Significance
Maturation of functional adult (α2β2) or fetal (α2γ2) hemoglobin (Hb) tetramers requires that a heme cofactor be incorporated into each globin. During erythropoiesis, Hb-α maturation is aided by the alpha Hb-stabilizing protein (AHSP), but what enables the maturation and heme insertion of the other globins is unknown. We found that chaperone hsp90 stabilizes the immature, heme-free forms of Hb-β and Hb-γ and then drives their heme insertion reactions in an ATP-dependent process. This finding fills an important gap in our understanding of hemoglobin maturation during erythropoiesis and also helps to explain how functional mature Hb can be expressed outside the circulation in nonerythroid cells and tissues that do not express AHSP.
Keywords: heme, erythropoiesis, hemoglobin, hemeprotein, nonerythroid
Abstract
Maturation of adult (α2β2) and fetal hemoglobin (α2γ2) tetramers requires that heme be incorporated into each globin. While hemoglobin alpha (Hb-α) relies on a specific erythroid chaperone (alpha Hb-stabilizing protein, AHSP), the other chaperones that may help mature the partner globins (Hb-γ or Hb-β) in erythroid cells, or may enable nonerythroid cells to express mature Hb, are unknown. We investigated a role for heat-shock protein 90 (hsp90) in Hb maturation in erythroid precursor cells that naturally express Hb-α with either Hb-γ (K562 and HiDEP-1 cells) or Hb-β (HUDEP-2) and in nonerythroid cell lines that either endogenously express Hb-αβ (RAW and A549) or that we transfected to express the globins. We found the following: (i) AHSP and hsp90 associate with distinct globin partners in their immature heme-free states (AHSP with apo-Hbα, and hsp90 with apo-Hbβ or Hb-γ) and that hsp90 does not associate with mature Hb. (ii) Hsp90 stabilizes the apo-globins and helps to drive their heme insertion reactions, as judged by pharmacologic hsp90 inhibition or by coexpression of an ATP-ase defective hsp90. (iii) In nonerythroid cells, heme insertion into all globins became hsp90-dependent, which may explain how mixed Hb tetramers can mature in cells that do not express AHSP. Together, our findings uncover a process in which hsp90 first binds to immature, heme-free Hb-γ or Hb-β, drives their heme insertion process, and then dissociates to allow their heterotetramer formation with Hb-α. Thus, in driving heme insertion, hsp90 works in concert with AHSP to generate functional Hb tetramers during erythropoiesis.
Hemoglobin (Hb) functions in oxygen delivery by virtue of its heme prosthetic group (1–3). Its expression and maturation is tightly coordinated during erythropoiesis and is subject to multiple levels of regulation (4–6), with disregulation manifesting in some forms of β-thalassemia (7–9) and anemia (10–12). In mammals, erythropoiesis involves a coordinated synthesis of partner globin chains, heme insertion, and globin interaction steps that ultimately create functional fetal (α2γ2) Hb or adult (α2β2) Hb tetramers (13). Our understanding of Hb maturation is still incomplete, and in particular, we do not know the details of Hb heme insertion or what partner proteins may assist the globins during this process. Previous studies identified a role for cell chaperones, specifically hsp70, in stabilizing Hb-α and preventing its aggregation in cells during maturation (14, 15). In addition, Hb-α maturation in erythrocytes is aided by the erythroid-specific α-hemoglobin–stabilizing protein (AHSP), which binds to nascent Hb-α to stabilize it against aggregation or degradation and to down-regulate its superoxide production once it has incorporated heme (16–18). However, what other chaperones, if any, might perform similar functions to assist maturation of the partner globin chains (Hb-γ and Hb-β), or might substitute for AHSP in nonerythroid cells that express Hb-α (19, 20), is unknown.
To address these questions, we studied Hb maturation as it occurs in the human erythroid leukemia cell line K562 (21, 22), in the human erythroid progenitor cells HiDEP-1 and HUDEP-2 during their differentiation, and in nonerythroid cell lines that either naturally or transiently expressed Hb, keying on a possible role for the cell chaperone hsp90 (23, 24). Our findings reveal that hsp90 chaperones heme insertion into Hb-β and Hb-γ, and in some circumstances, into Hb-α, and thus plays a fundamental role in forming mature functional fetal (α2γ2) or adult (α2β2) Hb tetramers in both erythroid and nonerythroid cells.
Results
Hsp90 Associates with Hb in Erythroid-Like Cells and Is Required for Heme Insertion.
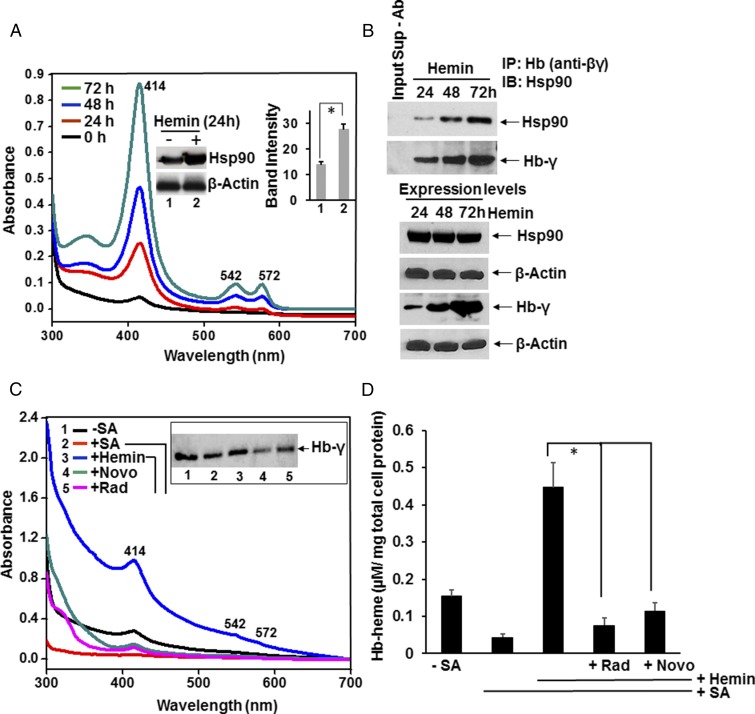
We first examined if hsp90 associates with Hb in the K562 human erythroid leukemia cell line (21, 22), which constitutively expresses fetal Hb (α2γ2) and can be induced to express greater levels when the cells are given hemin, which promotes their differentiation toward erythrocytes (21, 22). Cells were given hemin over a 3-d period, and the cell supernatants were subject to spectral analysis and immunoprecipitation (IP). The spectral traces of clarified cell supernatants (equal protein amounts) in Fig. 1A show that Hb protein expression increased steadily over the 3-d treatment and that this was associated with a twofold increase in hsp90 expression in the first 24 h (Inset). Hsp90 associated with fetal Hb in proportion to the level of Hb expression (Fig. 1B) in the resting cells and during their heme-induced induction.
Fig. 1.
