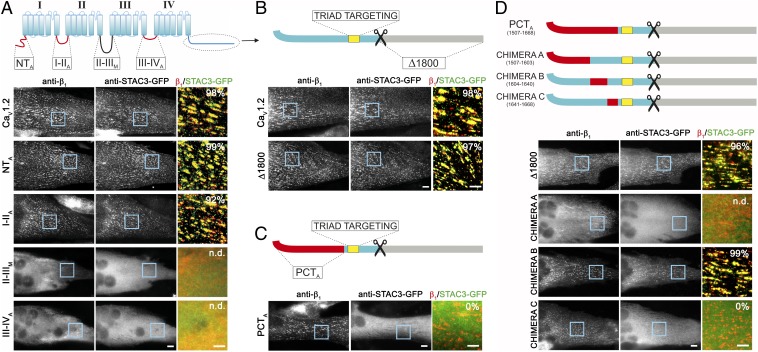
In dysgenic (CaV1.1−/−) myotubes, heterologously expressed CaV1 channel are targeted to junctions between the sarcoplasmic reticulum and the plasma membrane or T-tubules, henceforth collectively referred to as triads, resulting in a characteristically clustered distribution pattern (Fig. 1A). In the absence of a CaV1 subunit, heterologously expressed STAC-GFP proteins are diffusely distributed throughout the cytoplasm of the myotubes (5, 7). Upon reconstitution of dysgenic myotubes with CaV1.2, all three STAC isoforms, to different extents, redistributed from the cytoplasm to the triads, indicating that STAC proteins form complexes with CaV1.2 (7). Recently we identified the C1 domain of STAC3 as crucial interaction site with CaV1.2 (7). Here we set out to identify the corresponding interaction site in CaV1.2.
Fig. 1.
The PCT of CaV1.2 is crucial for the interaction with STAC3. (A–D, Upper) Cartoons showing the cytosolic domains of CaV1.2 (blue) substituted with the corresponding regions of CaV2.1 (N terminus, I-II loop, III-IV loop, PCT; red) or of the Musca channel (II-III loop; black). The CaV1.2 triad targeting sequence (yellow) is maintained in all chimeras. (Lower, Right) Representative immunofluorescence images of dysgenic myotubes expressing the indicated chimeric CaV1.2 channel and STAC3-GFP. (Scale bars, 10 μm.) (Lower, Right) Color overlay of CaVβ1a (red) and STAC3-GFP staining (green); 4× magnification of regions indicated by blue rectangle. (Scale bars, 5 μm.) The numbers indicate the percentage of myotubes in which STAC3-GFP was coclustered with the channel chimera (n = 3, n = 90). (A) STAC3-GFP showed triad targeting in the majority of the dysgenic myotubes expressing CaV1.2, CaV1.2-NTA or CaV1.2-I-IIA. CaV1.2-II-IIIM, and CaV1.2-III-IVA failed to localize in the triads of the myotubes, precluding analysis of STAC3-GFP association (n.d.). STAC3-GFP colocalized also with CaV1.2-Δ1800 (B), but not in myotubes expressing CaV1.2-PCTA (C), indicating that the PCT of CaV1.2 is crucial for the interaction with STAC proteins. (D) C-terminal chimera A was not targeted to the triads, preventing analysis of association of STAC3-GFP. Chimera B was targeted to the triads and STAC3-GFP colocalized in clusters, similarly to the control CaV1.2-Δ1800. Chimera C was targeted to the triads; however, it failed to recruit STAC3-GFP to the channel complex, indicating that amino acids 1,641–1,668 are crucial for the STAC interaction.
CaVα1 subunits contain five large cytoplasmic domains representing putative binding sites for cytoplasmic modulator and effector proteins: namely, the N terminus; the I-II, II-II, III-IV loops; and the C terminus (8, 9). We hypothesized that if any of these cytoplasmic regions of CaV1.2 contains sequences essential for the binding of STAC proteins, replacing it with the corresponding sequences of distantly related calcium channels would lead to loss of STAC association. Therefore, we constructed chimeras in which each of the cytoplasmic domains of CaV1.2 was replaced with the corresponding region of the neuronal CaV2.1 channel. Because the II-III loop of CaV2.1 is about three times longer than that of CaV1.2, a chimera with the II-III loop of the domestic fly channel (Musca domestica), which is very dissimilar in sequence but similar in size, was constructed instead (10) (Fig. 1A, Upper).
