Significance
Cell migration in multicellular organisms is crucial for embryonic development, immune responses, organ homeostasis, tissue regeneration, and tumor metastasis. However, mechanisms of cell locomotion in tissues are elusive. Here we report that single mammalian cells generate extracellular and membranous cytocapsular tubes as highways for directed cell transportation. Cytocapsular tubes interconnect and form tubular networks for directed cell transportation in multiple directions. Increased eukaryotic translation initiation factor eIF4E-mediated cap-dependent translation initiation promotes cytocapsular tube development. This study reveals that cytocapsular tubes provide membranous tubes for directed cell translocation.
Keywords: cytocapsula, cytocapsular tube, cell translocation, translation initiation, eIF4E
Abstract
Cell locomotion is essential for multicellular organism embryo development, organ homeostasis, tissue regeneration, immune responses, and tumor metastasis. Here we report that single mammalian cells can generate two extracellular membranous compartments: cytocapsulae and cytocapsular tubes. Cells migrate in cytocapsulae and engender cytocapsular tubes, which exhibit pleiotropic biological functions and provide tubular routes for directed cell transportation. Ultrastructural analysis by electron microscope revealed that nanoprotrusions surround and anchor cytocapsular tubes in place. Multiple cytocapsular tubes interconnect and form networks supporting directed cell transportation in diverse directions. Enhanced translation initiation factor eIF4E up-regulates translation of transcripts encoding proteins important for organelle development. Thus, this study proposes a mechanism of directed cell translocation in cytocapsular tubes, which may facilitate the management of diseases, including tumor metastasis.
Cell locomotion in multicellular organisms is critical for embryonic development (1–3), organ homeostasis (4), tissue regeneration (5), immunological responses (6, 7), wound repair (8), and tumor dissemination (9–11). Cells migrating in 3D microenvironments experience heterogeneous cells and extracellular matrices (ECM), which provide supporting scaffolds and guiding clues for locomotion directions but also form substantial environmental obstacles impeding cell motility (12, 13). To facilitate motility, cells adaptively generate temporospatial surface-connected organelles and compartments, including lamellipodia (14), filopodia (15), podosomes (16), invadopodia (17), blebs (18, 19), focal adhesions (20), dendritic pseudopodial protrusions, and type II epithelial bridges (21). The mechanics of cell relocation in multicellular organism are therefore unclear.
The cellular morphology, employed organelles, and mechanical and signaling control in 3D cell migration are often different from their 2D counterparts (22–24). Cells in locomotion in collagen-rich 3D matrices do not present lamellipodia or filopodia, but display highly dendritic pseudopodial protrusions, which are absent on rigid-plate surfaces (25, 26). An understanding of the mechanisms by which cells migrate in 3D microenvironments is a prerequisite for the development of pharmaceutical therapy for cell-migration–related diseases, such as tumor metastasis. We have investigated the mechanics by which cells migrate in 3D microenvironments.
Here, we report that single mammalian cells in 3D microenvironments can generate two membranous organelles of cytocapsulae and cytocapsular tubes. The cytocapsular tubes provide tubular pathways for directed cell transportation. Alloentry and ecellularization enable multiple cells to enter and leave cytocapsular tubes. Multiple cytocapsular tubes interconnect and form networks, supplying tube webs for directed cell transportation in diverse directions. Enhanced m7GTP RNA cap translation initiation increases PDGF-B and matrix metalloproteinase 3 (MMP-3) proteins, promoting cytocapsular tube elongation. This study reveals two organelles of cytocapsula and cytocapsular tubes and a mechanism of directed 3D cell transportation in cytocapsular tubes.
Results
Detection and Lifecycle of Cytocapsulae and Cytocapsular Tubes.
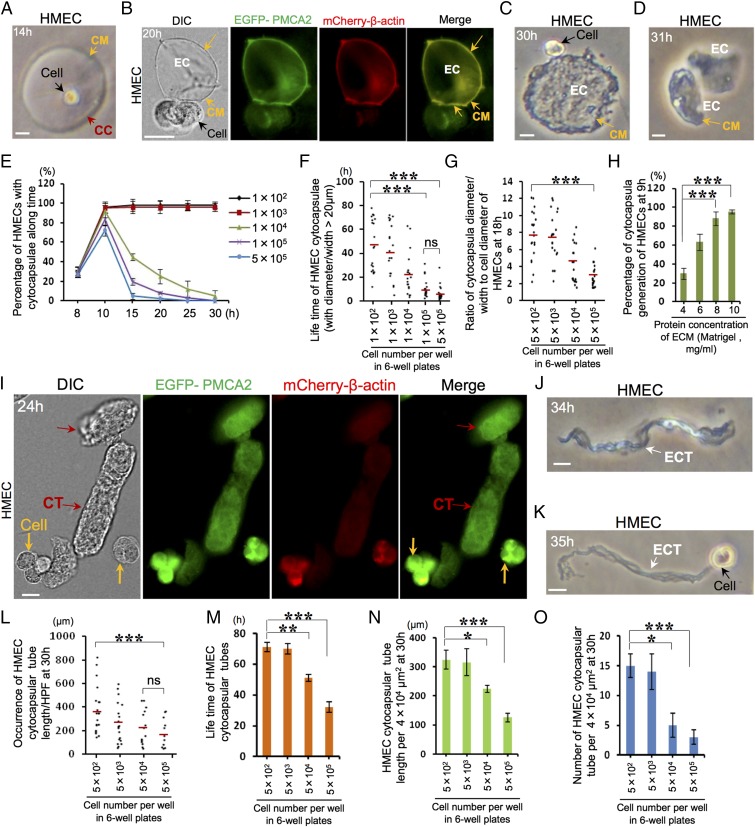
To investigate the mechanics of 3D cell locomotion, normal primary human mammary epithelial cells (HMECs) were implanted in a 3D Matrigel matrix, a reconstituted ECM surrogate. At 5 h postimplantation, HMECs in 3D Matrigel do not present flat, irregular morphologies but consistently display a small, stringently spherical morphology (Fig. S1A, Upper). At 12 h, the single spherical HMECs in 3D Matrigel, but not the cells in 2D environments, have generated round and extracellular bubble-like capsulae in variable sizes enclosing the cell (Fig. S1A, Lower Right). Next, we quantitated single cells with capsulae and found that ∼96% of single HMECs (1 × 103 cells per well in six-well plates; n = 3) engendered extracellular capsulae at 12 h (Fig. S1B). Next, we probed the development of the capsulae. Initially, at 9 h, single HMECs generated small, round, and extracellular capsulae enveloping the cell (Fig. S1C). These capsulae grow with taut membranes while cell size remains constant. At 14 h, the diameter/major axis of extracellular spherical/oval capsulae significantly increased (Fig. 1A) and reached up to 250 µm. The ratio of capsular diameter/major axis to cell diameter can reach up to 25. Subsequently, the stretched membranes of some large spherical/oval capsulae shrank, leading to slightly folded membranes, under which spherical cells had already interconverted into irregular morphologies with multiple small protrusions expanding and unfolding the local folded capsular membranes (Fig. S1D). To confirm whether the capsular surface is membranous, we transiently overexpressed in HMECs enhanced green fluorescence protein (EGFP) fusion with the plasma membrane protein Ca2+ ATPase 2 (EGFP–PMCA2). Indeed, EGFP–PMCA2 is distributed throughout both the plasma membrane of cells and extracellular capsulae membranes (Fig. S1E), which verified that the extracellular capsulae are enclosed by membranes. Over time, the single capsulae automatically ecellularized, leaving acellular and closed capsulae in multiple morphologies (spherical, oval, or irregular) with taut membranes (Fig. 1B and Fig. S1F). These observations evidenced that the capsular membranes independently locate outside the plasma membrane and that acellular capsulae can exist without the cell. We termed this previously unappreciated, single mammalian cell-generated, extracellular membranous capsula as a “cytocapsula.”