Hsp90 interacts with Hb and is needed for heme insertion during Hb maturation. (A and B) K562 cells were treated with 50 µM hemin over a 3-d period, and supernatants were generated every 24 h and used for absorption spectra and IPs. (A) Spectra show that Hb absorbance increases during the hemin treatment. (Inset) Western expression and cumulative densitometry data show that cell hsp90 expression increased after 24 h of hemin treatment. Values depicted are mean ± SD of n = 3 experiments (*P < 0.05, by one-way ANOVA). (B) IP shows bound hsp90 and Hb-γ (input 20%) retained on the beads. Lane 1 in the Upper two panels is a no-antibody control and indicates the input supernatant (Sup) from the 48-h time point minus the Hb(βγ) antibody (Ab). The Lower four panels show protein expression levels of hsp90 and Hb-γ (from 24 to 72 h of hemin treatment) from 8 and 15% SDS/PAGE, respectively, with β-actin as the loading control. (C and D) K562 cells were heme-deprived by treating with SA for 3 d and then were incubated for 3 h with 5 µM of hemin in the presence or absence of the hsp90 inhibitors radicicol or novobiocin. Cell supernatants (equal protein) were analyzed for absorption spectra and Hb-γ expression. (C) UV-visible spectra of the supernatants and effect of hsp90 inhibition. (Inset) Hb protein expression levels. (D) The calculated Hb-heme content of the supernatants as depicted in A, using the Soret absorbance at 414 nm. Results are mean ± SD of n = 3 experiments (*P < 0.05, by one-way ANOVA).
We next determined if two hsp90 inhibitors that work by different mechanisms (25) (radicicol or novobiocin) would acutely block heme insertion into heme-free Hb that had accumulated in K562 cells that had been cultured with succinyl acetone (SA) to make them heme-deficient (23, 24). The heme-deficient cells were given hemin (5 µM) with or without either inhibitor for 3 h, and then the level of heme-replete Hb that formed was determined by spectral analysis of the cell supernatants, which measured the heme content of Hb from the Soret absorbance peak at 414 nm (Fig. 1 C and D, equal amounts of total protein). The traces and associated Western blot show that the heme-deficient cells contained Hb that was predominantly heme-free but could incorporate the added heme over the 3-h period. Both hsp90 inhibitors inhibited the heme insertion, indicating that active hsp90 was required for the heme insertion into the apo-Hb.
Hsp90 Association Is Globin-Specific.
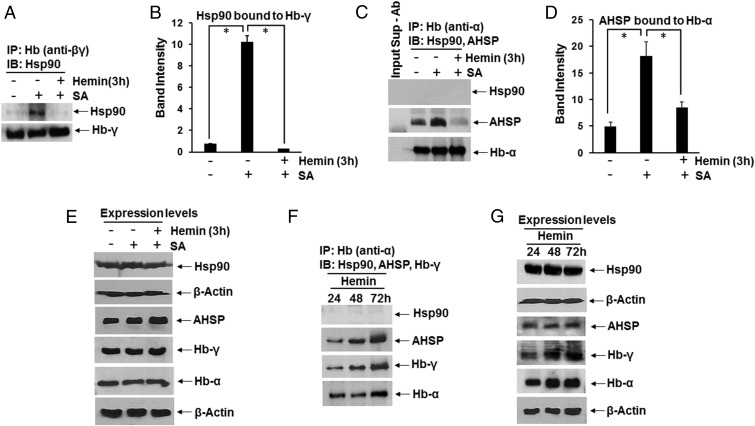
Supernatants from K562 cells that were cultured without any added heme were subject to IP using globin-specific antibodies, followed by Western analysis to observe the globin hsp90 or AHSP associations (Fig. 2). Very little hsp90 was found associated with Hb-γ in the cells under this circumstance, but the level of hsp90 association increased if the K562 cells had been made heme-deficient before the experiment. This increased hsp90 association was then lost when the heme-deficient K562 cells were given heme for 3 h, which allowed them to generate heme-replete Hb (Fig. 2 A and B). In comparison, hsp90 was not associated with Hb-α under any of the three conditions (Fig. 2C) and was instead associated with AHSP, which is known to bind exclusively to Hb-α (16–18) (Fig. 2 C and D). As seen for the hsp90 association with Hb-γ, the AHSP association with Hb-α was strongest under the heme-deficient condition and dropped off in the heme-replenished cells. Protein expression levels of hsp90, AHSP, Hb-γ, and Hb-α were unchanged under the different cell culture conditions (Fig. 2E). A specificity of hsp90 toward Hb-γ was also seen during the hemin-induced differentiation in the K562 cells (Fig. 2 F and G). Thus, hsp90 associated with Hb-γ only in its heme-free state and did not associate with Hb-α under any condition.
Fig. 2.
Hb-α and Hb-γ selectively interact with chaperone AHSP or hsp90. (A–E) K562 cells were cultured in normal media or were made heme-deficient by treating with SA for 72 h before experiments. In some cases, the heme-deficient cells were then incubated for 3 h with 5 µM of hemin. (F and G) Hb expression in K562 cells was induced by adding hemin (50 µM) for 72 h. Soluble cell supernatants were prepared every 24 h and analyzed by IP and Western blot to determine Hb-α and Hb-γ interactions with chaperons (Hsp90 and AHSP). (A) IP of Hb-γ showing bound hsp90 and Hb-γ (input 20%) retained on the beads. (B) Densitometric quantification of bound hsp90. (C) IP of Hb-α comparing bound hsp90 and AHSP (input 20%). Input Sup-Ab is a no-antibody control and indicates the input supernatant (Sup) minus the Hb(α) antibody (Ab). (D) Densitometry of AHSP bound to Hb-α. (E) Expression levels derived from 8% (Upper two panels) and 15% (Lower four panels) SDS/PAGE, with β-actin as loading control. In B and D, values depicted are mean ± SD of three independent experiments (n = 3) (*P < 0.05, by one-way ANOVA). (F) IP of Hb-α showing bound AHSP and Hb-γ (input 20%) retained on the beads. (G) Expression levels in cell supernatants as indicated from 8% (Upper two panels) and 15% (Lower four panels) SDS/PAGE.
Hsp90 Drives Globin Maturation During Erythropoiesis.