To analyze the incorporation of STAC proteins into the calcium channel complex, we coexpressed the channel chimeras with STAC3-GFP, the isoform that previously showed the highest degree of colocalization with CaV1.2 in dysgenic myotubes (7). We labeled STAC3-GFP with a GFP antibody and the channel chimeras with an antibody against the β1a subunit, as this antibody combination could be applied to all channel constructs. The two chimeras in which either the N terminus or the I-II loop of CaV1.2 was replaced by that of CaV2.1 (CaV1.2-NTA and CaV1.2-I-IIA) were localized in the triads of the myotubes, as expected. STAC3-GFP coclustered with the channel complex in the great majority of the myotubes (99 ± 1% for CaV1.2-NTA and 92 ± 1% for CaV1.2-I-IIA), similarly to the wild-type CaV1.2 (98 ± 1%), indicating that these CaV1.2 intracellular domains are not essential for the association with STAC proteins (Fig. 1A). Unfortunately, the chimeras in which the II-III loop was replaced with that of the Musca channel (CaV1.2-II-IIIM) or the III-IV loop with that of CaV2.1 (CaV1.2-III-IVA) failed to target to the triads of the myotubes (Fig. 1A). Electrophysiological analysis revealed that CaV1.2-II-IIIM was not functionally expressed, and that the currents of CaV1.2-III-IVA were dramatically reduced compared with CaV1.2 (Fig. S1 and Table S1). Because CaV1.2-II-IIIM and CaV1.2-III-IVA were not effectively targeted to the triads, the potential involvement of these cytoplasmic loops in the association of CaV1.2 with STAC proteins could not be assessed using this approach.
Examining the importance of the large C terminus for the interaction with STAC proteins required a more elaborate approach. Because the C terminus is subjected to proteolytic cleavage (11), we first examined the importance of the distal C terminus for STAC3-GFP binding. The C-terminally truncated CaV1.2-Δ1800 localized in the triads of dysgenic myotubes and STAC3-GFP colocalized in clusters in the great majority of the myotubes (97 + 1%), indicating that the distal C terminus is dispensable for the interaction with STAC proteins (Fig. 1B). Next, we replaced the proximal C-terminal (PCT) region with the corresponding region of CaV2.1, yet sparing the triad targeting signal (amino acids 1,681–1,700) (12). As expected, this chimera, CaV1.2-PCTA, was localized in the triads of the myotubes. However, STAC3-GFP failed to associate with this chimeric channel and remained diffusely distributed throughout the cytoplasm (Fig. 1C). Thus, the PCT of CaV1.2 contains specific sequences that are essential for the association of STAC with the calcium channel.
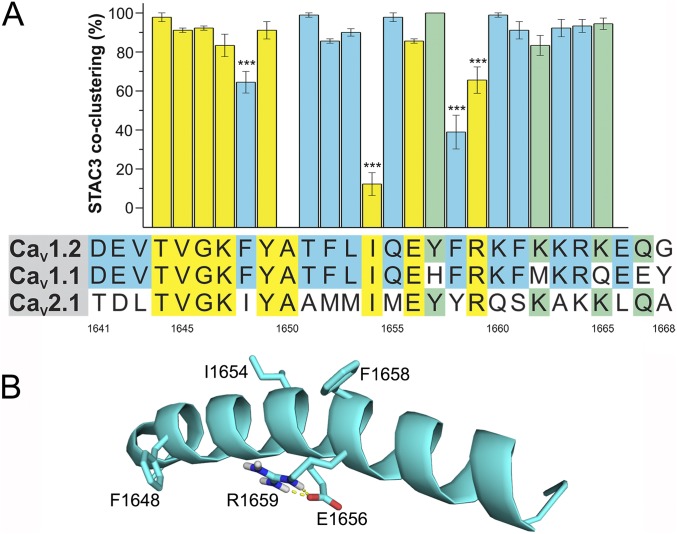
STAC Proteins Interact with the IQ Domain (1,641–1,668) of CaV1.2.
To narrow down the sequence in the PCT of CaV1.2 critical for STAC association, we further subdivided CaV1.2-PCTA (1,507–1,668) into three smaller regions and replaced them with the corresponding sequences of CaV2.1 (Fig. 1D): region A (1,507–1,603), which forms a conserved structural unit (13); region B (1,604–1,640), containing the pre-IQ domain and extending to the end of the currently solved calcium channel structure (13); and region C (1,641–1,668), the remaining 28 amino acids containing the IQ CaM binding domain. Chimera A failed to target into the triads of the myotubes, precluding analysis of its potential role in STAC3-GFP interactions (Fig. 1D). Chimera B was targeted to the triads, and STAC3-GFP associated with it at a level (99 ± 1%) comparable to the wild-type CaV1.2-Δ1800 channel (96 ± 2%). Thus, an involvement of this sequence in CaV1.2-STAC association can be excluded. Most importantly, chimera C was correctly targeted to the triads, but STAC3-GFP failed to associate with the channel complex in all analyzed myotubes (0 ± 0%).
To examine whether the importance of this CaV1.2 sequence (amino acids 1,641–1,668) is specific for STAC3 or it is also essential for recruiting other STAC isoforms into the channel complex, we tested STAC1-GFP association to chimera C. While STAC1-GFP colocalized in clusters with CaV1.2-Δ1800 in 70 ± 3% of the differentiated myotubes, it completely failed to associate with chimera C (0 ± 0%), but remained diffusely localized in the cytoplasm (Fig. S2). Thus, binding to the C-terminal 1,641–1,668 sequence may be a general mechanism for the association of STAC proteins to CaV1.2.
STAC3 Recruitment into the CaV1.2 Channel Complex Requires Conserved and Nonconserved Residues of the IQ Motif.