Fig. 1.
The lifecycle of cytocapsulae and cytocapsular tubes. (A–D) The lifecycle of cytocapsulae proceeds from extracellular cytocapsulae generation and growth (A), ecellularization of cytocapsulae (B), shrinkage and deflation of acellular cytocapsulae (C), to autodecomposition of acellular cytocapsulae (D). Cytocapsula (CC), cytocapsular membrane (CM; orange arrow), and ecellulated cytocapsulae (EC), are shown. (E–G) Quantitation of cytocapsula generation (n = 3) (E), lifetime (red lines indicate the mean; n = 2) (F), and growth (red lines indicate the mean; n = 2) (G). (H) Effects of ECM protein concentrations on HMEC cytocapsula generation; n = 3. (I–K) The lifecycle of cytocapsular tubes proceeds from cell migration in the cytocapsula, cytocapsular membrane deformation and cytocapsular tube formation, ecellularization, and deflation (I), shrinkage and twisting (J), to membrane degradation and autodecomposition of acellular cytocapsular tubes (K). Cell (orange arrows), cytocapsular tubes (CT; red arrows), and ecellulated cytocapsular tubes (ECT; white arrows) are shown. (L and M) Quantitation of cytocapsular tube elongation (red lines indicate the mean; n = 2) (L) and lifetime (n = 3) (M). (N and O) Quantitation of cytocapsular tube density in length (n = 3) (N) and number (n = 3) (O). (Scale bars: 10 µm.) ns, no significance, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
After ecellularization, the growth of acellular cytocapsulae terminated (Fig. S1F), strongly suggesting that cytocapsula’s generation and growth depends on the intraluminal cell. Meanwhile, the big gap/distance between the plasma membrane and extracellular capsular membrane (Fig. 1A) indicated that the capsular components originated from the intraluminal cell are released/delivered outside the plasma membrane for cytocapsular building. Subsequently, the acellular cytocapsulae continued to shrink and deflate, and the cytocapsular membranes folded, forming large, deflated, and concave discs (Fig. 1C) or short, flat tubes (Fig. S1G). After about 0.5–1 h, the acellular cytocapsulae’s membranes degraded, and cytocapsulae self-decomposed (Fig. 1D and Fig. S1 H and I). These observations suggested that the lifecycle of a cytocapsula proceeds through several successive and distinct phases: from initiation, growth, ecellularization, deflation and shrinkage, membrane degradation, to autodecomposition.
Cells in 3D environments in vivo are surrounded by ECM and other cells. Thus, we next investigated the time dependence of cytocapsula generation and growth in cellular crowded microenvironments at increasing cell densities. From 8–10 h, the average percentages of HMECs with cytocapsulae increased in all indicated cell densities (Fig. 1E). By contrast, after 10 h, the average percentages of HMECs with cytocapsulae decreased significantly at the cell densities of 1 × 104, 1 × 105, and 5 × 105 cells per well (in six-well plates) but not at densities of 1 × 102 or 1 × 103 cells per well (Fig. 1E). These data strongly suggested that cell contacts at high cell density diminish cytocapsula generation and significantly increase cytocapsula decomposition. Furthermore, the average lifetime of HMEC cytocapsulae (diameter/ width >20 µm) is ∼46.6 h at 1 × 102 cells per well (in six-well plates) and ∼6.5 h at 5 × 105 cells per well (Fig. 1F). These data suggested that cell contacts at high cell density suppressed cytocapsula duration. On the other hand, at 30 h with cell density of 5 × 105 cells per well, there are still ∼1.2‰ of HMECs associated with cytocapsulae (Fig. 1E), indicating that cell contact inhibition at high cell density does not completely suppress cytocapsula generation or entirely induce cytocapsula decomposition. Next, we quantitatively assessed the cell contact effects on cytocapsula growth with a series of increased cell densities. Elevated cell density statistically reduces the average ratio of cytocapsula diameter/width to cellular diameter (Fig. 1G), indicating that at high cell density cell contact decreases cytocapsula growth in diameter. Protein concentration in the ECM influences biochemical, biophysical, and biomechanical signals of ECM and broadly affects cellular activities. Next, we examined the effects of protein concentrations on cytocapsula generation. In a series of increased protein concentrations (in the range from 4–10 mg/mL) in Matrigel, percentages of HMECs with cytocapsulae increased (Fig. 1H), suggesting that elevated protein concentration in the ECM among the indicated range promotes cytocapsula generation.
Over time, single cells migrate in their cytocapsulae, deform cytocapsular membranes, and generate elongated cytocapsulae, forming membranous tubes of variable length (Fig. 1I). Afterward, the intraluminal cells were expulsed from cytocapsular tubes. Ecellularization of cytocapsular tubes produced acellular cytocapsular tubes (Fig. 1I). Then, the long, acellular cytocapsular tubes automatically shrank, and membranes severely folded, forming deflated, coiled, twisted, and membrane-condensed strands (Fig. 1J). Subsequently, the membranes of the long, shrunken, acellular cytocapsular tubes degraded, followed by tube autodecomposition (Fig. 1K). These sequentially developmental stages of cytocapsular tubes of single cells demonstrated that the lifecycle of cytocapsular tubes progressively proceeds from single-cell migration in its cytocapsula to cytocapsular membrane deformation, cytocapsula elongation, cytocapsular tube formation, ecellularization, and acellular cytocapsular tube autodecomposition.
Single HMECs generated cytocapsular tubes can reach up to 820 µm in length (Fig. 1L). Increased cell density decreases average cytocapsular tube lengths (Fig. 1L) but not the average width (∼11 µm in diameter/width) (Fig. S2A). Moreover, high cell density leads to reduced average lifetime of cytocapsular tubes (Fig. 1M) but not their average decomposition time (∼32 min) after decellularization (Fig. S2B). In addition, high cell density diminishes average cytocapsular tube density (in length and number) (Fig. 1 N and O). On the other hand, at 32 h and with a high cell density of 5 × 105 cells per well, multiple long (>360 µm in length) HMEC cytocapsular tubes (5–15 µm in diameter/width) still existed (Fig. 1 L and M), strongly suggesting that some cytocapsular tubes can endure compacted microenvironments.