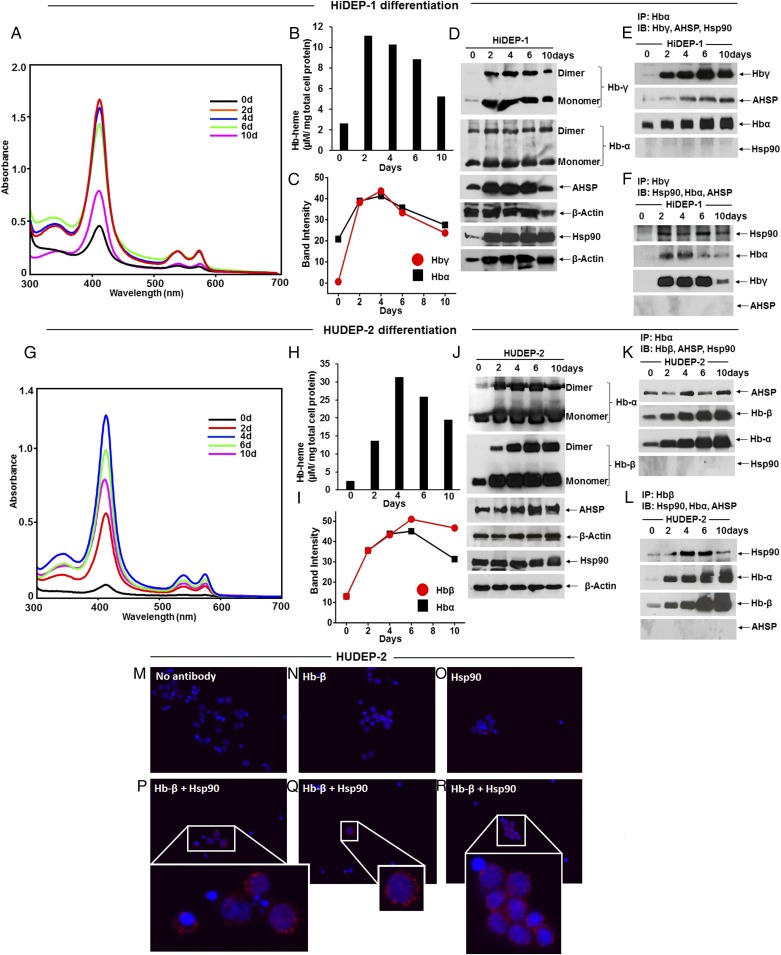
We then performed experiments with the human erythroid progenitor cells HiDEP-1 and HUDEP-2, which can be induced by culturing in differentiation media with erythropoietin (EPO) and growth factors to undergo full differentiation to erythroid cells that produce fetal (Hb-α/γ) or adult (Hb-α/β) hemoglobins, respectively (26). We studied the cells over a 16-d differentiation period (Fig. S1). Fig. 3 A–D and G–J shows spectroscopic and Western results that document the time-dependent buildup of heme-containing and total Hb proteins along with the expression levels of other proteins of interest in the HiDEP-1 and HUDEP-2 cells. As expected, the levels of total and heme-replete globins increased during the differentiation period, with the heme-replete globin levels peaking between days 2–6 (Fig. 3 A, B, G, and H and Figs. S2 and S3). The pulldown results in Fig. 3 E, F, K, and L show that the globins maintained their individual specificities in associating with either AHSP or hsp90. Hsp90-associated Hb-γ or Hb-β and AHSP-associated Hb-α proteins were observed over the entire course of the differentiation, although the chaperone association levels decreased with time in the HUDEP-2 cells (Fig. S4). An association between hsp90 and Hb-β could also be visualized in intact HUDEP-2 cells using the proximity ligation assay (PLA) protocol (Fig. 3 M–R).
Fig. 3.
Distinct Hsp90 and AHSP globin interactions occur in erythroid progenitor cells during red blood cell differentiation. Erythroid progenitor cells HiDEP-1 or HUDEP-2 were induced to differentiate for a 16-d period, cell aliquots were harvested before or during differentiation, and the soluble supernatants were analyzed. (A and G) Representative UV-visible spectra of cell supernatants (equal protein) created from cells before (day = 0) or during differentiation. (B and H) The calculated Hb heme content in HiDEP-1 and HUDEP-2 supernatants depicts a mean of n = 2 experiments. (C and I) Mean densitometry of Hb protein expression levels as depicted in D and J, from n = 2 experiments, during differentiation. (D and J) Hb, AHSP, and hsp90 expression levels depicted in 15 and 8% SDS/PAGE, respectively, during differentiation, with β-actin as loading control. (E, F, K, and L) IP showing AHSP bound to Hb-α or hsp90 bound to Hb-β and Hbα bound to Hbγ/Hbβ (input 20% in all cases). Western blots and IPs are representative, and the differentiation experiments were repeated two times with similar results. (M–R) Representative images from PLAs showing colocalization of hsp90 and Hb-β in undifferentiated (day = 0) HUDEP-2 cells. (M) Control using no primary antibody, (N) Hb-β antibody alone, (O) Hsp90 antibody alone, and (P–R) PLA images with both Hb-β and hsp90 antibodies with Insets showing close-ups. Red dots indicate the location and extent of interaction.
We next investigated if hsp90 inhibition would impact Hb-β heme insertion and Hb maturation in HUDEP-2 cells. The cells were made heme-deficient and then had hemin added with or without the hsp90 inhibitors radicicol or AUY-922. Fig. 4 shows that the heme deficiency led to a buildup of apo-Hb–β and a diminished level of mature Hb-α/β in the cells without altering the expression levels of either globin or related proteins of interest. Both hsp90 inhibitors blocked heme insertion into apo-Hb–β and prevented the concomitant formation of mature Hb-α/β. IP experiments in Fig. 4D show that hsp90 dissociated from Hb-β after the heme reconstitution, but did not do so when radicicol was also present to prevent heme insertion into Hb-β. AUY-922, unlike radicicol, allowed dissociation of the hsp90–apo–Hbβ complex, despite preventing heme insertion (Fig. 4 A, C, and D). In all cases, the IP data (Fig. 4D) showed that the hsp90 association with Hb-β inversely correlated with an elevated Hb-αβ association, which significantly fell in the presence of both hsp90 inhibitors. Together, our results establish that hsp90 chaperones Hb maturation in differentiating erythroid cells by exclusively associating with either apo-Hb–γ or apo-Hb–β and by driving their heme insertion reactions in an ATP-dependent manner. The hsp90 then dissociates after heme insertion takes place to allow heterotetramer formation between the heme-replete Hb-γ or Hb-β and Hb-α.
Fig. 4.
Hsp90 inhibitors acutely block heme insertion and Hb maturation in HUDEP-2 cells. HUDEP-2 cells were made heme-deficient by a 5-d culture with SA and then were incubated for 3 h with 5 µM of hemin in the presence or absence of the hsp90 inhibitors radicicol (5 µM) or AUY-922 (300 nM), and the soluble cell supernatants (equal protein) were analyzed. (A) UV-visible spectra of the supernatants. (B) Hb and hsp90 protein expression levels as analyzed in 15 and 8% SDS/PAGE, respectively, with β-actin as loading control. (C) The Hb heme content of supernatants as calculated from spectra in A, mean ± SD of n = 3 experiments (*P < 0.05, by one-way ANOVA). (D) IP of Hb-β in each supernatant compares levels of bound hsp90 or Hb-α (input 20% in all cases).
Hsp90 Drives Globin Maturation in Nonerythroid Cells.