The 28-amino acid residues of CaV1.2 that in chimera C were replaced by the corresponding sequence of CaV2.1 contain the IQ domain (1,644–1,665) flanked on each side by three additional amino acids (Fig. 2A). To identify the residues that are important for interacting with STAC proteins, we performed a systematic alanine scanning mutagenesis of the 22 amino acids comprising the IQ domain and analyzed whether mutation of individual residues affected the ability of STAC3-GFP to associate with CaV1.2-Δ1800 clusters in the triads of dysgenic myotubes (Fig. 2A and Table S2). When we mutated the residues that are conserved between CaV1.1 and CaV1.2, both of which associate with STAC3 but are different in CaV2.1, which does not associate with STAC (in blue in Fig. 2A), we identified two amino acid substitutions that significantly reduced the STAC3-GFP coclustering compared with the wild-type channel (96 ± 1%): that is, F1658 (39 ± 9%) and F1648 (64 ± 6%). Conversely, mutation of the residues that are not conserved between CaV1.1 and CaV1.2 (in green in Fig. 2A) had no effect on STAC3 association. When we extended the mutagenesis to the residues that are conserved among all three calcium channels (in yellow in Fig. 2A), we identified two additional mutations that significantly reduced the ability of STAC3-GFP to associate with the calcium channel, I1654 (12 ± 6%) and R1659 (66 ± 7%). Because I1654 and R1659 are conserved both in calcium channels that recruits STAC3 (CaV1.1 and CaV1.2) and in a calcium channel that does not (CaV2.1), these residues are important for STAC association but do not contribute to the channel specificity of the interaction. F1658 and F1648, on the other hand, are conserved only in STAC interacting channels and therefore may determine the specificity of the interaction.
Fig. 2.
Identification of the amino acid residues crucial for the interaction with STAC proteins. (A) Amino acid sequences of the IQ domain of CaV1.2, CaV1.1, and CaV2.1: yellow, residues conserved in all three paralogs; green, conserved between CaV1.2 and CaV2.1; blue, conserved between CaV1.2 and CaV1.1. Numbers indicate the amino acid position of CaV1.2. The bar graph shows the percentage of transfected myotubes in which STAC3-GFP coclustered with the CaV1.2 IQ point mutant. Values obtained for each point mutation are indicated in Table S1. ANOVA F(21, 49) = 32.41, P < 0.0001. The three asterisks (***) represent values for Tukey post hoc analysis. (B) Position of F1648A, I1654A, F1658A, and R1659A in the secondary structure of the IQ domain of CaV1.2 (PDB ID code 2BE6).
Coexpression of STAC3 Abolishes CDI in CaV1.2.
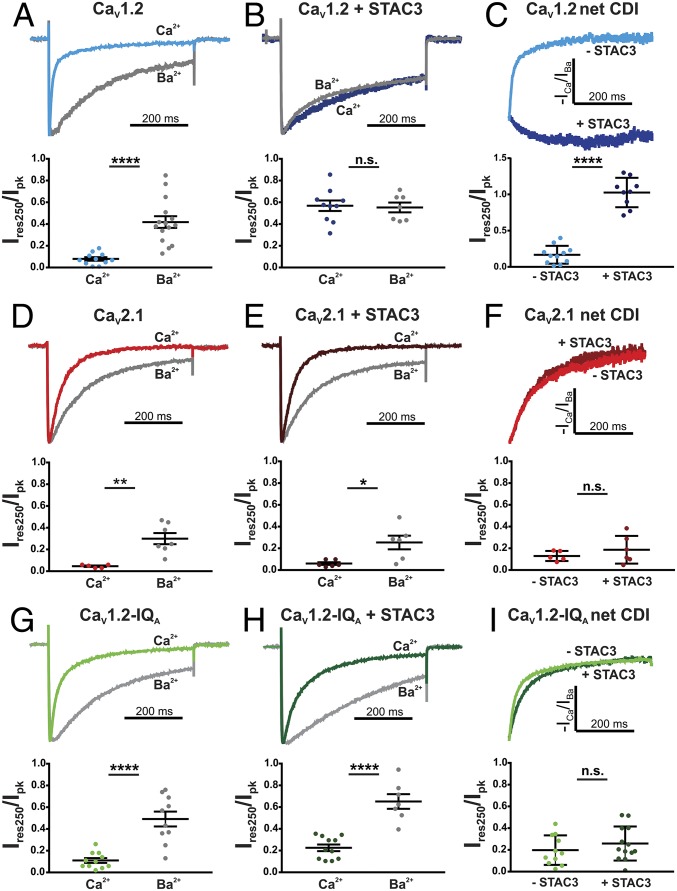
Earlier X-ray crystallography demonstrated that the IQ domain of CaV1.2 forms an α-helix (14). The two residues, I1654 and F1658, mutation of which caused a larger decrease in STAC3 association are positioned side-by-side in adjacent turns of the helix and oriented in the same direction. In contrast, the two residues, F1648 and R1659, mutation of which caused a milder decrease of STAC3 association, are oriented in the opposite direction. Structure modeling suggests that R1659 stabilizes the helical domain structure by an intramolecular interaction with E1656 (Fig. 2B). Importantly, several earlier studies demonstrated that the two crucial residues for STAC association, I1654 and F1658, make contacts with the C-lobe of Ca/CaM and are thus important for its function in CDI (14–16). Therefore, we hypothesized that association of STAC proteins with the CaV1.2 C terminus may interfere with binding of CaM or its allosteric effects on channel conformation, and thereby prevent CDI. To test this hypothesis, we analyzed the inactivation properties of CaV1.2 currents expressed in the absence and presence of STAC3 in tsA201 cells using whole-cell patch-clamp recordings.