Next, we assessed cytocapsular generation in HMLER (CD44high/CD24low)FA subpopulations of breast cancer stem cells (BCSCs) (27). At the low cell densities of 1 × 102 and 1 × 103 cells per well, ∼99% of BCSCs generated cytocapsulae at 15 h in 3D Matrigel (Fig. S2C). Consistently, cell contact at high cell density (1 × 104, 1 × 105, and 5 × 105 cells per well) increases BCSC cytocapsular decomposition after 10 h (Fig. S2C). At 30 h and at a high cell density of 5 × 105 cells per well, there were ∼1.1% BCSCs with cytocapsulae, which is about one order of magnitude more than that of HMECs under the same conditions (Fig. 1E and Fig. S2C). These results suggest that BCSC cytocapsulae have greater ability than HMEC cytocapsulae to withstand inhibition by cell contact. BCSC cytocapsular tubes are statistically longer than those of HMECs, and single BCSC cytocapsular tubes can reach up to 1,000 µm in length (Fig. 1L and Fig. S2D). In comparison with HMEC cytocapsular tubes, BCSC cytocapsular tubes possess higher ability to survive in compact environments with high cell density (Fig. 1 L and M and Fig. S2 D and E).
Ultrastructure of Cytocapsular Tubes.
Next, using transmission electron microscopy (TEM), we performed 3D ultrastructure analysis of cytocapsular tubes with a series of continuous cross-sectioned specimens. The data reveal that the cytocapsular tube is a membrane-enclosed complex with a number of finger-shaped nanoprotrusions outside, surrounding the tubes. In the cytocapsular tube lumens, there are some small, isolated, and single-layered, membrane-enclosed vesicles of various morphologies (Figs. S3A, S4A, and S5A). Based on differential morphologies, sizes, and electron density of intraluminal fluids, these small membranous vesicles can be categorized into four types: (i) irregular and branched, thin (30–80 nm in width), and long (80–400 nm in length) (Fig. 2 A and B and Fig. S3 C and D); (ii) irregular with no branch (28–80 nm in width, 50–300 nm in length); (iii) round and small (40–80 nm in diameter); and (iv) round, small (40–100 in diameter) with less electron density of intraluminal fluids (Figs. S3 C and D and S4 B and C). The specific functions of these vesicles are unknown.
Fig. 2.
Ultrastructural analysis of cytocapsular tubes. (A) An enlarged TEM image of part of a transverse section of the HMEC cytocapsular tube shown in Fig. S4A. The cytocapsular tube (CT), cytocapsular tube membrane (CTM; black arrow), the longitudinal section (red arrows) and transverse section (cyan arrow) of the cytocapsular tube nanoprotrusion (NP), and Matrigel matrix (MM) are shown. The four framed areas (1–4) are shown in B (1), C (4), and Fig. S4 B and C (2 and 3, respectively). (B) TEM image of 1 in A: a membranous, branched, irregular vesicle (BIV; black arrows) with two branches (B1 and B2) in the cytocapsular tube lumen. (C) TEM image of 4 in A: a part of a cytocapsular tube nanoprotrusion (NP). The bilayer lipid nanoprotrusion membranes (NPM; green arrows) are shown. (D) A schematic diagram of a cytocapsular tube with its sectioned and exposed tube membranes and an epithelial cell and vesicles in the cytocapsular tube lumen. (E) Quantitation of cytocapsular tube nanoprotrusion diameters. The total number of nanoprotrusions is 141. (F) Quantitation of cytocapsular tube nanoprotrusion lengths. The total number of nanoprotrusions is 117.
In the transverse-section view of the cytocapsular tube membranes, there are large tubular membrane folds (Fig. S4A). Cytocapsular tube membranes protrude outside and form a number of nanoscale (110–130 nm in diameter/width), long (sectioned fragments, 300–2,500 nm in length), and finger-shaped nanoprotrusions extending into the ECM in diverse directions (Fig. 2 A, C, E, and F and Figs. S3 A and B, S4A, and S5). There are plenty of transverse sections and longitudinal sections of cytocapsular tube nanoprotrusions around the transverse sections of cytocapsular tubes, indicating that the long cytocapsular tube nanoprotrusions perpendicular, heterotopic, or parallel to the tubes cross over each other in different layers. Cytocapsular tube nanoprotrusions present in straight lines, turns, and curves (Fig. 2 A and C and Figs. S3A, S4A, and S5). All cytocapsular tube nanoprotrusions directly stem from cytocapsular tube membranes without branches or interconnections (Fig. 2 A and D and Figs. S3A, S4A, and S5). These nanoprotrusions form large, long, and thick networks around the cytocapsular tubes and anchor them in place (Fig. 2 and Figs. S3 and S5).
Cytocapsulae and Cytocapsular Tubes Are Previously Unknown Facultative Organelles.
Cytocapsulae and cytocapsular tubes are neither extensions of the plasma membrane nor cell-surface connected. They are generated by single cells, surrounded by their own engendered membrane that is distinct from the plasma membrane, and enclose the cell that generated them (Figs. 1A and 2D, Fig. S1D, and Dataset S1). Besides their pleiotropic biological functions, the cytocapsulae and cytocapsular tubes have unique characteristics: their extracellular membranes envelope the cell and permit ecellularization and cell entry (Dataset S1). On the other hand, the cytocapsular tube, but not the cytocapsula, supplies long tubular avenues for directed transportation of multiple cells (Dataset S1). The 29 items of comparative differences and similarities in multiple aspects between cytocapsula/cytocapsular tube and other six facultative organelles comprehensively demonstrated that cytocapsulae and cytocapsular tubes are two facultative organelles that are distinct from all previously described organelles (Figs. 1 and 2, Figs. S1–S5, and Dataset S1).
Cytocapsula Ecellularization and Autoentry.