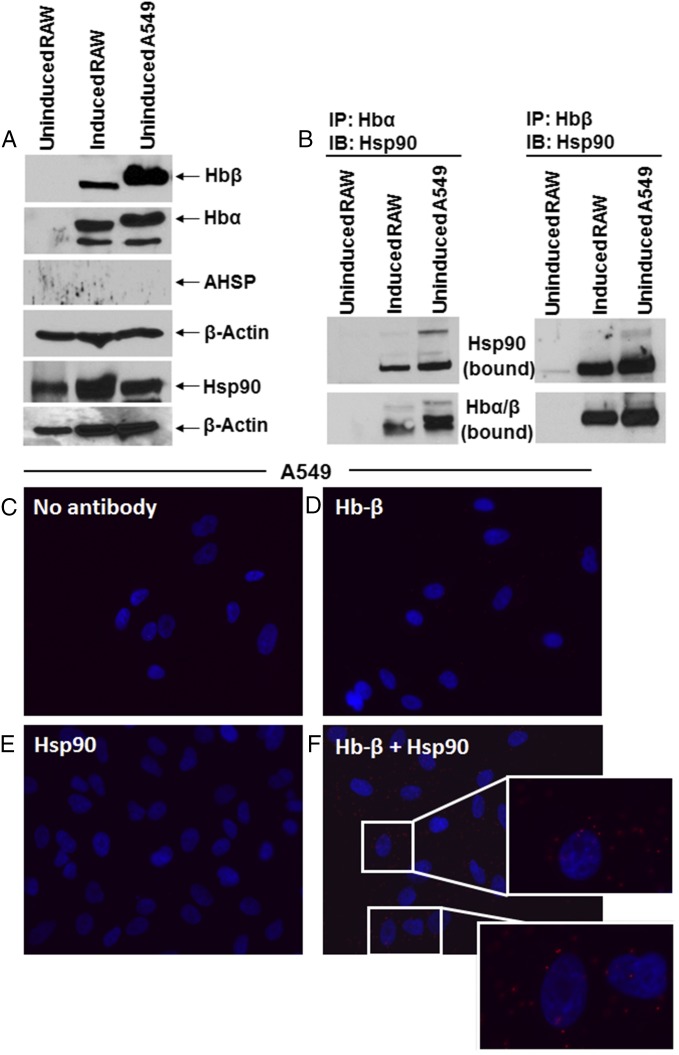
Hb-α and Hb-β are also expressed singly or in combination in diverse nonerythroid cell types, none of which naturally express AHSP (9, 19, 20). To investigate hsp90 roles in this circumstance, we first probed the mouse macrophage (RAW 264.7) and human lung epithelium (A549) cell lines and confirmed that they express heme-containing Hb-α and Hb-β (Fig. 5A and Fig. S5), either endogenously (A549) or after being immune-activated (RAW) (19, 27). AHSP protein was not expressed, and in this circumstance the antibodies directed against either Hb-α or Hb-β pulled down hsp90 in both cell types (Fig. 5B). The hsp90–globin interaction was confirmed by the proximity ligation assay (Fig. 5 C–F). These findings suggested that hsp90 may support maturation of both Hb-α and Hb-β in nonerythroid cells in the absence of AHSP. To further investigate, we transfected HEK cells to express Myc-tagged Hb-α or Hb-β, alone or in combination and with or without cotransfected AHSP. Individual transfection of Hb-α or Hb-β led HEK cells to express similar levels of either globin (Fig. 6 A and B). Cotransfection with AHSP boosted Hb-α expression several fold, consistent with it stabilizing Hb-α (16–18), but repressed the expression of Hb-β (Fig. 6 A and B and Fig. S6). When the HEK cells were cotransfected with Hb-α and Hb-β, their expression levels increased beyond an additive effect (Fig. 6 A and B). Western analysis showed that a boost in Hb-α expression level was similar to when it was coexpressed with AHSP (Fig. 6A), suggesting that there is a reduced reliance on AHSP when Hb-α and -β are coexpressed in nonerythroid cells. Regarding hsp90, Fig. 6C shows that hsp90 associated with Hb-β that was expressed either singly or together with Hb-α, whereas hsp90 did not associate with Hb-α when it was expressed alone or in combination with AHSP. This suggests that the chaperone specificity toward individual globins remains intact in nonerythroid cells.
Fig. 5.
Hsp90-globin associations in nonerythroid cells that naturally express Hb. RAW and A549 cell supernatants were analyzed for expression of Hb, hsp90, and ASHP, and the hsp90-globin associations were determined. The RAW cells were either resting or induced by a 16-h culture with IFN-γ + LPS before lysis. (A) Representative Western blots comparing protein expression levels, derived from 15% (Upper four panels) or 8% (Lower two panels) SDS/PAGE with β-actin as loading control. (B) IPs comparing hsp90 bound to Hbα or to Hb-β as indicated (input 20% in all cases). (C–F) Representative PLA images indicating colocalization of hsp90 and Hb-β in A549 cells. (C) PLA controls using no primary antibody, (D) Hb-β antibody alone, (E) Hsp90 antibody alone, (F) PLA images with both Hb-β and hsp90 antibodies with Insets showing close-ups. Red dots indicate the location and extent of the interaction.
Fig. 6.
AHSP and Hb-α have opposite impacts on Hb-β expression in nonerythroid cells. HEK cells transiently expressing Myc-tagged globins either individually or in combination with AHSP were analyzed for protein expression and protein associations. (A) Expression levels of Myc-tagged globins, AHSP, and loading control β-actin in HEK cells under conditions as indicated. (Right) Western blots probed with specific Hb antibodies. (B) Corresponding densitometry of protein expression levels. Values depicted are mean ± SD of three independent experiments (n = 3) (*P < 0.05, by one-way ANOVA). (C) IPs showing bound hsp90 and AHSP associated with Myc-Hb (input 10%) retained on the beads. (Lower three panels) The corresponding expression levels of individual globins and β-actin as indicated.
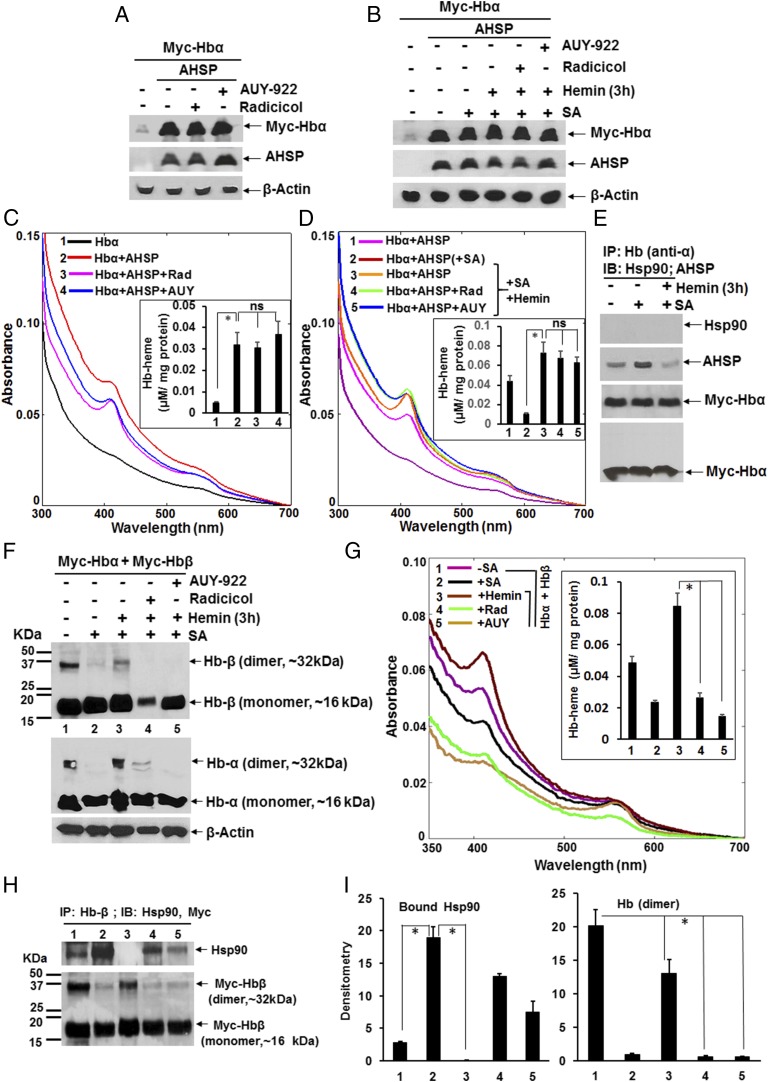
When the hsp90 inhibitors radicicol or AUY-922 were added to the HEK cells during the 28-h cotransfection of Hb-α and AHSP, neither inhibitor altered the expression level of either protein (Fig. 7A) or inhibited heme insertion into the Hb-α (Fig. 7C). Likewise, adding either hsp90 inhibitor to heme-depleted HEK cells that were expressing apo-Hb–α and AHSP did not prevent the insertion of added hemin into the apo-Hb–α (Fig. 7 B and D). In this circumstance, IP experiments showed that AHSP was associated with Hb-α before and more weakly after the heme insertion took place, and no hsp90 was found associated with Hb-α under any circumstance (Fig. 7E). This established that Hb-α heme insertion can remain hsp90-independent if AHSP is coexpressed along with it in nonerythroid cells.