First, we compared current inactivation with calcium and barium as charge carriers. As previously shown, in cells transfected with CaV1.2, calcium currents show fast inactivation kinetics and are strongly reduced at the end of a 500-ms depolarization step, whereas barium currents showed significantly slower and weaker inactivation (Fig. 3A) (15, 17). The inactivation measured with barium as a charge carrier represents voltage-dependent inactivation; the additional component with calcium as a charge carrier is CDI and depends on the interaction of Ca/CaM with the IQ domain of CaV1.2 (18). Inactivation in calcium and barium was quantitatively assessed as the residual current amplitude after 250 ms of the 500-ms voltage step normalized to the peak current (Ires250/Ipk). Thus, CDI of CaV1.2 is evident in the significant difference in Ires250/Ipk between calcium and barium currents (P < 0.0001) (Fig. 3A and Table S3). Notably, upon coexpression of STAC3, this difference in inactivation in calcium and barium was completely abolished (P = 0.82) (Fig. 3B), indicating that in the presence of STAC3 the remaining inactivation of CaV1.2 currents is purely voltage-dependent inactivation. Coexpression of STAC3 had no significant effect on the voltage-dependence of current activation (Fig. S3 and Table S4). To directly compare net CDI of CaV1.2 in the presence and absence of STAC3, we calculated the quotient of the normalized ICa and mean IBa over time (19, 20) (Fig. 3C). This analysis clearly demonstrates the robust CDI of CaV1.2 in the absence of STAC3 and its complete loss upon coexpression of STAC3 (P < 0.0001). This effect is reminiscent of the effects of CaV1.2 mutations affecting CaM binding (15) or of dominant-negative CaM mutants (17), and thus indicates a role of STAC3 in blocking the function of CaM in CaV1.2.
Fig. 3.
STAC3 coexpression abolishes CDI of CaV1.2 but not of CaV2.1 in an IQ domain dependent manner. Representative normalized calcium and barium currents at Vmax of tsA201 cells expressing CaV1.2 (A), CaV2.1 (D), or CaV1.2-IQA (G). Internal calcium buffering with 0.5 mM EGTA. Significant differences in the relative residual current amplitude after 250 ms (Ires250/Ipk) in calcium and barium indicate robust CDI (****P < 0.0001 for CaV1.2; **P = 0.002 for CaV2.1; ****P < 0.0001 for CaV1.2-IQA). (B) Upon coexpression of STAC3, the CDI of CaV1.2 currents was abolished, as in calcium and barium currents inactivated to the same extent and Ires250/Ipk were comparable (n.s., P = 0.82). In contrast, CDI of CaV2.1 and CaV1.2-IQA was unaffected by coexpression of STAC3 (*P = 0.012 and ****P < 0.0001, respectively) (E–H). Representative normalized net CDI (−ICa/IBa) of CaV currents in the absence or the presence of STAC3 and relative Ires250/Ipk demonstrate that STAC3 coexpression abolishes the CDI of CaV1.2 (****P < 0.0001) (C), but not of CaV2.1 and CaV1.2-IQA (n.s., P = 0.32 and P = 0.37, respectively). (F–I) P values are from unpaired t test.
In contrast to CaV1.2, STAC3 does not associate with the C terminus of CaV2.1 (Fig. 1C). Therefore, we expected that coexpression of STAC3 and CaV2.1 will not block CDI of CaV2.1 currents. Indeed, calcium and barium currents recorded in the absence or presence of STAC3 showed a similar degree of inactivation (Fig. 3 D and E). The mean residual currents Ires250/Ipk of calcium and barium did not differ in the absence (P = 0.002) or in the presence of STAC3 (P = 0.012). In addition, analysis of net CDI of CaV2.1 showed no significant difference in Ires250/Ipk with and without STAC3 (P = 0.32) (Fig. 3F), demonstrating that STAC3 has no effect on CDI of CaV2.1 currents.
To examine whether inhibition of CDI of CaV1.2 currents depends on the specific interaction of STAC3 with the CaV1.2 IQ domain, we constructed a CaV1.2-IQA chimera, in which the IQ domain of the full-length C terminus of CaV1.2 has been substituted with the corresponding sequence of CaV2.1. If our hypothesis was correct, this sequence substitution should abolish the effect of STAC3 on the CDI of CaV1.2. We first examined whether CaM is still able to bind to this IQ motif chimera and cause inactivation. This was the case, as in the absence of STAC3, we observed a significant difference in Ires250/Ipk between calcium and barium currents (P < 0.0001) (Fig. 3G). Most importantly, upon coexpression of STAC3 the sizable difference between calcium and barium currents was maintained (P < 0.0001) (Fig. 3H), indicating that, as hypothesized, STAC3 did not abolish CDI in the CaV1.2-IQA chimera. Furthermore, comparison of the net CDI of CaV1.2-IQA currents in the presence or the absence of STAC3 revealed no significant difference (P = 0.37) (Fig. 3I). Together with our microscopy association analysis, this demonstrates that both the association of STAC3 and CaV1.2 in the triads of dysgenic myotubes and the STAC3-induced suppression of CDI in CaV1.2 currents expressed in tsA201 cells depend on the integrity and specificity of the IQ domain.