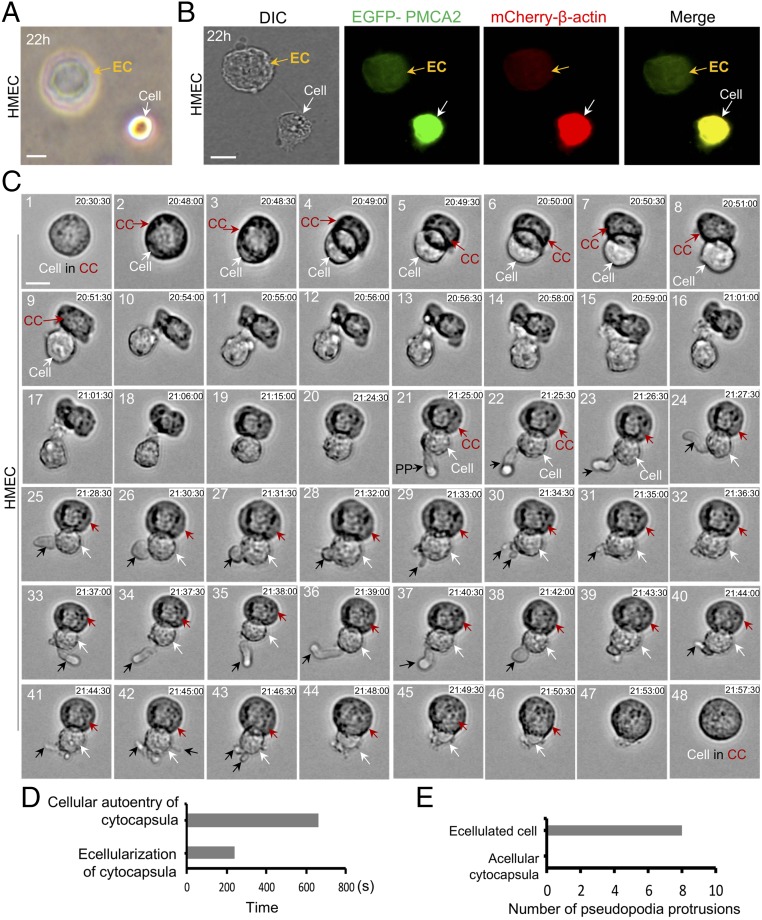
In most instances, after cytocapsular ecellularization, the cell is completely separated from its acellular cytocapsula. The acellular cytocapsulae collapse and form deflated, concave discs (Fig. 3 A and B). Sometimes, cytocapsula ecellularization results in incomplete separation of the expulsed cell from its acellular cytocapsula (Fig. 3C and Movie S1). Initially, a small part of the round cell is expulsed outside the cytocapsula membrane, and the latter wraps the leftover part of the cell inside (Fig. 3C, 2 and 3). After about 30 s, half of the cell is evicted (Fig. 3C, 4). Then, after about 90 s, a major part of the cell is expelled, and the cytocapsula size is reduced in diameter due to the contraction of cytocapsula membrane (Fig. 3C, 5–7). Subsequently, the contracted cytocapsula membranes constantly push the cell out. After about 90 s, the cell is expulsed, and the acellular cytocapsula is deformed into an irregular morphology (Fig. 3C, 8–10). The total time of cytocapsular ecellularization is about 240 s. There remain some connections between the cell and the acellular cytocapsula (Fig. 3C, 10). After about 29 min, the deformed acellular cytocapsula remodels and reforms into a spherical morphology (Fig. 3C, 11–20 and Movie S1).
Fig. 3.
Ecellularization and autoentry of cytocapsulae. (A) Ecellularization of cytocapsulae with complete separation. An ecellulated cytocapsula (EC) is shown. (B) DIC and fluorescence microscope examination of cytocapsula ecellularization with complete separation. (C) Real-time analysis of ecellularization with incomplete separation and autoentry of a cytocapsula with a time-lapse DIC microscope. The DIC images are taken from Movie S1. The cell (white arrows), cytocapsula (CC; red arrows), and pseudopodia protrusions (PP; black arrows) are shown. (D) Quantitation of the time of ecellularization and autoentry of cytocapsulae in C. (E) Quantitation of the number of pseudopodia protrusions of acellular cytocapsulae and repulsed cells in C. (Scale bars: 10 µm.)
Subsequently, the expulsed cells with incomplete separation reenter the acellular cytocapsula and reunite into reclosed cytocapsulae with the cells inside (Fig. 3C, 43–48). The total time of the autoentry procedure is about 660 s, which is ∼2.8-fold longer than the ecellularization process (Fig. 3D). The significant time difference between cytocapsular ecellularization and autoentry indicates the divergence and asymmetry of the two opposite procedures of cell exit and reentry. The opening, reclosing, and reuniting of cytocapsulae during the ecellularization and auto-reentry procedures suggested that cytocapsular membranes possess potent capacities in mobility, self-assembly, and recovery. The evicted cells actively and repeatedly generate and retract multiple pseudopodia protrusions (dendritic or not) of variable size and length, which can quickly protrude, elongate, swing, twist, turn, and retract (Fig. 3C, 21–44, Fig. 3E, and Movie S1). By contrast, acellular cytocapsulae lack the ability to independently generate pseudopodia protrusion (Fig. 3C, 21–44 and Fig. 3E).
Alloentry of Cytocapsulae and Cytocapsular Tubes.
In the alloentry of cytocapsulae, cells can enter cytocapsulae created by other cells, leading to a single cytocapsula harboring two or more cells (Fig. S6 A and B). Next, we investigated alloentry of cytocapsular tubes. Initially, a protrusion of a cell enters a cytocapsular tube of another cell, and cytocapsular tube membranes clasp the cell tip (Fig. 4A, 1 and 1′ and Movie S2). Subsequently, after about 90 s, half of the cell moves into the cytocapsular tube. Cytocapsular tube membranes tightly enclose the inserted cell part. The tight grasp of tube membranes creates a deep and ring-shaped furrow in the entering cell, which exhibits an asymmetric, dumbbell-shaped morphology (Fig. 4A, 2 and 2′). The cell continues to move into the cytocapsular tube. Subsequently, after about 150 s, the majority of the cell has entered the tube. The tight clasp of the tube membranes continues to make a deep ring-shaped furrow in the entering cell (Fig. 4A, 3 and 3′). After about another 150 s, the whole cell has inserted into the cytocapsular tube. The reclosed cytocapsular tube membrane of the other cell now completely envelopes the entered cell (Fig. 4A, 4 and 4′). The average total time of alloentry of cytocapsular tubes is about 420 ± 50 s (n = 3), which is less than that required for cytocapsulae alloentry (about 630 ± 30 s) (n = 3) (Fig. 4B). Subsequently, the cells that have entered cytocapsular tubes formed by other cells migrate actively and bidirectionally in the cytocapsular tubes in vitro (Fig. 4A, 5 and 5′ and Movie S2). These data demonstrated that alloentry substantially enables cytocapsulae and cytocapsular tubes to accommodate multiple cells and permits cell migration in cytocapsular tubes.
Fig. 4.
Alloentry of cytocapsular tubes, cell migration in cytocapsular tubes, and cytocapsular tube connection formation. (A) Real-time analysis with a time-lapse DIC microscope of cell entry into the cytocapsular tube (CT) of another cell. The double-ended red arrow shows the distance cell 1 migrates in the cytocapsular tube of cell 2 (blue arrow). The furrow (black triangles) is shown. (B) Quantitation of the time needed for alloentry into cytocapsulae and cytocapsular tubes; n = 3. (C) Quantitation of cell migration speeds of HMECs and BCSCs (single and multiple cell streaming) in the 3D Matrigel and cytocapsular tubes; n = 3. (D) DIC and fluorescence images of HMEC cytocapsular tube interconnection. Two cytocapsular tubes (CT1 and CT2) interconnect and form a cytocapsular tube connection node (CTN). (E) Quantitation of cytocapsular tube connection nodes in HMECs and BCSCs; n = 3. (F) Quantitation of the number of cytocapsular tubes per connection node in HMECS and BCSCs; n = 3. (G) Quantitation of cytocapsular tube densities of HMECs and BCSCs; n = 3. (H) Quantitation of the effects of cell and tube connection on the lifetime of BCSC cytocapsular tubes; n = 3. (Scale bars: 10 µm.) *P < 0.05; **P < 0.01; ***P < 0.001.