Fig. 7.
AHSP-supported heme insertion into Hb-α is immune to hsp90 inhibition, whereas Hb maturation in nonerythroid cells is hsp90-dependent. HEK cells were transfected with Myc-Hb-α ± AHSP and ± radicicol (5 µM) or AUY-922 (300 nM) for 28 h, or HEK cells were heme-deprived by culturing with SA and then were transfected with Myc-Hb-α and AHSP or with Myc-Hb-α + Myc-Hb-β for 42 h, followed by hemin (5 µM) treatment for 3 h, in the presence or absence of the hsp90 inhibitors. Soluble cell supernatants (equal protein) were analyzed for protein expression, Hb heme content, and Hb protein associations. (A, B, and F) Protein expression levels under the indicated conditions. (C, D, and G) UV-visible spectra of the supernatants as indicated. Insets compare the calculated Hb heme contents. (E and H) IPs as indicated depicting AHSP or hsp90 bound to Hb-α and hsp90 bound to Hb-β, respectively (input 20% in all cases). (H) (Upper) Bound hsp90. (Lower) Myc-Hbβ retained on the beads. (I) Densitometric quantification of hsp90 and dimeric Myc-Hb protein bands as depicted in H. Values depicted in all bar graphs are mean ± SD of three independent experiments (n = 3) (*P < 0.05, by one-way ANOVA; ns, not statistically significant). Molecular markers (kDa) indicate the position of protein standards on the gels, and the experimental conditions designated 1–5 are constant from F–I.
We next probed hsp90 involvement in heme-depleted HEK cells that were cotransfected to express apo-Hb–α and apo-Hb–β in the absence of AHSP. The heme-depleted cells expressed normal levels of Hb-α and Hb-β proteins that, based on spectroscopic measures, were ≥60% heme-free, and a subsequent culture for 3 h with added heme resulted in good heme incorporation into both apo-Hb–α and apo-Hb–β (Fig. 7 F and G). Heme incorporation restored the stabilization of a PAGE-resistant Hb dimer species that had been lost in the heme-depleted condition (Fig. 7F). Moreover, the spectral measures indicated that radicicol or AUY-922 inhibited the incorporation of the added hemin into both apo-Hb–α and apo-Hb–β during the 3-h reconstitution period (Fig. 7G). Thus, Hb-α heme incorporation became hsp90-dependent in nonerythroid cells, where no AHSP is coexpressed. Pulldowns using an Hb-β–specific antibody showed that there was a strong hsp90 association with apo-Hb–β in the heme-depleted cells, which was lost after heme insertion took place, unless the hsp90 inhibitor radicicol was present during the heme reconstitution period (Fig. 7H, Upper, and Fig. 7I, Left). Also, the association of hsp90 with apo-Hb–β inversely correlated with the stabilization of the PAGE-resistant Hb dimer species (Fig. 7H, Lower, and Fig. 7I, Right).
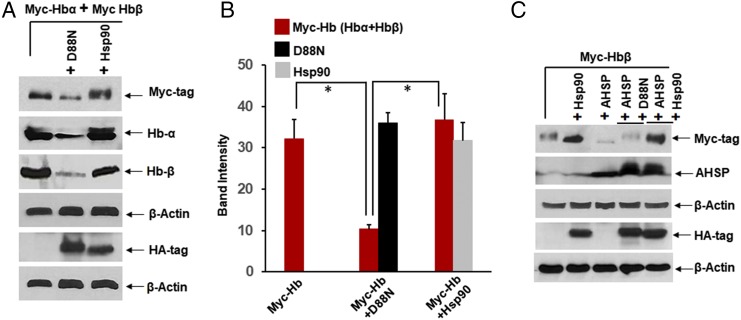
To investigate hsp90 involvement by an additional means, we cotransfected the HEK cells with Myc-tagged Hb-α and Hb-β, along with HA-tagged wild-type hsp90 or an ATPase-defective hsp90 variant (D88N hsp90) that acts as a dominant negative effector of heme insertion into other hemeproteins (23, 24). As shown in Fig. 8 A and B, the cotransfection with D88N hsp90, but not with wild-type hsp90, severely diminished buildup of Hb-α and Hb-β proteins in the cells over the 42-h period. This suggests that active hsp90 helps to stabilize both globins in nonerythroid cells. Indeed, in HEK cells transfected to express Hb-β alone, overexpression of hsp90 aided Hb-β protein buildup (Fig. 8C) and also rescued its expression from the down-regulation caused by coexpression with AHSP, whereas coexpression of the D88N hsp90 variant was ineffective at rescue (Fig. 8C). This shows that the ATPase activity of hsp90 plays a role in stabilizing Hb-β when it is expressed in the absence of Hb-α or in the face of AHSP coexpression.
Fig. 8.
Hsp90 ATPase activity is essential for Hb maturation in nonerythroid cells and can rescue Hb-β expression from inhibition by AHSP. HEK cells were transfected with Myc-Hb-β alone or in combination with Myc-Hb-α and along with HA-tagged D88N or wild-type hsp90, and/or untagged AHSP, and analyzed for protein expression. (A and C) Expression levels of proteins as indicated. (B) Corresponding densitometry of Myc-Hb or HA-tagged hsp90 protein expression levels as shown in A. Western blots are representative, and values depicted are mean ± SD of three independent experiments (n = 3) (*P < 0.05, by one-way ANOVA).
Discussion
Although a role for AHSP in Hb-α maturation has been established (16–18), what chaperones might aid partner globin maturation (Hb-γ and Hb-β) has been unclear. Our study reveals that hsp90 enables maturation of both Hb-γ and Hb-β by associating with and stabilizing their immature, heme-free forms and by driving their heme insertion reactions in an ATP-dependent manner. This was demonstrated in the erythroid-like K562 cell line and in two human erythroid progenitor cell types (HiDEP-1 and HUDEP-2) during their in vitro differentiation to mature erythroid cells, implying that hsp90’s role is relevant for globin maturation during erythropoiesis. Thus, hsp90 appears to act as a counterpart to AHSP by chaperoning Hb-γ and Hb-β for their heme insertion reactions, as is required to form functional fetal (α2γ2) and adult (α2β2) tetramers during erythropoiesis.