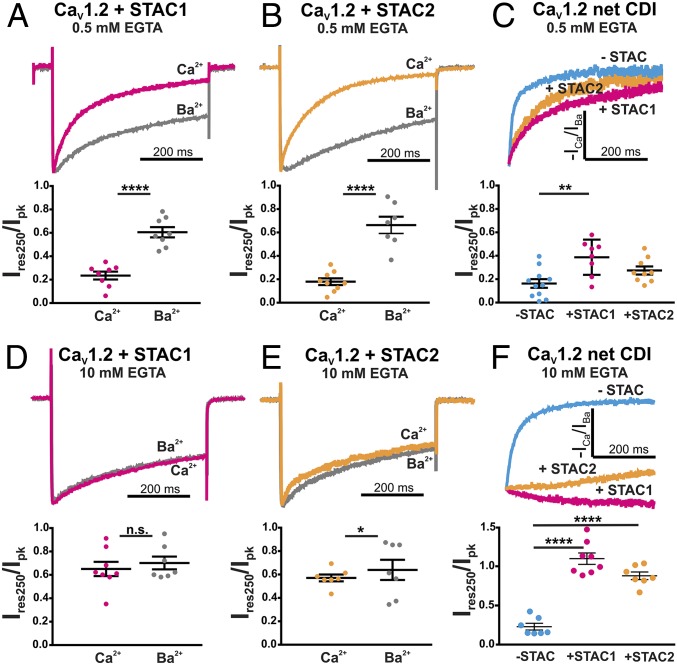
Neuronal STAC1 and STAC2 Isoforms Reduce CDI of CaV1.2 Calcium Currents.
So far we tested the effect of STAC3 on the inactivation of CaV1.2, because it most potently associates with CaV1.2, as evidenced by its superior recruitment to CaV1.2 clusters in the triads of dysgenic myotubes (7), although CaV1.2 and STAC3 are probably not expressed together in native tissue (1). Conversely, STAC1 and STAC2 are expressed in a variety of tissues, including the brain, where CaV1.2 is also abundant. We therefore addressed the question as to whether the neuronal isoforms, STAC1 and STAC2, are also able to oppose CDI of CaV1.2. When recorded with mild cytoplasmic calcium buffering (0.5 mM EGTA), coexpression of STAC1 or STAC2 in tsA201 cells did not result in a total loss of CDI, as significant differences in Ires250/Ipk were detected between calcium and barium currents in the presence of both STAC1 and STAC2 (Fig. 4 A and B and Table S3) (P < 0.0001). Nevertheless, when comparing the net CDI of CaV1.2 calcium currents in the absence or presence of STAC1 or STAC2, it became evident that CDI was reduced and this reduction of Ires250/Ipk was significant in the case of STAC1 (P = 0.029) (Fig. 4C). Further analysis of the kinetics of calcium current inactivation demonstrated that coexpression of either STAC1 or STAC2 caused a significant decrease in the fraction of the fast component of inactivation (P = 0.0001) (Fig. S4A), without significantly shifting the voltage dependence of activation (Fig. S4B and Table S4). When we repeated this experiment with higher internal calcium buffering (10 mM EGTA), coexpression of STAC1 resulted in a complete loss of CDI, and coexpression of STAC2 in a nearly complete loss of CDI (Fig. 4 D–F and Table S3), without significantly shifting the voltage dependence of activation (Fig. S4D and Table S4). Together, these results demonstrate that STAC1 and STAC2 also interfere with CDI of CaV1.2 channels, although at a somewhat lower degree compared with STAC3, and that the extent of the inhibition depends on the internal calcium buffering capacity of the cell. The lower extent of this functional effect correlates with the lower ability of STAC1 and STAC2 to associate with the CaV1.2 complex (7).
Fig. 4.
STAC1 and STAC2 impede CDI of CaV1.2 currents. Representative normalized calcium and barium CaV1.2 currents in the presence of STAC1 (A) or STAC2 (B) using mild intracellular calcium buffering (0.5 mM EGTA). Significant differences in Ires250/Ipk in calcium and barium indicate CDI (****P < 0.0001, unpaired t test). (C) Analysis of the net CDI (−ICa/IBa) of CaV1.2 indicates that with 0.5 mM EGTA in the internal solution coexpression of STAC1 and STAC2 slows down CDI to different extents. ANOVA F(2, 24) = 7.44; P = 0.0029; P values in the figure are from Tukey post hoc analysis, **P = 0.002. (D) With strong intracellular calcium buffering (10 mM EGTA) coexpression of STAC1 resulted in loss of CDI, as the calcium and barium currents inactivated similarly and Ires250/Ipk were not significantly different (n.s., P = 0.70). (E) Coexpression of STAC2 strongly reduced CDI (*P = 0.02). (F) Representative normalized net CDI of CaV currents and relative Ires250/Ipk demonstrate that STAC1 abolishes and STAC2 severely reduces the CDI of CaV1.2. ANOVA F(2, 19) = 1.319; P < 0.0001; P values in the figure are from Tukey post hoc analysis, ****P < 0.0001.