Cytocapsular Tubes Provide a Highway for Cell Transportation.
Next, we assessed cell migration in cytocapsular tubes in vivo. We examined the xenografted and excised BCSC tumors with immunohistochemistry analysis. As expected, in the compact BCSC tumor tissues, there were long (up to 500 µm in length in sectioned specimens), large (5–25 µm in diameter) cytocapsular tubes accommodating multiple cells (Fig. S6 C–F). These data strongly suggest that BCSCs generate substantial, long, cytocapsular tubes and that multiple BCSCs enter and migrate within the tubes in vivo. Next, we quantified the migration speeds of both single and multiple cells in the 3D Matrigel and cytocapsular tubes. The average migration speeds of single HMECs and BCSCs in cytocapsular tubes are ∼7.5-fold faster than in 3D Matrigel (Fig. 4C). In addition, the average migration speeds of multiple HMECs and BCSCs (cell streaming) in cytocapsular tubes are ∼1.6-fold faster than in 3D Matrigel (Fig. 4C). These data strongly suggest that both single and multiple cells migrate faster in cytocapsular tubes than in the 3D environments. These results demonstrate that cytocapsular tubes form highways for directed 3D cell transportation.
Next, we probed mechanics of cytocapsular tube elongation. Single HMECs in their cytocapsulae migrate forward, push, deform and generate elongated cytocapsular membranes, and engender cytocapsular tubes along the migration direction. After 30 min, the cell has generated a short (∼20 µm in length), large (∼8 µm in diameter/width) membranous tube behind the cell (Fig. S7A, 2 and 3 and Movie S3). The elongated, continuous, and seamless cytocapsular tube (∼40 µm in length) lies inactively in the 3D Matrigel and cannot automatically move, change its location, or adjust its movement direction (Fig. S7A, 2–4). Subsequently, after about 26 min, the cell actively changes migration direction and turns. The cell contracts its cytocapsular tube and generates shrunken, membrane-condensed cytocapsular tube fragments (Fig. S7A, 5 and Movie S3). After about 58 min, the cell has actively dragged its contracted cytocapsular tube and pulled it to cross over ECM surfaces and multiple round cytocapsulae in the ECM without tube membrane breakage, interruption, or interception (Fig. S7A, 6–10 and Movie S3), reflecting the extracellular cytocapsular tube’s toughness against fragility under the cell’s drag and pull. These observations (Fig. S7 A and B) suggested that the cell migrates in its cytocapsula, pushes and deforms cytocapsular membranes, secrets and/or delivers component molecules outside the cell, generates new, elongated cytocapsular membranes, shapes tube morphology, and forms long cytocapsular tubes.
Two or more HMEC cytocapsular tubes of variable length interconnect and form tubular networks via connection nodes (Fig. 4D). At 38 h, BCSCs developed many more cytocapsular tube connection nodes than did HMECs (Fig. 4E). In addition, the average number of cytocapsular tubes per connection node of BCSCs is larger than that of HMECs (Fig. 4F). The cytocapsular tube density of BCSCs is about threefold higher than that of HMECs (Fig. 4G). These data demonstrate that the BCSCs have a more aggressive capacity to generate interconnected cytocapsular tube networks. The lifetime of cell-containing BCSC cytocapsular tubes, isolated or connected, is much longer than that of acellular BCSC cytocapsular tubes, indicating that the intraluminal cells facilitate maintaining tubes against decomposition (Fig. 4H). Statistically, connected BCSC cytocapsular tubes with cells stay intact longer than isolated BCSC cytocapsular tubes with cells (Fig. 4H). These results suggest that cytocapsular tube maintenance is tightly controlled and mediated by the intraluminal cells. Importantly, BCSC cytocapsular tubes interconnect and form tubular networks in tumors in vivo, and multiple cells migrate in these tubular networks (Fig. S7C). These data demonstrate that cytocapsular tube networks provide membranous tubular webs for directed cell locomotion in diverse directions.
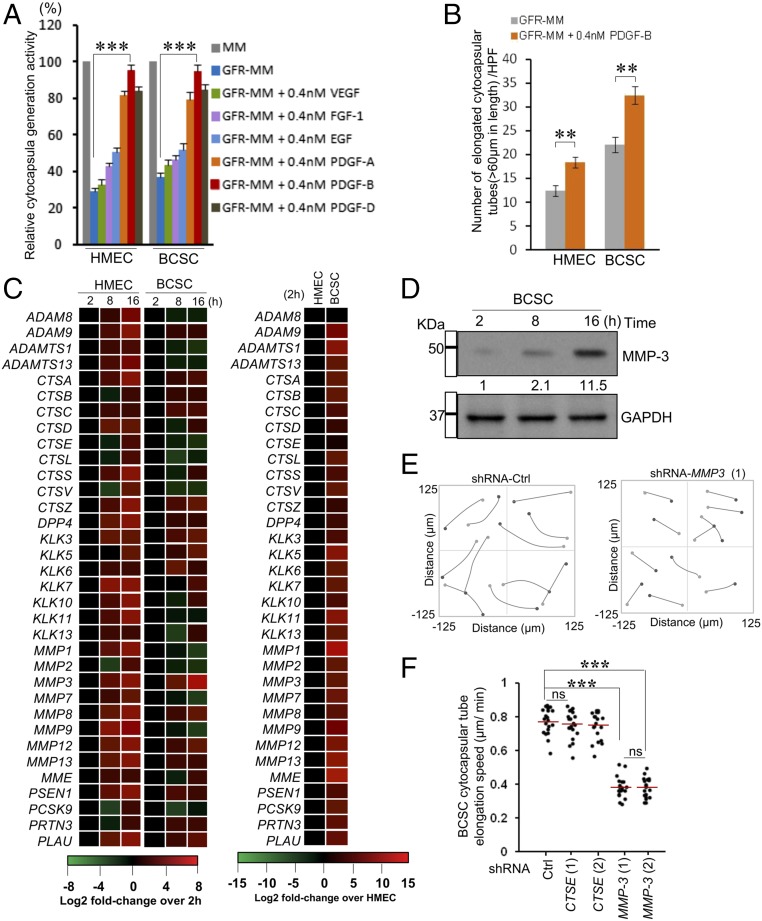
PDGF-B and MMP3 Promote Cytocapsular Tube Development.