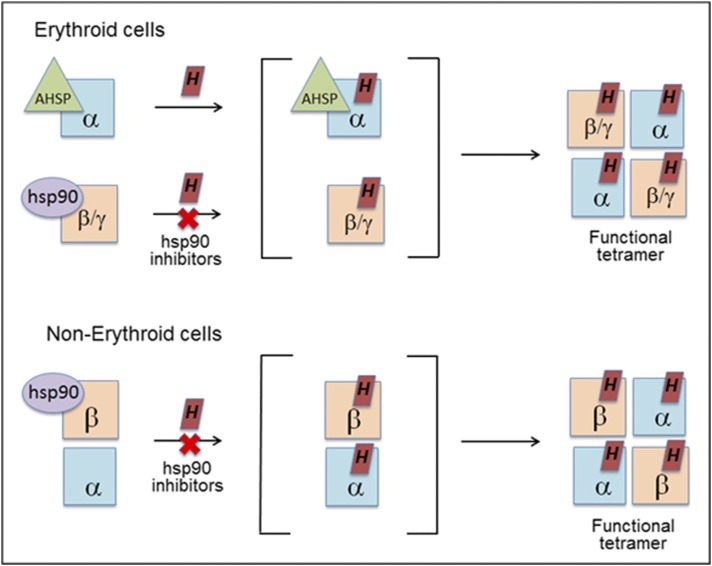
Under all circumstances, the globin associations of hsp90 or AHSP remained specific. Consider that heme insertion into Hb-β and Hb-γ was always hsp90-dependent whether AHSP was expressed or not and that Hb-β never associated with AHSP when they were coexpressed. However, we did find that Hb-β expression was actually reduced when AHSP was coexpressed with it in nonerythroid cells, and this effect could be prevented by hsp90 overexpression or by coexpression with Hb-α. Thus, AHSP antagonizes Hb-β expression when they are expressed alone together, which may explain why this expression pattern is never observed in physiologic settings. In comparison, hsp90 was never found associated with Hb-α in cells that expressed Hb-α alone or together with AHSP, and Hb-α heme insertion under these circumstances was immune to hsp90 inhibitors. Together, our findings support the view that Hb-α and Hb-β maturation are independently chaperoned in erythroid cells (28) and confirm that Hb-α maturation in erythroid cells is independent of hsp90. However, when Hb-α was coexpressed with Hb-β in nonerythroid cells either naturally or as a consequence of transfection, we saw that its heme insertion then became hsp90-dependent, possibly as a consequence of hsp90 enabling the Hb-β maturation. Thus, hsp90 is needed for heme insertion into at least two (Hb-β and Hb-γ) and as many as three (Hb-α) globins, depending on the circumstances under which they are expressed (i.e., with or without AHSP), and therefore hsp90 plays an unexpectedly broad role in globin maturation. This concept may help explain how Hb maturation can succeed in nonerythroid cells, which all lack AHSP expression. A model for Hb maturation that incorporates our current findings is presented in Fig. 9.
Fig. 9.
Model for chaperone involvement in Hb maturation. In erythroid cells, the immature, heme-free globins associate with their specific chaperones (AHSP or hsp90). Hsp90 helps drive heme insertion into its globin partner, and after the chaperones dissociate, the heme-replete globins interact to form functional hetero-tetramers. In nonerythroid cells, the heme-free globins associate in a complex with hsp90. In this case, heme insertion into the globins is entirely hsp90-dependent and allows them to form functional tetramers. See Discussion for details.
Hsp90-assisted heme insertion into Hb-β and Hb-γ appears to follow the tenets established for maturation of other heme proteins: Hsp90 associates with and stabilizes the immature heme-free form of the protein, and after helping to drive heme insertion, dissociates and is replaced by a protein partner, which generates a functional mature form (23, 24). This mechanism operates in erythroid cells (HUDEP-2) that were made heme-deficient when expressing Hb-α and -β: The heme-free Hb-β associated with hsp90 rather than Hb-α, and the Hb-α/β interaction significantly increased post heme insertion with a concurrent hsp90 dissociation from the heme-replete complex. In another example (soluble guanylate cyclase β1 subunit, sGCβ1), evidence suggests that the exclusivity of its hsp90 versus partner protein association arises in part from competition for a common binding domain on sGCβ1 (29, 30). Similarly, there is overlap between the interaction sites of AHSP and Hb-β with Hb-α (13), and this likely explains why AHSP can bind only to heme-free or heme-replete Hb-α before it is has formed an oligomer with Hb-β (13). These events can be further driven by our finding that AHSP has an inhibitory effect on Hb-β expression (Fig. 6 and Fig. S6), which can be protected and stabilized by hsp90, enabling Hb-α–ASHP and Hb-β–hsp90 to mature as parallel complexes before chaperon dissociation post heme insertion and subsequent Hb dimer and tetramer formation. We are currently working to identify the interaction sites of hsp90 and Hb-β to understand how hsp90 chaperones heme insertion during its maturation.
The discovery that hsp90 participates in Hb maturation has several biomedical and therapeutic implications. Posttranslational modifications that alter hsp90 function like phosphorylation (31, 32) and cysteine nitrosylation (32, 33) are known to undergo changes in inflammatory diseases or cancer (32, 34). Whether such modifications help hsp90 control Hb maturation during erythropoiesis can now be investigated. The hsp90 inhibitors that are being developed for cancer treatment might unintentionally block Hb maturation in the recipient. Indeed, anemia has been commonly reported as a side effect during the clinical trials of hsp90 inhibitor drug candidates (35, 36). Regarding Hb expression in nonerythroid cells, the Hb-α expressed in pulmonary endothelial cells was recently shown to regulate NO control of pulmonary blood pressure and to present an effective target for therapeutic intervention (37). Hb-β expression in lung tissues was found to be antimetastatic (38), while its expression in a variety of tumor cells increased their metastatic potential, possibly by enhancing tumor-cell survival during blood-born dissemination (39, 40). In light of our current findings, hsp90 could impact Hb maturation under all of these circumstances and thus presents a locus for controlling the diverse biological roles of Hb.
Materials and Methods
Reagents.
All chemicals were purchased from Sigma, Fischer, and StemCell Technologies. Hsp90 inhibitors (radicicol, novobiocin, and AUY-922), heme biosynthesis inhibitor SA, protein synthesis inhibitor (cycloheximide), and hemin were all purchased from Sigma. Chemicals and growth factors required for culture and differentiation of human erythroid progenitor cells were purchased from StemCell Technologies and Sigma. Dexamethasone (DEX), doxicyclin (DOX), stem cell factor (SCF), and EPO were purchased from StemCell Technologies while insulin, heparin, and holo-transferrin were purchased from Sigma. Molecular mass markers were purchased from BioRad. Human erythroid leukemia cells (K562), HEK293T, RAW 264.7, and A549 cells were purchased from the American Type Culture Collection. The human immortalized erythroid progenitor cells HiDEP-1 and HUDEP-2 were obtained from Y. Nakamura, RIKEN BioResource Center, Ibaraki, Japan. Dominant-negative hsp90 mutant (D88N) and wild-type hsp90 constructs were gifts from Bill Sessa, Yale University, New Haven, CT.