Finally, we examined whether CDI of CaV2.1 currents is unaffected by STAC1 coexpression, as it is by STAC3. Indeed, the difference between CaV2.1 calcium and barium currents was not diminished by coexpression of STAC1 and the calculated net CDI of CaV2.1 was not significantly different with and without STAC1 (Fig. S5 and Tables S3 and S4). Thus, muscle and neuronal STAC isoforms specifically alter CDI of CaV1.2, but not that of CaV2.1. Due to its efficient interaction with the CaV1.2 IQ domain, STAC3 completely abolishes CDI, whereas the weaker interactions of the STAC1 and STAC2 isoforms essentially slow down CaV1.2 current inactivation kinetics to different degrees, depending on the internal calcium buffering.
Discussion
The results of our study demonstrate that: (i) amino acids 1,641–1,668 in the C terminus of CaV1.2 are essential for the recruitment of STAC proteins to the calcium channel when expressed in the triads of dysgenic myotubes; (ii) this sequence contains the IQ CaM interaction domain and that the critical residues responsible for interaction with STAC proteins overlap with those known to bind the C-lobe of CaM; (iii) that, in variance to the interactions with CaM, the IQ/STAC interaction is specific for the family of CaV1 channels; (iv) STAC proteins inhibit CDI of CaV1.2, but not of CaV2.1, and substitution of the CaV1.2 IQ domain with that of CaV2.1 abolishes this effect; and (v) the potency of STAC/CaV1.2 association and of quenching CDI differs between the three STAC isoforms congruently.
The importance of the CaV1.2 IQ domain for the interaction with STAC proteins is established by its essential role in recruiting STAC to the triads and in inhibition of CDI in response to STAC coexpression. This twofold effect is not surprising, considering the known role of this IQ motif as the binding site for the effector of CDI, the C-lobe of the calcium sensor CaM (14), and considering our finding that STAC and CaM interactions depend on the same amino acids in the IQ motif (Fig. 2). However, two of the four crucial residues for STAC association are not conserved in CaV2 channels and, consequently, the STAC/IQ interaction is specific for CaV1 channels. Thus, we identified STAC proteins as specific modulators of L-type calcium channels.
In a previous study we demonstrated the importance of the C1 domain of STAC proteins for their recruitment to the channel complex in dysgenic myotubes (7). Furthermore, CDI of CaV1.2 can be abolished by a truncated STAC3 containing the C1 domain but lacking the SH3 domains (6). Thus, it is likely that the C1 domain in STAC proteins and the IQ domain in CaV1 channels are the corresponding sites responsible for the association of STAC with the channel complex in the triads and for interference with CDI. This, however, does not imply that the IQ domain is the only site in CaV1 channels involved in interactions with STAC proteins. For example, additional interaction sites may reside in any of the three cytoplasmic sequences (II-III loop, III-IV loop, PCT), which were unamenable for testing in our triad targeting assay. Indeed, in a recent X-ray analysis study, we solved the structure of tandem-SH3 domains of STAC in complex with the II-III loop of CaV1.1. Introducing the NAM mutation in the SH3-1 domain or mutations in the corresponding binding site of the II-III loop both abolished the interaction and impaired EC coupling (6). However, we and others previously showed that the NAM mutation in STAC3 did not prevent STAC3-CaV1.1 association in skeletal myotubes (2–4, 7). Moreover, our present results demonstrate that in the absence of the CaV1.2 IQ domain possible additional STAC binding site are not sufficient for recruiting heterologously expressed STAC proteins into the triads of dysgenic myotubes. Therefore, the interaction of STAC3 and CaV1.1 seems to depend on at least two distinct sites with different functions. The C1/IQ interaction is crucial for the stable association of the two proteins in skeletal muscle triads and for the modulation of inactivation properties of CaV1.2 currents, whereas the SH3-1/II-III loop interaction is of functional importance for EC coupling.
The molecular mechanism by which STAC proteins modulate CDI in CaV1.2 requires further investigation. Because STAC association to CaV1.2 and CaM C-lobe–mediated CDI depend in part on the same IQ motif residues, STAC might block CDI by competing with CaM for the same binding site. Because both calcium-free and calcium-occupied CaM can bind to the IQ domain, blocking either interaction may be sufficient for abolishing CDI. Alternatively, the high affinity of Ca/CaM binding to the IQ domain (21) favors the possibility that STAC proteins interact with the Ca/CaM/IQ domain complex at the C terminus of CaV1.2, thus preventing the conformational changes that trigger CDI. We further observed that the three STAC isoforms oppose CDI of CaV1.2 currents in tsA201 cells to different degrees and that the rank order of how effectively each STAC isoform inhibited CDI (STAC3 > STAC1 > STAC2) correlated well with the efficiency at which they were recruited to the CaV1.2 channel complex in myotubes (7). Thus, association of STAC proteins at the IQ domain with different affinities may explain their different potencies to interfere with CaM modulation.