Using growth factor-reduced Matrigel significantly decreased cytocapsula initiation (Fig. 5A). Then, we examined vascular endothelial growth factor (VEGF), fibroblast growth factor-1 (FGF-1), epidermal growth factor (EGF), and PDGFs (28), which are important for cell motility and growth (29). Exogenous PDGF-B, but not VEGF, considerably increases cytocapsula generation in HMECs and BCSCs (Fig. 5A). In addition, PDGF-B promoted HMEC and BCSC cytocapsular tube elongation (Fig. 5B). These data strongly suggest that PDGF-B regulates cytocapsula generation and cytocapsular tube development.
Fig. 5.
PDGF-B and MMP3 regulate cytocapsular tube elongation. (A) Quantitation of cytocapsula generation. Percentages of cells with cytocapsula in a high-performance field (200×) were measured; n = 3. (B) Quantitation of cytocapsular tubes; n = 3. (C) Heat map of the transcriptional changes of 34 MMP genes during cytocapsular tube development; n = 3. (D) Western blotting of cellular MMP-3 in BCSCs during cytocapsular tube development. (E) BCSC cytocapsular tube elongation with and without transient knockdown of MMP-3 (1), derived from stacks of images by a time-lapse DIC microscope during cytocapsular tube development. (F) Quantitation of the speed of cytocapsular tube elongation in E. Red lines indicate mean; n = 2. ns, P > 0.05; **P < 0.01; ***P < 0.001.
Matrix metalloproteinases (MMPs) cleave cell-surface receptors, release ligands, and are important for cell migration (30–32). Cell migration in cytocapsulae is essential for the generation of elongated cytocapsular tubes (Fig. S7A). Next, we assessed whether MMPs are involved in cytocapsular tube development. Using quantitative real-time PCR (qPCR), we examined the expression of 34 MMP genes during cytocapsular tube growth. These genes were variably expressed, and among them MMP-3 transcription was consistently elevated (Fig. 5C). Furthermore, cellular MMP-3 levels were elevated up to ∼11.5-fold during cytocapsular tube elongation (Fig. 5D). On the contrary, transient knockdown of MMP-3, but not CTSE, significantly reduced the average elongation speed of BCSC cytocapsular tubes from ∼0.75 µm/min to ∼0.37 µm/min (n = 23 elongated cytocapsular tubes) (Fig. 5 E and F). These data demonstrated that MMP-3 mediates cytocapsular tube elongation.
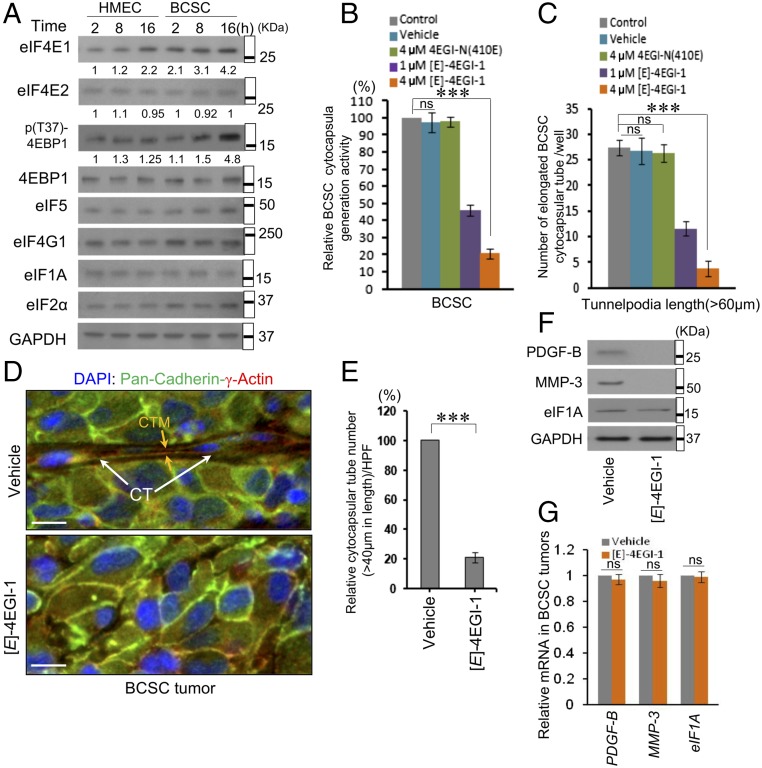
Cap-Dependent Translation Promotes Cytocapsular Tube Development.
Initiation is the rate-limiting step in translation (33, 34). To investigate the translation initiation mechanism driving cytocapsula development, whose construction requires plenty of proteins, we tested seven key initiation factors. Cellular eIF4E1, whose availability is tightly controlled, constantly elevates during cytocapsular tube development (Fig. 6A). The cap-binding protein eIF4E1 (namely, eIF4E) is essential for mRNA recruitment to the ribosome (35). Furthermore, the phosphorylation of 4EBP1, whose hyperphosphorylation promotes dissociation of 4EBP1–eIF4E and augments eIF4E–eIF4G interaction (36), increases during cytocapsular tube growth (Fig. 6A). The eIF4E–eIF4G interaction inhibitor [E]-4EGI-1 (37), but not the vehicle or the inactive analog 4EGI-N (410E), inhibited cytocapsular tube generation and elongation in BCSCs (Fig. 6 B and C). [E]-4EGI-1 decreased cytocapsular tube numbers (Fig. 6 D and E) and suppressed protein levels of PDGF-B and MMP-3, but not eIF1A, in BCSC tumors (Fig. 6F). In addition, the inhibitor did not decrease mRNA levels of these genes in BCSC tumors (Fig. 6G). These results demonstrated that enhanced eIF4E-mediated cap-dependent translation initiation up-regulates the translation of mRNAs encoding proteins promoting cytocapsular tube development.
Fig. 6.
Translation initiation factor eIF4E1-enhanced translation promotes cytocapsular tube development. (A) Western blotting assays of HMECs and BCSCs during cytocapsular tube development. (B and C) Quantitation of cytocapsula generation (n = 3) (B) and cytocapsular tube elongation (n = 3) (C). (D) Immunohistochemistry assays of cytocapsular tubes in BCSC tumors with compound treatments. The cytocapsular tube (CT; white arrows) and cytocapsular tube membrane (CTM; orange arrow) are shown. (Scale bars: 10 µm.) (E) Quantitation of cytocapsular tubes in D (n = 5 specimens from five mice of each group, one specimen per mouse). The cytocapsular tube numbers in the mice in the control group was set as 100%, and the relative cytocapsular tube quantitation is shown. (F) Western blotting assays in D. (G) Quantitation of gene transcription in D; ns, P > 0.05; ***P < 0.001.
Discussion
Cell locomotion in multicellular organisms is critical for embryonic development, tissue formation, organ homeostasis, immune responses, wound healing, tissue regeneration, and tumor metastasis. Here we report that single mammalian cells generate two organelles, the extracellular membranous cytocapsulae and cytocapsular tubes, and that cytocapsular tubes provide tubular freeways for 3D cell transportation (Fig. S8).