Antibodies.
Rabbit polyclonal hsp90 antibody was obtained from Cell Signaling Technology while mouse monoclonal hsp90 and rabbit polyclonal AHSP antibodies were obtained from Origene. Goat polyclonal Hb-α and Hb-βγ antibodies or mouse monoclonal Hb-α, Hb-β, and Hb-γ were purchased from Santa Cruz Biotechnology. Rabbit polyclonal Hb-β and Hb-γ antibodies were obtained from Sigma and Abcam, respectively. Mouse monoclonal Myc, HA, and β-actin antibodies were purchased from Sigma.
Cell Culture, Transient Transfection, and Growth/Differentiation of Erythroid Progenitor Cells.
All cell lines were grown and harvested as previously described (23, 41). Cultures (50–60% confluent) of K562 cells expressing basal levels of Hb-γ were treated with SA for 72 h, pretreated with the hsp90 inhibitors radicicol (10 µM) or novobiocin (250 µM) for 30 min, along with cycloheximide (10 μg/mL), and then given hemin (5 µM) for an additional 3 h before being harvested. In similar experiments, HEK cells pretreated with SA (48 h) and transiently expressing (42 h) Myc-Hbα alone with AHSP or both Myc-Hbα and -β in the absence of AHSP were treated with hsp90 inhibitors, radicicol (5 µM), or the very potent water-soluble AUY-922 (42) (300 nM) before hemin (5 µM for 3 h) addition. Control untreated cultures not receiving SA were included in all experimental setups. Other experiments included transfection of Myc-Hbα and AHSP in HEK cells in the presence or absence of hsp90 inhibitors (radicicol, 5 µM, or AUY-922, 300 nM), and cells were harvested by the 28th hour. In separate experiments, K562 cells were treated with hemin (50 µM) from 0 to 3 d to boost fetal Hb (γ) expression before being harvested. In other cases, HEK cells were transfected in various combinations with Myc-Hbα, Myc-Hbβ, AHSP, D88N, and Hsp90 for 42 h before being harvested. For experiments involving endogenous Hb expression in nonerythroids, RAW and A549 cells were grown as previously described (23, 43). Macrophages (RAW) were induced by IFN-γ + LPS as previously mentioned (23), and all cells were harvested for biochemical analyses as indicated.
For the culture and differentiation of erythroid progenitor cells, immortalized human erythroid progenitor cells HiDEP-1 and HUDEP-2 were cultured as described earlier (26) following protocols obtained from Y. Nakamura, RIKEN BioResource Center, Ibaraki, Japan. In brief, the HiDEP-1 and HUDEP-2 cells were allowed to proliferate vigorously in Stem Span SFEM media (StemCell Technologies) containing EPO (3 IU/mL), SCF (50 ng/mL), DEX (10−6 M), and DOX (1 µg/mL). The cells were then split into the desired number of T75 flasks and induced to differentiate by switching to differentiation media (IMDM media from Sigma, I:3390) containing 2% FBS + 3% AB human serum (Atlanta Biologicals), EPO (3 IU/mL), insulin (10 µg/mL), holo-transferrin (500 µg/mL), and heparin (3 U/mL). The cells were allowed to differentiate under these conditions at intervals between 0 and 16 d and were harvested for various biochemical analyses. For heme-insertion studies involving HUDEP-2 cells expressing Hb-α and Hb-β, cells were grown in StemSpan media containing growth additives as described earlier and then heme-deprived by treating with SA (400 µM) for 5 d, pretreated with hsp90 inhibitors radicicol (10 µM) or AUY 922 (300 nM) for 30 min, along with cycloheximide (10 μg/mL), and then given hemin (5 µM) for an additional 3 h before being harvested. In all cases wherever applicable, cell supernatants were assayed for protein expression by Western blot, binding assays by IPs, heme insertion by heme staining, and soret absorption by UV-visible spectroscopy, as indicated.
Western Blots, Heme Staining, and IPs.
For Western blots, standard protocols were followed as previously mentioned (23, 24, 29). Heme staining of cell supernatants (50 μg) from differentiating HiDEP-1/HUDEP-2 or from RAW/A549 (150 µg) cells was done as previously described (41). For IPs, 500 μg of the total cell supernatant was precleared with 20 µL of protein G-Sepharose beads (Amersham) for 1 h at 4 °C, beads were pelleted, and the supernatants were incubated overnight at 4 °C with 3 μg of anti–Hb-βγ, Hb-α, or Myc antibodies. Protein G-Sepharose beads (20 µL) were then added and incubated for 1 h at 4 °C. The beads were microcentrifuged (6,000 × g), washed three times with wash buffer (50 mM Hepes, pH 7.6, 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40) and then boiled with SDS buffer and centrifuged. The supernatants were then loaded on SDS/PAGE gels and Western-blotted with specific antibodies. Band intensities on Western blots were quantified using Image J quantification software (NIH).
UV-Visible Absorption Spectroscopy.
UV-visible absorption spectra of cell supernatants were recorded at room temperature between 350 and 700 nm on a Shimadzu spectrophotometer. Equal amounts of total protein supernatants were used for respective wavelength scans. The heme content for Hb was determined from the Soret absorption peak at 414, using the extinction coefficient of 342.5 mM−1⋅cm−1 and a manipulation to account for the variable absorbance contributions that were attributable to sample turbidity. This involved creating a baseline for each scan by drawing a line that connected the absorbance values at 380 and 470 nm. The additional Soret absorbance at 414 nm above this baseline was then used to calculate the Hb heme content.
Proximity Ligation Assay.
PLAs (Duolink; Sigma) for colocalization were performed according to the manufacturer’s protocol using primary antibodies (anti–Hb-β, 1:25; and anti–Hsp90, 1:200 for HUDEP-2 and 1:400 for A549), followed by a pair of oligonucleotide-labeled secondary antibodies. The assay detects positive signal only when the epitopes of the target proteins are in close proximity (<40 nm). The signal from each of the detected pair of PLA probes was then imaged using fluorescence microscopy (excitation/emission for Duolink red: 594/624; excitation/emission for Dapi: 360/460). One or both primary antibodies were omitted for negative controls.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grants GM 097041 (to D.J.S.) and HL081064 (to D.J.S. and A.G.); a Research Centre for Excellence Grant from the Cleveland Clinic (to A.G. and D.J.S.); and an American Heart Association Postdoctoral Fellowship (to E.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1717993115/-/DCSupplemental.