Although we mapped the interaction site of STAC proteins using CaV1.2, several lines of evidence suggest that the IQ domain is also required for association of STAC3 with CaV1.1 and that this might be of functional importance in skeletal muscle. First, while we demonstrated that STAC3 interacts with CaV1.1 as stably as the β1a subunit (7), the measured affinity of the STAC SH3-1/II-III loop interaction is more than 100-fold weaker, suggesting that this site alone would not be sufficient for a stable interaction (6). Second, the four IQ residues critical for the STAC interaction are conserved among the IQ domains of CaV1.1 and CaV1.2, and mutation of three residues not conserved between these two isoforms did not weaken the STAC3/CaV1.2 complex (Fig. 2). Therefore, it is likely that in skeletal muscle triads STAC3 association at the IQ motif of CaV1.1 interferes with CaM function.
The notion that in skeletal muscle triads STAC3 association with the C terminus of CaV1.1 prevails over CaM function is consistent with the essential functional role of STAC3 in EC coupling, as well as with the lacking inactivation of skeletal muscle calcium currents (22). Conversely, the earlier observation showing that mutation of the CaV1.1 IQ motif to alanines (IQAA) resulted in the loss of EC coupling (23) could readily be explained by its disruptive effect on the association of STAC3 with CaV1.1. Because in skeletal muscle L-type calcium currents are limited by several other mechanisms (24), negative feedback regulation of calcium influx by CDI may be irrelevant. Finally, interference of STAC3 with CaM function in skeletal muscle may also explain why inactivation kinetics of CaV1.2 are dramatically reduced when it is expressed in skeletal myotubes, which endogenously express STAC3 (25–29).
Interestingly, cardiac muscle, in which CaV1.2 is of central importance, does not express STAC proteins (1). In this tissue, limiting calcium influx by CDI appears to be of major importance, as mutations in CaV1.2 and auxiliary subunits that prolong the activation of the cardiac channel result in arrhythmias, long QT disease, and Timothy syndrome (30). Interference with CDI by competing STAC proteins would be expected to exert similar undesired effects of prolongation of calcium currents.
In contrast, modulation of CDI in L-type calcium channels may be desirable in other excitable cells, including neurons. There, Ca/CaM-dependent regulation of presynaptic CaV2 channels is subject to modulation by a variety of calcium-binding proteins, which compete for the same interaction domain (31). Similarly, STAC proteins could serve the complementary function by acting as modulators of CDI on the postsynaptic CaV1 channels. Indeed, the expression patterns of STAC1 and STAC2 overlap with those of CaV1.2 in several areas of the brain, as well as in smooth muscle and adrenal cells (1). Their weaker association with CaV1.2 and their incomplete block of CDI compared with STAC3 is consistent with such a modulatory function, allowing inactivation of CaV1.2 to be tuned by both the expression levels of STAC1 or STAC2 and by the specific calcium buffering capacity in different neuronal cell types. In conclusion, the identification of the IQ domain of CaV1.2 as a crucial determinant for binding STAC proteins, and the observation that STAC proteins differentially oppose to CaV1.2 CDI suggest a role of STAC proteins as CaV1 regulators and adds them to the diverse group of proteins that tune the calcium entry in excitable cells.
Materials and Methods
Cloning Procedures.
A detailed description of the constructs used is available in the SI Materials and Methods.
Immunostaining and Image Processing.
Dysgenic myotubes were cultured, transfected and labeled as previously described (7). A detailed description is available in the SI Materials and Methods.
Electrophysiological Recordings and Data Analysis.
tsA201 cells expressing β3 and α2δ-1 were cultured as previously described (32). A detailed description of whole-cell patch clamp recordings and data analysis is available in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Stefania Monteleone for help with molecular modeling; Dr. Jörg Striessnig for helpful discussion; and Ariane Benedetti, Martin Heitz, and Katharina Heinz for excellent technical assistance. This work was supported by Austrian Science Fund (FWF) Grants T855 (to M.C.), W1101 and P27031 (to B.E.F.), and P27809 (to N.O.), and by the RYR-1 Foundation (F.V.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1715997115/-/DCSupplemental.