It is unanticipated that single mammalian cells engender large, extracellular, and membranous cytocapsulae enveloping the cell and generate cytocapsular tubes functioning as membranous tube highways for directed cell relocation and dissemination. Why have cytocapsulae and cytocapsular tubes not been recognized previously? They are, in fact, difficult to see. The generation and development of cytocapsulae and cytocapsular tubes depend greatly on both the 3D environment and cellular activity, and in this study they were brought into focus only when the biochemical, biophysical, and biomechanical characteristics (e.g., polymerization, density, and viscoelasticity) of the Matrigel matrix were precisely controlled. The temporospatial appearance, self-degradation, and autodecomposition of cytocapsulae and cytocapsular tubes further increase difficulty in recognizing and identifying them. Cytocapsulae and cytocapsular tubes are independently located outside the plasma membrane, without cell-surface connection, which substantially differentiates them from many cell-surface–connected organelles/compartments, such as lamellipodia (38), filopodia, podosomes, invadopodia, focal adhesions, nanotubes, blebs, type II epithelial bridges, pseudopodia protrusions, cilia (39), and so forth. The extracellular and membranous cytocapsulae and cytocapsular tubes envelope the cell and permit cell entry and ecellularization, which significantly distinguishes them from all the cell-secreted/shed membranous vesicles, including exosomes (40), microvesicles, shedding microvesicles, apoptotic bodies (41), and others. In addition, the biomembranous cytocapsulae and cytocapsular tubes are biochemically and structurally different from the outer membranes of Gram-negative bacteria (42) and all the nonmembranous extracellular compartments or matrices, such as cell walls (43) and polysaccharide-layered bacterial capsules (44). The comprehensive analyses of the similarities and differences of cytocapsulae and cytocapsular tubes demonstrate that cytocapsulae and cytocapsular tubes are two related but distinct organelles that originate from single mammalian cells. Therefore, the two facultative organelles—cytocapsulae and cytocapsular tubes—may open avenues to understanding the mechanisms underlying biological evolution at the organelle level.
Cytocapsular membranes provide substantial biomembranous scaffolds supporting cellular adhesion, attachment, detachment, morphological transition, and motility plasticity. On the other hand, cytocapsulae envelop the cells, physically shielding them from the extracytocapsular microenvironments, which thus facilitates the stringently spherical cells to interconvert into relaxed, irregular morphologies, and might serve as protective coverings against environmental stresses. Cytocapsular tubes supply membranous tubes that accommodate directed migration and bidirectional locomotion of multiple cells. More importantly, cytocapsular tubes interconnect and form tubular networks, which significantly increase cell-migration directions, amplify cell-spreading areas, and augment directed 3D cell migration efficiency. Although cell contact inhibition at high cell density diminishes cytocapsula generation, increases cytocapsula decomposition, and reduces cytocapsular tube duration, ∼1.2‰ normal cells (HMECs) and up to 1.1% BCSCs still generate cytocapsulae and engender long capsular tubes for directed migration of multiple cells. Therefore, compared with the situation in which all cells are unavoidably exposed to and experience heterogeneous obstacles sourced from compacted and heterogeneous 3D ECM and cells in vivo, the condition in which a small percentage of cells generates homogeneous membranous cytocapsular tubes serving as highways for the directed transportation of multiple cells is an efficient one, at least as an alternative pattern. Cytocapsulae and cytocapsular tubes exhibit potentials for pleiotropic biological functions and other functions whose elucidation will require further work.
The enlargement of cytocapsulae depends on the activities of the intraluminal cell, and acellular cytocapsulae terminate growth. On the other hand, acellular cytocapsulae and cytocapsular tubes proceed to rapid autodegradation and self-decomposition. In addition to intracellular organelles, eukaryotic cells contain extracellular organelles that are released or shed into the microenvironment, such as exosomes (45). Interestingly, there are a number of various, single-layered membrane-enclosed vesicles in the cytocapsular tube lumens. Therefore, these membranous vesicles may functionally link to the carriers or cargoes that deliver/shuttle cell-originated cytocapsular components and to containers that include lysates whose release results in the tightly controlled autodecomposition of cytocapsulae and cytocapsular tubes.
The phenomenon that, after ecellularization, acellular cytocapsular tubes shrink, contract, and become deflated, shortened, twisted, and folded strands is in agreement with the lack of intraluminal skeleton in cytocapsular tubes. The absence of microfilament networks in the cytocapsular tube lumens is consistent with the active migration of single and multiple cells in cytocapsular tubes. On the other hand, the presence of microfilament scaffolds under cytocapsular tube membranes agrees with the long cytocapsular tubes' ability to withstand being dragged by the cell across ECM surfaces without breakage, interruption, or interception.
Cell migration in cytocapsulae drives cytocapsular membrane deformation and elongation and cytocapsular tube formation and elongation. PDGF-B significantly promotes cytocapsula generation and cytocapsular tube elongation, which is consistent with the observations that PDGF-BB can functionally interact with all of the homo- and heterodimers of PDGFRs (-αα, -αβ, and -ββ) with high affinity and that PDGF-B is critical for cell migration, membrane generation, and movement. MMP-3 promotes cytocapsular tube elongation, which is in agreement with the requirement that the intraluminal cell be detached for cell migration in the cytocapsular tubes and with MMP-3’s broad functions in cell-surface receptor cleavage, molecular lysis, and adhesion release.
The building of membranes and other components in cytocapsulae and cytocapsular tubes requires plenty of variable proteins. Consistently, the tightly controlled eIF4E levels are constantly elevated, and phosphorylation of 4EBP1 increases during the cytocapsula generation and cytocapsular tube development. On the contrary, 4EGI-1, an eIF4E–eIF4G interaction inhibitor, effectively suppresses BCSC cytocapsular tube generation in vitro and in BCSC tumors in vivo. These results agree with the finding that the growth and maintenance of cytocapsulae and cytocapsular tubes depend on the intraluminal cell(s).
In summary, the two organelles, cytocapsulae and cytocapsular tubes, uncovered in this study present pleiotropic biological functions, including supplying tubular pathways and networks for cell transportation, relocation, and migration, which may provide insights into the mechanisms of the processes involved in cell translocation in the development and pathogenesis of diseases, including tumor metastasis.
Materials and Methods
Cells and Reagents.
Primary normal HMECs were ordered from ATCC (PCS-600-010). BCSCs of the HMLER (CD44high/CD24low)FA subpopulation were prepared as previously described (27). FITC-conjugated anti-CD44 antibody (G44-26; BD Biosciences) and phycoerythrin-conjugated anti-CD24 antibody (ML15; BD Biosciences) were used for cell sorting with flow cytometry. The MEGM Mammary Epithelial Cell Growth Medium BulletKit (Clonetics MEGM Mammary Epithelial Cell Growth Medium plus SingleQuots Kit package) was ordered from Lonza (CC-3150). Matrigel Membrane Matrix (CB-40234) was purchased from Corning. BD Matrigel Matrix Growth Factor Reduced (GFR; catalog no. 356230) was ordered from BD Bioscience.
Animals.