References
- 1.Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood. 2008;112:3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varnado CL, et al. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18:2314–2328. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doctor A, Stamler JS. Nitric oxide transport in blood: A third gas in the respiratory cycle. Compr Physiol. 2011;1:541–568. doi: 10.1002/cphy.c090009. [DOI] [PubMed] [Google Scholar]
- 4.Orkin SH. Regulation of globin gene expression in erythroid cells. Eur J Biochem. 1995;231:271–281. doi: 10.1111/j.1432-1033.1995.tb20697.x. [DOI] [PubMed] [Google Scholar]
- 5.Khandros E, Weiss MJ. Protein quality control during erythropoiesis and hemoglobin synthesis. Hematol Oncol Clin North Am. 2010;24:1071–1088. doi: 10.1016/j.hoc.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantú I, Philipsen S. Flicking the switch: Adult hemoglobin expression in erythroid cells derived from cord blood and human induced pluripotent stem cells. Haematologica. 2014;99:1647–1649. doi: 10.3324/haematol.2014.116483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 8.Kong Y, et al. Loss of alpha-hemoglobin-stabilizing protein impairs erythropoiesis and exacerbates beta-thalassemia. J Clin Invest. 2004;114:1457–1466. doi: 10.1172/JCI21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss MJ, et al. Role of alpha-hemoglobin-stabilizing protein in normal erythropoiesis and beta-thalassemia. Ann N Y Acad Sci. 2005;1054:103–117. doi: 10.1196/annals.1345.013. [DOI] [PubMed] [Google Scholar]
- 10.Crispino JD, Weiss MJ. Erythro-megakaryocytic transcription factors associated with hereditary anemia. Blood. 2014;123:3080–3088. doi: 10.1182/blood-2014-01-453167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sankaran VG, Weiss MJ. Anemia: Progress in molecular mechanisms and therapies. Nat Med. 2015;21:221–230. doi: 10.1038/nm.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–1843. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 13.Mollan TL, Yu X, Weiss MJ, Olson JS. The role of alpha-hemoglobin stabilizing protein in redox chemistry, denaturation, and hemoglobin assembly. Antioxid Redox Signal. 2010;12:219–231. doi: 10.1089/ars.2009.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh MK, Yu J. Accumulation of a heat shock-like protein during differentiation of human erythroid cell line K562. Nature. 1984;309:631–633. doi: 10.1038/309631a0. [DOI] [PubMed] [Google Scholar]
- 15.Banerji SS, Theodorakis NG, Morimoto RI. Heat shock-induced translational control of HSP70 and globin synthesis in chicken reticulocytes. Mol Cell Biol. 1984;4:2437–2448. doi: 10.1128/mcb.4.11.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kihm AJ, et al. An abundant erythroid protein that stabilizes free alpha-haemoglobin. Nature. 2002;417:758–763. doi: 10.1038/nature00803. [DOI] [PubMed] [Google Scholar]
- 17.Feng L, et al. Structure of oxidized alpha-haemoglobin bound to AHSP reveals a protective mechanism for haem. Nature. 2005;435:697–701. doi: 10.1038/nature03609. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, et al. An erythroid chaperone that facilitates folding of alpha-globin subunits for hemoglobin synthesis. J Clin Invest. 2007;117:1856–1865. doi: 10.1172/JCI31664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu L, Zeng M, Stamler JS. Hemoglobin induction in mouse macrophages. Proc Natl Acad Sci USA. 1999;96:6643–6647. doi: 10.1073/pnas.96.12.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straub AC, et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dean A, Ley TJ, Humphries RK, Fordis M, Schechter AN. Inducible transcription of five globin genes in K562 human leukemia cells. Proc Natl Acad Sci USA. 1983;80:5515–5519. doi: 10.1073/pnas.80.18.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huo XF, et al. Differential expression changes in K562 cells during the hemin-induced erythroid differentiation and the phorbol myristate acetate (PMA)-induced megakaryocytic differentiation. Mol Cell Biochem. 2006;292:155–167. doi: 10.1007/s11010-006-9229-0. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Chawla-Sarkar M, Stuehr DJ. Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process. FASEB J. 2011;25:2049–2060. doi: 10.1096/fj.10-180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Stuehr DJ. Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme. Proc Natl Acad Sci USA. 2012;109:12998–13003. doi: 10.1073/pnas.1205854109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zhang T, Schwartz SJ, Sun D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: Mechanisms, clinical perspective and more potential. Drug Resist Updat. 2009;12:17–27. doi: 10.1016/j.drup.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurita R, et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS One. 2013;8:e59890. doi: 10.1371/journal.pone.0059890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton DA, Rao KM, Dluhy RA, Baatz JE. Hemoglobin is expressed by alveolar epithelial cells. J Biol Chem. 2006;281:5668–5676. doi: 10.1074/jbc.M509314200. [DOI] [PubMed] [Google Scholar]
- 28.Weiss MJ, dos Santos CO. Chaperoning erythropoiesis. Blood. 2009;113:2136–2144. doi: 10.1182/blood-2008-09-115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh A, Stasch JP, Papapetropoulos A, Stuehr DJ. Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content. J Biol Chem. 2014;289:15259–15271. doi: 10.1074/jbc.M114.559393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar A, et al. Heat shock protein 90 associates with the Per-Arnt-Sim domain of heme-free soluble guanylate cyclase: Implications for enzyme maturation. J Biol Chem. 2015;290:21615–21628. doi: 10.1074/jbc.M115.645515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W, et al. Dynamic tyrosine phosphorylation modulates cycling of the HSP90-P50(CDC37)-AHA1 chaperone machine. Mol Cell. 2012;47:434–443. doi: 10.1016/j.molcel.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prodromou C. Regulatory mechanisms of Hsp90. Biochem Mol Biol J. 2017;3:2. doi: 10.21767/2471-8084.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Ruiz A, et al. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA. 2005;102:8525–8530. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillai RN, Ramalingam SS. Hsp90 inhibitors. J Thorac Oncol. 2012;7(Suppl 5):S407–S408. doi: 10.1097/JTO.0b013e31826df2bb. [DOI] [PubMed] [Google Scholar]
- 36.Do K, et al. Phase I study of the heat shock protein 90 (Hsp90) inhibitor onalespib (AT13387) administered on a daily for 2 consecutive days per week dosing schedule in patients with advanced solid tumors. Invest New Drugs. 2015;33:921–930. doi: 10.1007/s10637-015-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez RA, et al. Targeting pulmonary endothelial hemoglobin α improves nitric oxide signaling and reverses pulmonary artery endothelial dysfunction. Am J Respir Cell Mol Biol. 2017;57:733–744. doi: 10.1165/rcmb.2016-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maman S, et al. The beta subunit of hemoglobin (HBB2/HBB) suppresses neuroblastoma growth and metastasis. Cancer Res. 2017;77:14–26. doi: 10.1158/0008-5472.CAN-15-2929. [DOI] [PubMed] [Google Scholar]
- 39.Capulli M, et al. Increased expression of a set of genes enriched in oxygen binding function discloses a predisposition of breast cancer bone metastases to generate metastasis spread in multiple organs. J Bone Miner Res. 2012;27:2387–2398. doi: 10.1002/jbmr.1686. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, et al. Expression of β-globin by cancer cells promotes cell survival during blood-borne dissemination. Nat Commun. 2017;8:14344. doi: 10.1038/ncomms14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waheed SM, et al. Nitric oxide blocks cellular heme insertion into a broad range of heme proteins. Free Radic Biol Med. 2010;48:1548–1558. doi: 10.1016/j.freeradbiomed.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oki Y, et al. Experience with HSP90 inhibitor AUY922 in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica. 2015;100:e272–e274. doi: 10.3324/haematol.2015.126557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh A, et al. Soluble guanylate cyclase as an alternative target for bronchodilator therapy in asthma. Proc Natl Acad Sci USA. 2016;113:E2355–E2362. doi: 10.1073/pnas.1524398113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.