References
- 1.Nelson BR, et al. Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc Natl Acad Sci USA. 2013;110:11881–11886. doi: 10.1073/pnas.1310571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horstick EJ, et al. Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat Commun. 2013;4:1952. doi: 10.1038/ncomms2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linsley JW, et al. Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3. Proc Natl Acad Sci USA. 2017;114:E228–E236. doi: 10.1073/pnas.1619238114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polster A, Nelson BR, Olson EN, Beam KG. Stac3 has a direct role in skeletal muscle-type excitation-contraction coupling that is disrupted by a myopathy-causing mutation. Proc Natl Acad Sci USA. 2016;113:10986–10991. doi: 10.1073/pnas.1612441113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polster A, Perni S, Bichraoui H, Beam KG. Stac adaptor proteins regulate trafficking and function of muscle and neuronal L-type Ca2+ channels. Proc Natl Acad Sci USA. 2015;112:602–606. doi: 10.1073/pnas.1423113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong King Yuen SM, Campiglio M, Tung CC, Flucher BE, Van Petegem F. Structural insights into binding of STAC proteins to voltage-gated calcium channels. Proc Natl Acad Sci USA. 2017;114:E9520–E9528. doi: 10.1073/pnas.1708852114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campiglio M, Flucher BE. STAC3 stably interacts through its C1 domain with CaV1.1 in skeletal muscle triads. Sci Rep. 2017;7:41003. doi: 10.1038/srep41003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campiglio M, Flucher BE. The role of auxiliary subunits for the functional diversity of voltage-gated calcium channels. J Cell Physiol. 2015;230:2019–2031. doi: 10.1002/jcp.24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabner M, et al. Insect calcium channels. Molecular cloning of an alpha 1-subunit from housefly (Musca domestica) muscle. FEBS Lett. 1994;339:189–194. doi: 10.1016/0014-5793(94)80413-3. [DOI] [PubMed] [Google Scholar]
- 11.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakada T, et al. The proximal C-terminus of α(1C) subunits is necessary for junctional membrane targeting of cardiac L-type calcium channels. Biochem J. 2012;448:221–231. doi: 10.1042/BJ20120773. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, et al. Structure of the voltage-gated calcium channel Ca(v)1.1 at 3.6 Å resolution. Nature. 2016;537:191–196. doi: 10.1038/nature19321. [DOI] [PubMed] [Google Scholar]
- 14.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–1115. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zühlke RD, Pitt GS, Tsien RW, Reuter H. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the(alpha)1C subunit. J Biol Chem. 2000;275:21121–21129. doi: 10.1074/jbc.M002986200. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Johny M, Yue DT. Calmodulin regulation (calmodulation) of voltage-gated calcium channels. J Gen Physiol. 2014;143:679–692. doi: 10.1085/jgp.201311153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ -dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- 18.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 19.Barrett CF, Tsien RW. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type calcium channels. Proc Natl Acad Sci USA. 2008;105:2157–2162. doi: 10.1073/pnas.0710501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Findeisen F, Minor DL., Jr Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. J Gen Physiol. 2009;133:327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Findeisen F, Rumpf CH, Minor DL., Jr Apo states of calmodulin and CaBP1 control CaV1 voltage-gated calcium channel function through direct competition for the IQ domain. J Mol Biol. 2013;425:3217–3234. doi: 10.1016/j.jmb.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bannister RA, Beam KG. Ca(V)1.1: The atypical prototypical voltage-gated Ca2+ channel. Biochim Biophys Acta. 2013;1828:1587–1597. doi: 10.1016/j.bbamem.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroffekova K. The IQ motif is crucial for Cav1.1 function. J Biomed Biotechnol. 2011;2011:504649. doi: 10.1155/2011/504649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flucher BE, Tuluc P. How and why are calcium currents curtailed in the skeletal muscle voltage-gated calcium channels? J Physiol. 2017;595:1451–1463. doi: 10.1113/JP273423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Biase V, et al. Surface traffic of dendritic CaV1.2 calcium channels in hippocampal neurons. J Neurosci. 2011;31:13682–13694. doi: 10.1523/JNEUROSCI.2300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tuluc P, Kern G, Obermair GJ, Flucher BE. Computer modeling of siRNA knockdown effects indicates an essential role of the Ca2+ channel alpha2delta-1 subunit in cardiac excitation-contraction coupling. Proc Natl Acad Sci USA. 2007;104:11091–11096. doi: 10.1073/pnas.0700577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kugler G, Weiss RG, Flucher BE, Grabner M. Structural requirements of the dihydropyridine receptor alpha1S II-III loop for skeletal-type excitation-contraction coupling. J Biol Chem. 2004;279:4721–4728. doi: 10.1074/jbc.M307538200. [DOI] [PubMed] [Google Scholar]
- 28.Tanabe T, Mikami A, Numa S, Beam KG. Cardiac-type excitation-contraction coupling in dysgenic skeletal muscle injected with cardiac dihydropyridine receptor cDNA. Nature. 1990;344:451–453. doi: 10.1038/344451a0. [DOI] [PubMed] [Google Scholar]
- 29.Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–569. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- 30.Liao P, Soong TW. CaV1.2 channelopathies: From arrhythmias to autism, bipolar disorder, and immunodeficiency. Pflugers Arch. 2010;460:353–359. doi: 10.1007/s00424-009-0753-0. [DOI] [PubMed] [Google Scholar]
- 31.Catterall WA, Leal K, Nanou E. Calcium channels and short-term synaptic plasticity. J Biol Chem. 2013;288:10742–10749. doi: 10.1074/jbc.R112.411645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortner NJ, et al. Lower affinity of isradipine for L-type Ca2+ channels during substantia nigra dopamine neuron-like activity: Implications for neuroprotection in Parkinson’s disease. J Neurosci. 2017;37:6761–6777. doi: 10.1523/JNEUROSCI.2946-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.