HMLER (CD44high/CD24low)FA subpopulation cells were injected into the mammary glands of 1.5-mo-old, nonobese diabetic/severe combined immunodeficiency female mice (five mice in each group; strain name: NOD.CB17-Prkdcscid/J; the Jackson Laboratory) for the analysis of cytocapsular tube formation in tumors, as approved by the Harvard Medical Area Standing Committee on Animals.
Time-Lapse Differentiation Interference Contrast Microscopy, TEM, Transient Transfection, qPCR, and Transient Gene Knockdown.
Time-lapse differentiation interference contrast (DIC) microscopy analyses of cytocapsula elongation and cell migration were performed using a Nikon Ti motorized inverted microscope and a digital Hamamatsu ORCA-ER cooled CCD camera with a 20× lens. HMEC cultures with cytocapsulae in Matrigel Matrix (>40 µm in depth) were analyzed with TEM. Plasmids of EGFP-hPMCA2z/b (no. 47584; Addgene) and/or mCherry–β-actin (no. 54967; Addgene) were cotransfected into HMECs using Lipofectamine 2000 (Thermo Fisher Scientific, catalog no. 11668027). qPCR assays were performed using gene-specific primers (IDT Company), iQ SYBR Green Supermix (Bio-Rad), and 7900HT Fast Real-Time PCR. The transient gene knockdown effects of two shRNAs in each set were evaluated: RHS4533-EG4314 (shRNA-MMP-3, including five shRNAs), and RHS4533-EG1510 (shRNA-CTSE, including five shRNAs).
Details of the development of cytocapsulae and cytocapsular tubes, Western blotting, imaging acquisition, immunohistochemistry staining, and quantification and statistical analysis are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Harvard Nikon Imaging Center for imaging facilities, the Electron Microscope Center of the Cell Biology Department of Harvard Medical School for support with electron microscope technique, and the Pathology Department of Brigham and Women’s Hospital of Harvard Medical School for histology service. This work was supported by NIH Grant CA200913.
Footnotes
Conflict of interest statement: The authors are cofounders of and hold equity in Cellmig Biolabs, Inc. to support future basic research on cell migration.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717910115/-/DCSupplemental.
References
- 1.Le Douarin NM. Cell migrations in embryos. Cell. 1984;38:353–360. doi: 10.1016/0092-8674(84)90490-2. [DOI] [PubMed] [Google Scholar]
- 2.Reig G, Pulgar E, Concha ML. Cell migration: From tissue culture to embryos. Development. 2014;141:1999–2013. doi: 10.1242/dev.101451. [DOI] [PubMed] [Google Scholar]
- 3.Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: Strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryant DM, Mostov KE. From cells to organs: Building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 7.Weninger W, Biro M, Jain R. Leukocyte migration in the interstitial space of non-lymphoid organs. Nat Rev Immunol. 2014;14:232–246. doi: 10.1038/nri3641. [DOI] [PubMed] [Google Scholar]
- 8.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 9.Friedl P, Alexander S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Friedl P, Wolf K. Tumour-cell invasion and migration: Diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 11.Barbolina MV, et al. Microenvironmental regulation of ovarian cancer metastasis. Cancer Treat Res. 2009;149:319–334. doi: 10.1007/978-0-387-98094-2_15. [DOI] [PubMed] [Google Scholar]
- 12.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Franz CM, Jones GE, Ridley AJ. Cell migration in development and disease. Dev Cell. 2002;2:153–158. doi: 10.1016/s1534-5807(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: Characteristics, formation and function. Nat Rev Mol Cell Biol. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattila PK, Lappalainen P. Filopodia: Molecular architecture and cellular functions. Nat Rev Mol Cell Biol. 2008;9:446–454. doi: 10.1038/nrm2406. [DOI] [PubMed] [Google Scholar]
- 16.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 18.Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–1022. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012;24:116–124. doi: 10.1016/j.ceb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Zani BG, Indolfi L, Edelman ER. Tubular bridges for bronchial epithelial cell migration and communication. PLoS One. 2010;5:e8930. doi: 10.1371/journal.pone.0008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker BM, Chen CS. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu PH, Giri A, Sun SX, Wirtz D. Three-dimensional cell migration does not follow a random walk. Proc Natl Acad Sci USA. 2014;111:3949–3954. doi: 10.1073/pnas.1318967111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedl P, Sahai E, Weiss S, Yamada KM. New dimensions in cell migration. Nat Rev Mol Cell Biol. 2012;13:743–747. doi: 10.1038/nrm3459. [DOI] [PubMed] [Google Scholar]
- 25.Jayatilaka H, et al. Synergistic IL-6 and IL-8 paracrine signalling pathway infers a strategy to inhibit tumour cell migration. Nat Commun. 2017;8:15584. doi: 10.1038/ncomms15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giri A, et al. The Arp2/3 complex mediates multigeneration dendritic protrusions for efficient 3-dimensional cancer cell migration. FASEB J. 2013;27:4089–4099. doi: 10.1096/fj.12-224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi T, et al. Quantitative phosphoproteomic analysis reveals system-wide signaling pathways downstream of SDF-1/CXCR4 in breast cancer stem cells. Proc Natl Acad Sci USA. 2014;111:E2182–E2190. doi: 10.1073/pnas.1404943111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heldin CH, Ostman A, Rönnstrand L. Signal transduction via platelet-derived growth factor receptors. Biochim Biophys Acta. 1998;1378:F79–F113. doi: 10.1016/s0304-419x(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 29.Noble M, Murray K, Stroobant P, Waterfield MD, Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 30.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf K, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 32.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 33.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 34.Gross JD, et al. Ribosome loading onto the mRNA cap is driven by conformational coupling between eIF4G and eIF4E. Cell. 2003;115:739–750. doi: 10.1016/s0092-8674(03)00975-9. [DOI] [PubMed] [Google Scholar]
- 35.Marintchev A, et al. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell. 2009;136:447–460. doi: 10.1016/j.cell.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moerke NJ, et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell. 2007;128:257–267. doi: 10.1016/j.cell.2006.11.046. [DOI] [PubMed] [Google Scholar]
- 38.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satir P. CILIA: Before and after. Cilia. 2017;6:1. doi: 10.1186/s13630-017-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 41.Théry C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 42.Dong H, Tang X, Zhang Z, Dong C. Structural insight into lipopolysaccharide transport from the gram-negative bacterial inner membrane to the outer membrane. Biochim Biophys Acta. 2017;1862:1461–1467. doi: 10.1016/j.bbalip.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Chebli Y, Geitmann A. Cellular growth in plants requires regulation of cell wall biochemistry. Curr Opin Cell Biol. 2017;44:28–35. doi: 10.1016/j.ceb.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Yoshida K, et al. Role of bacterial capsule in local and systemic inflammatory responses of mice during pulmonary infection with Klebsiella pneumoniae. J Med Microbiol. 2000;49:1003–1010. doi: 10.1099/0022-1317-49-11-1003. [DOI] [PubMed] [Google Scholar]
- 45.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: From biogenesis and secretion to biological function. Immunol Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.